Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Carmelia Milano.
Melatonin is a lipophilic hormone synthesized and secreted mainly in the pineal gland, acting as a neuroendocrine transducer of photoperiodic information during the night. In addition to this activity, melatonin has shown an antioxidant function and a key role as a regulator of physiological processes related to human reproduction. Several studies have evaluated its role in ovarian dysfunction, ovarian ageing, embryo maturation and gynecological cancer.
- melatonin gestational period
- melatonin in pregnancy
1. Melatonin in Pregnancy
Melatonin has an important role in human reproduction, it is essential in each phase of ovulation, fertilization, embryo implantation and finally as a pregnancy regulator [1]. The role of melatonin in the first time of reproduction has been deeply investigated. The study by Carlomagno G. et al., 2018, reviewed several in vitro and in vivo studies, confirming that the antioxidant action by melatonin to remove free radicals from the oocyte and the embryo, together with the immunomodulator action, is essential to support implantation and proliferation. This is demonstrated by the presence of melatonin in higher concentrations in ovary tissue and by MT1 and MT2 receptors expressed in the ovary and placenta tissue during embryo implantation for improving the success and quality of embryo development. Throughout gestation, serum melatonin concentrations in the mother display fluctuations in both physiological and pathological pregnancies [20][2]. In adults, melatonin levels remain low throughout the day. In the early evening, the levels begin to increase, peaking between 02:00 and 03:00 and then falling back down to low daylight concentrations again in the morning [21][3]. This production followed the 24 h rhythm and relies on a series of gene transcription and translation feedback loops by a group of circadian genes known as “clock genes”, which are contained in the placenta and that are modulated especially by melatonin hormone and glucocorticoids ones [22][4]. In fact, the circadian rhythm is amplified during pregnancy likely due to de novo melatonin placental synthesis. Particularly, a significant increase in serum melatonin occurs after 24 weeks after implantation, increasing again after 32 weeks [23][5]. The relationship between the circadian clock system and the immune system is important during pregnancy. Wai M.G.C. et al., 2017 explained in an animal and human study that immunity and clock gene control is important during pregnancy, because through the modulation in T-cell responses, melatonin exerts potential beneficial effects in suppressing various diseases with inflammatory origins, including preterm labor, gestational diabetes, and preeclampsia (Figure 21). Furthermore, in the third trimester of pregnancy, melatonin crosses the placenta and blood–brain barrier from the maternal circulation to the fetus and melatonin receptors are widespread in the fetus in both central and peripheral tissue from early fetal development [19][6]. Lastly, after birth, the values return to physiological levels within two days [23][5]. Another potential application of melatonin could be its use in the treatment of insomnia during pregnancy, but wthe researchers did not find trials where the primary outcomes suggested the safety or efficacy of melatonin for insomnia or other sleep disorders during pregnancy.
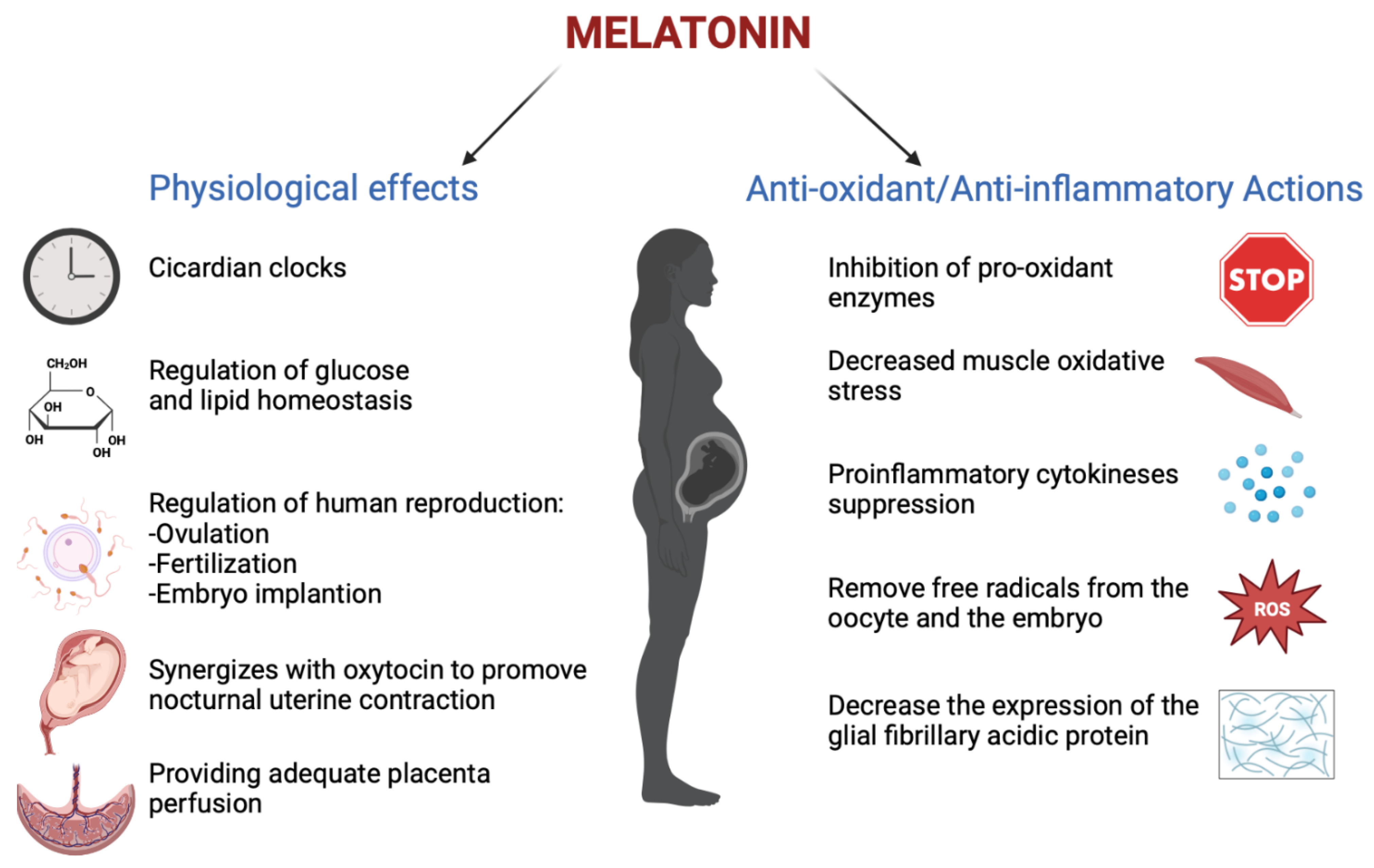
Figure 21. The physiological functions performed by melatonin in pregnancy (Created with BioRender.com on 1 January 2022).
2. Melatonin and Placenta
As mentioned previously, melatonin serum concentration is higher in pregnant women due to de novo placental synthesis [23][5]. In fact, the placenta represents the source of melatonin and it also contains a melatonin receptor. The surface of the placenta includes two different types of cells: mononuclear villous cytotrophoblast and the multinucleated syncytiotrophoblast. The fusion of the cells villous cytotrophoblasts forms the syncytiotrophoblast by the melatonin regulated process. Specifically, the melatonin preserves the apoptosis of villous cytotrophoblast to promote the development of the syncytiotrophoblast due to paracrine, autocrine and/or intracrine actions of the MT1 and MT2 receptors in the placenta [24][7]. Additionally placental melatonin acts with the MT1 and MT2 receptors and by the ROS (reactive oxygen species) to diminish placental oxidative damage [23,25][5][8]. Because melatonin maintains the turnover of the syncitiotrophoblast and protects the placenta from the antioxidant action, it has obtained the role of regulator of placental homeostasis [24][7].
In a recent case-control study by Ejaz H. et al., 2021, the serum level of melatonin and its major metabolite 6-OHMS in normal pregnant women was determined during each trimester of pregnancy and immediately after delivery (Table 21).
Table 21. This table shows melatonin levels characteristic for pregnancy, newborns and mother`s milk. The melatonin plasma level increases significantly during pregnancy with highest levels in the third trimester and decreases abruptly after delivery. Subsequently, it is secreted in the breastmilk and its concentration in the serum of newborn becomes low after early days.
| Serum melatonin concentration | |
| I TRIMESTER OF PREGNANCY | Up to 611.4 pg/mL |
| II TRIMESTER OF PREGNANCY | Up to 1246.4pg/mL |
| III TRIMESTER OF PREGNANCY | Up to 1372 pg/mL (Ejaz H. et al., 2021) |
| AT DELIVERY | Up to 158 pg/mL (Biran V., 2019) |
| Serum melatonin concentration | |
| NEWBORN AT BIRTH | Up to 184 pg/mL |
| NEWBORN ON 3 DAYS AFTER BIRTH | Up to 75 pg/mL (Biran V., 2019) |
| Milk melatonin concentration | |
| MOTHER MILK | Up to 20 pg/mL (Biran V., 2019) |
Blood samples were obtained from a cohort of 26 healthy pregnant women during each trimester of pregnancy, from 15 women scheduled for elective cesarean section (CS) before and after delivery, along with placental samples, and from 30 healthy non-pregnant women as controls. It showed that the levels of serum melatonin were significantly higher during pregnancy than in non-pregnant women and increased throughout pregnancy. In women undergoing CS, serum melatonin decreased markedly 24 h after delivery. Similar results were seen for serum levels of 6-OHMS, and placental tissue 6-OHMS levels correlated with the week of gestation at delivery.
In conclusion, maternal melatonin production is higher in pregnant than in non-pregnant women, increases significantly during pregnancy with the highest levels in the third trimester, and decreases abruptly after delivery [26][9]. These results suggest that the placenta is a major source of melatonin and supports a physiological role for melatonin in pregnancy. Because the placenta plays a key role in the numerous pathologies of pregnancy, its production of melatonin has also been studied in the context of high-risk pregnancy, as it is underlined in the review by Laste G. et al., 2021.
3. Melatonin in High-Risk Pregnancy
A high-risk pregnancy is defined as a pregnancy in which the mother or the baby may be at increased risk for health problems during the pregnancy and/or during and after delivery. A high-risk pregnancy may involve chronic health problems, such as diabetes or high blood pressure; infections; complications from a previous pregnancy, or other issues that might arise during pregnancy. [24][7]. The mechanisms involved in pregnancy complications have been studied, demonstrating the role of melatonin in these processes [23,24,26,27][5][7][9][10]. Particularly, the review by Last G. et al., 2021 reported that melatonin is important in gestational diabetes and preeclampsia.
3.1. Melatonin and Gestational Diabetes
Gestational diabetes mellitus (GDM) is one of the most common complications in pregnancy. Its previous definition was “any degree of glucose intolerance with onset or first recognition during pregnancy”. GDM is responsible for many risks for the mother, such as preeclampsia, primary cesarean delivery, preterm delivery, hydramnios, and for the child, including macrosomia, neonatal hypoglycemia, shoulder dystocia/birth injury, neonatal respiratory problems, hyperbilirubinemia, hypocalcemia, and intensive neonatal care. An additional long-term risk for the mother is a 30–70% recurrence risk of gestational diabetes, which is also largely dependent on weight gain between pregnancies, in addition to a 7-fold increase in the risk of diabetes occurrence after 5–10 years, as well as an increased risk in metabolic syndrome or cardiovascular disease [28][11].
In gestational diabetes, it was shown that a decrease in melatonin levels increased glucose transport to embryos, which could then augment the oxidative state of cells by decreasing free radical scavenging, increasing oxidative metabolism, or both. The formation of oxidative stress species would be a direct consequence of hyperglycemia, leading to various diabetic embryopathies [29][12]. In addition, recent reports demonstrated that melatonin receptor 1B (MTNR1B) gene polymorphisms may influence insulin secretion and pancreatic glucose sensing, causing gestational diabetes mellitus (GDM) [30,31][13][14]. The review by Laste G et al., 2021 analyzes 12 articles, of which the main findings are the association between MTNR1B polymorphisms and gestational diabetes mellitus. It cites one of the first reviews by Zhang and colleagues [32][15], which found a higher frequency of G alleles in MTNR1B rs10830963 and T alleles in both MTNR1B rs1387153 and rs1801278 in GDM patients than in healthy controls. Additionally, a meta-analysis by Huang and colleagues [33][16] indicated that the variant G allele of the MTNR1B rs10830963 polymorphism significantly increased the risk of GDM. Then, in Alharbi K.K. et al., 2019, MTNR1B variants were found to be related to insulin secretion and impaired β-cell function and GDM could develop when a genetic predisposition to pancreatic islet β-cell impairment is unmasked by increased insulin resistance during pregnancy. Finally, the review by Liao and colleagues [34][17] showed that MTNR1B is likely involved in the regulation of glucose homeostasis during pregnancy. MTNR1B rs10830963 and rs1387153 were shown to influence fasting plasma glucose (FPG) [32,35][15][18] and were associated with a higher risk of type 2 diabetes. In conclusion, this revisewarch states that decreased melatonin levels were found to be positively correlated with an increased risk of glycemic disorder, and melatonin administration was found to reduce the risk of glycemic disturbance [36][19].
3.2. Melatonin and Preeclampsia
Preeclampsia is an expressive gestational disorder that affects 3 to 10% of pregnancies, classically characterized by high blood pressure and proteinuria [37][20]. It is characterized by new-onset hypertension which usually occurs after 20 weeks of gestation and evidence of end-organ dysfunction. The end-organ disease resulting from preeclampsia is varied and can include proteinuria, acute kidney injury, hepatic dysfunction, hemolysis, thrombocytopenia, and, less frequently, liver rupture, seizures (eclampsia), stroke, and death. There are several risk factors for developing preeclampsia such as a history of preeclampsia in a prior pregnancy, diabetes, hypertension, obesity, and multiple pregnancies [38][21]. The placenta has always been a central figure in the etiology of preeclampsia because the removal of the placenta is necessary for symptoms to regress [39][22]. Impaired placenta function may cause preeclampsia, leading to oxidative stress and pro-inflammatory biomarkers. In preeclamptic placentas, the expression of melatonin-synthesizing enzymes, melatonin levels, and melatonin receptors is reduced [40][23]. It is plausible that melatonin, an antioxidant with anti-inflammatory and anti-apoptotic effects, protects cells/tissues by indirectly increasing gene expression and reducing errors in nuclear deoxyribonucleic acid (DNA) [41][24]. The review by Chuffa LGA et al., 2019 documents that melatonin levels and its receptor are depressed during severe preeclampsia. In the first trimester of gestation, the MT1 receptor is more important for promoting villous cytotrophoblast syncialization by protecting trophoblastic cells against oxidative injuries and increasing apoptosis in altered ones.
Experimental evidence supports the role of melatonin in providing adequate placental perfusion while preventing vascular damage, inflammation, and local oxidative stress. At pharmacologically relevant levels, melatonin reduces ROS (Reactive oxygen species) and hypertension in preeclamptic tissue and may be considered useful as a natural adjuvant for the treatment of preeclampsia. The review by Man GCW et al., 2017 states that melatonin inhibits the VEGF (vascular endothelial growth factor) expression and hypoxia-induced factor-1α(HIF-1α), a mediator of VEGF, which is predominantly active within the vascular endothelial cells. That the VEGF detrimentally influences the outcome of preeclampsia under the influence of the clock genes network is entirely speculative. As VEGF is predominantly active within the vascular endothelial cells, it lends itself as a prime candidate to this speculative, but plausible, association of a “clock” determining factor for preeclampsia. In fact, normal blood pressure is known to vary in a circadian manner, but in those with preeclampsia, this circadian relationship is lost, probably influenced by circadian rhythm under the melatonin. Hobson S.R. et al., 2018, observed that melatonin supplementation prolonged gestation and reduced the dosage of antihypertensive drugs, suggesting there is likely to be a new and promising paradigm shift in terms of diagnostics and therapeutics [42][25].
3.3. Melatonin and Intrauterine Growth Restriction (IUGR)
Fetal growth restriction (FGR) or IUGR is a common fetus and neonatal complication of preeclampsia [43][26]. In this pregnancy condition, the fetus does not reach its biological growth potential as a consequence of impaired placental function, which may be because of a variety of factors. Fetuses with FGR are at risk for perinatal morbidity and mortality and poor long-term health outcomes such as impaired neurological and cognitive development, and cardiovascular and endocrine diseases in adulthood. It is usually defined by the statistical deviation of fetal size after a population-based study; its algorithm was summarized in “Delphi procedure consensus criteria for defining fetal growth restriction”. SGA, instead, differs from FGR, principally because it also encompasses a majority of constitutionally small but healthy fetuses at lower risk of abnormal perinatal outcomes (Gordijn SJ., 2016).
Pregnancy complicated by hypertensive disorders, including preeclampsia, has been shown to substantially increase the risk of FGR in subsequent small-for-gestational-age newborns [43][26]. In some recent studies, it was established that in pregnant women with placenta insufficiency manifested as intrauterine fetal growth restriction syndrome, the blood concentrations of PlGF (Placental growth factor) significantly decrease. PIGF has a pro-angiogenic effect on the placental tissue; it also stimulates the proliferation of the trophoblast, and it is known as a predictor and a diagnostic marker of preeclampsia and is responsible for the angiogenesis in the placenta. Recently, it was found that in the case of IUGR, melatonin concentrations in maternal blood significantly decrease, resulting in strengthening of the pro-inflammatory immunity, increasing the levels of the anti-inflammatory cytokines, which was significant in the case of IUGR [44][27]. In the case-control study by Berbets A.M. et al., 2020, whether the level of melatonin, cytokines, and PlGF in umbilical blood after birth is different in the case of IUGR compared to normal fetuses was investigated. The study was conducted on 14 women whose pregnancies were complicated with IUGR. The control group consisted of 13 women who had uncomplicated pregnancies. The umbilical blood was taken immediately after a baby’s birth from the placental side of the clamped and cut umbilical cord before the birth of the placenta. In the end, it was confirmed that the level of melatonin and PIGF in the umbilical blood taken during the third period of labor from pregnant women whose pregnancies were complicated with IUGR is considerably decreased compared to the patients with uncomplicated pregnancies. This is probably caused by the placenta’s altered production of melatonin. Larger studies are needed to confirm this correlation and to confirm melatonin as a new marker of placenta function.
References
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802.
- Kivela, A. Serum melatonin during human pregnancy. Acta Endocrinol. 1991, 124, 233–237.
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24.
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The circadian clock controls toll-like-receptor 9-mediated innate and adaptive immunity. Immunity 2012, 36, 251–261.
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yagamata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to the feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33.
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The regulations and role of circadian clock and melatonin in uterine receptivity and pregnancy-An immunological perspective. Am. J. Reprod. Immunol. 2017, 78, e12715.
- NIH 2017. What Is a High-Risk Pregnancy? Available online: https://www.nichd.nih.gov/health/topics/pregnancy/conditioninfo/high-risk (accessed on 31 January 2017).
- Lanoix, D.; Guérin, P.; Vaillancourt, C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: New insights into the role of this hormone in pregnancy. J. Pineal Res. 2012, 53, 417–425.
- Ejaz, H.; Figaro, J.K.; Woolner, A.M.F.; Thottakam, B.M.V.; Galley, H.F. Maternal Serum Melatonin Increases During Pregnancy and Falls Immediately After Delivery Implicating the Placenta as a Major Source of Melatonin. Front. Endocrinol. 2021, 11, 623038.
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307.
- Spaight, C.; Gross, J.; Horsch, A.; Puder, J.J. Gestational Diabetes Mellitus. Endocr. Dev. 2016, 31, 163–178.
- Liu, S.; Guo, Y.; Yuan, Q.; Pan, Y.; Wang, L.; Liu, Q.; Wang, F.; Wang, J.; Hao, A. Melatonin prevents neural tube defects in the offspring of diabetic pregnancy. J. Pineal Res. 2015, 59, 508–517.
- Firneisz, G.; Rosta, K.; Al-Aissa, Z.; Hadarits, O.; Harreiter, J.; Nádasdi, Á.; Bancher-Todesca, D.; Németh, L.; Igaz, P.; Rigó, J., Jr.; et al. The MTNR1B rs10830963 Variant in Interaction with Pre-Pregnancy BMI is a Pharmacogenetic Marker for the Initiation of Antenatal Insulin Therapy in Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3734.
- Nisa, H.; Qi, K.H.T.; Leng, J.; Zhou, T.; Liu, H.; Li, W.; Wang, L.; Li, N.; Hu, G.; Qi, L. The Circadian Rhythm-Related MTNR1B Genotype, Gestational Weight Gain, and Postpartum Glycemic Changes. J. Clin. Endocrinol. Metab. 2018, 103, 2284–2290.
- Zhang, Y.; Sun, C.M.; Hu, X.Q.; Zhao, Y. Relationship between melatonin receptor 1B and insulin receptor substrate 1 polymorphisms with gestational diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2014, 4, 6113.
- Huang, B.; Wang, Y.; Qin, L.; Wei, Q.; Liu, N.; Jiang, M.; Yu, H.-P.; Yu, X.-Y. A functional polymorphism rs10830963 in melatonin receptor 1B associated with the risk of gestational. Biosci. Rep. 2019, 39, BSR20190744.
- Liao, S.; Liu, Y.; Tan, Y.; Gan, L.; Mei, J.; Song, W.; Chi, S.; Dong, X.; Chen, X.; Deng, S. Association of genetic variants of melatonin receptor 1B with gestational plasma glucose level and risk of glucose intolerance in pregnant Chinese women. PLoS ONE 2012, 7, e40113.
- Liu, C.; Wu, Y.; Li, H.; Qi, Q.; Langenberg, C.; Loos, R.J.; Lin, X. MTNR1B rs10830963 is associated with fasting plasma glucose, HbA1C and impaired beta-cell function in Chinese Hans from Shanghai. BMC Med. Genet. 2010, 11, 59.
- Laste, G.; da Silva, A.A.; Gheno, B.R.; et Rychcik, P.M. Relationship between melatonin and high-risk pregnancy: A review of investigations published between the years 2010 and 2020. Chronobiol. Int. 2021, 38, 168–181.
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011.
- Ma’ayeh, M.; Costantine, M.M. Prevention of preeclampsia. Semin. Fetal Neonatal Med. 2020, 25, 101123.
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113.
- Sagrillo-Fagundes, L.; Assunção Salustiano, E.M.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520.
- Cheung, R.T.F. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J. Pineal Res. 2003, 34, 153–160.
- Genario, R.; Morello, E.; Bueno, A.A.; Santos, H.O. The usefulness of melatonin in the field of obstetrics and gynecology. Pharmacol. Res. 2019, 147, 104337.
- McKinney, D.; Boyd, H.; Langager, A.; Oswald, M.; Pfister, A.; Warshak, C.R. The impact of fetal growth restriction on latency in the setting of expectant management of preeclampsia. Am. J. Obstet. Gynecol. 2016, 214, 395.e1-7.
- Berbets, A.M.; Barbe, A.M.; Andriiets, O.A.; Andriiets, A.V.; Yuzko, O.M. Melatonin Levels Decrease in the Umbilical Cord in Case of Intrauterine Growth Restriction. J. Med. Life 2020, 13, 548–553.
More
