You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 2 by Beatrix Zheng.
Herbs and spices have been historically characterized as plant components utilized in the diet for their fragrant characteristics, but have no or minimal nutritional value. Herbs and spices, on the other hand, have been found as sources of different phytochemicals with strong antioxidant activity. As a result, phytoconstituents from herbs and spices can be formulated in various forms of nano-drug delivery systems. The nanodelivery systems for various phytoconstituents are discussed below.
- dietary polyphenols
- nanotechnology
- nanoformulation
- nanophytomedicine
- nanodelivery
1. Solid-Lipid Nanoparticles
Solid-lipid nanoparticles (SLNs) are concurrent to nano-emulsions with the minor difference of having lipids playing the role as a solid phase. SLNs are the sub-micron (50–1000 nm)-sized colloidal nano-carriers composed of physiologically compatible lipids dispersed in an aqueous solution having surfactants dissolved in it. Some of the methods by which SLNs are prepared are micro fluidization (also called high-pressure homogenization) and ultra-sonication [1]. The merits of SLNs are that they are of submicron size, providing a large surface area, increased drug payload, and enhanced interfacial interaction. Colloidal nanocarriers such as nanoemulsion, microemulsions, polymeric nanoparticles, and liposomes do not provide efficient targeting effects on the site of action and controlled release profile, and these are some of the limitations encountered by SLNs. Drug payload is achieved in two ways. Firstly, the drug can be integrated into the polymeric core, and secondly, it can be attached to the surface of the polymeric part. A major advantage perceived in SLNs is that they entrap the lipophilic drug in a stable form without the use of harmful organic solvents [2]. Other advantages of SLNs include their unique size-related aspects and their ability for drug incorporation; both lipophilic and hydrophilic drugs can be loaded. Large scale production is easier to achieve, as well as higher bioavailability with minimal toxic effects.
2. Nano-Emulsions
Nano-emulsions include mixtures of two or more immiscible liquids, of which one is water and the other may be any type of oil used in the formulation. These types of nano-formulations are prepared from chemical or mechanical process. When a chemical process is used, nanoemulsion droplets are formed as a result of the interface of hydrophobic compounds with emulsifiers, whereas a mechanical process includes high shear homogenization of large emulsion droplets to form nano-droplets. A basic concept which differs from conventional emulsion with nanoemulsion is the size and shape of emulsion droplets formed (ranging from 20–200 nm) [3].
3. Nano-Crystals
Nano-crystals are sub-micron (20 to 850 nm)-sized colloids dispersed in a dissimilar phase consisting of drug molecules. These nano-crystals are also formulated using chemical or mechanical methods, with an advantage of reduced particle size within a nano-range and provide increased surface area to remain in contact with the dissolving phase. Some of the merits of nanocrystals include improved saturation solubility and dissolution rate with greater drug loading, which provide more benefits compared to conventional dosage forms [4].
4. Nano-Polymersomes
Nano-polymersomes (NPS) are vesicles of polymers with a size ranging between 10 nm to 1 µm, which are forms of an aqueous core as a result of self-assembling amphiphilic copolymers. The tuneable properties of NPS allow for flexible biomedical advantages; e.g., as drug delivery vehicles or as artificial organelles. The synthetic process is the same as polymeric nanoparticles [5]. In case of NPS, a wide variety of biodegradable and stimuli-responsive polymers are utilized in drug encapsulation and improving the release behavior owing to their tuneable properties. NPS have an excellent capability in incorporating both types of drug molecules; i.e., lipophilic (in the polymeric membrane bilayer) and hydrophilic (in the aqueous core). The major advantages over nano-lipid carriers (NLCs) are that NPS provide a more stable vesicular formulation, a wide range of usages, and a controlled drug release profile [6][7].
5. Liposomes
Liposomes are biodegradable sphere-shaped phospholipid bilayer vesicles with an aqueous core (Figure 1). They are formed by the self-assembly of phospholipids. The organized structure of liposomes allows the loading of hydrophilic drug molecules in the inner aqueous core and lipophilic drug molecules into the phospholipid bilayer. Liposomes are synthesized by different conventional methods, some of which are the thin film hydration method, reverse phase evaporation, the solvent injection method, and the detergent removal method. Liposomes are categorized as unilamellar, multilamellar, and multivesicular vesicles based on the size of the vesicle and number of phospholipid bilayers [8][9][10]. From the literature search, it was found that liposomal forms of polyphenols have improved therapeutic benefits. Researchers reported that catechin liposomes showed increased bioavailability and cerebral distribution when compared to catechin alone [11]. Curcumin liposomes have a prolonged antioxidant effect compared to the uncomplexed curcumin [12]. Researchers have developed a quercetin liposomal formulation and observed that the formulation showed increased solubility, bioavailability, and in vivo antitumor efficacy [13][14][15].
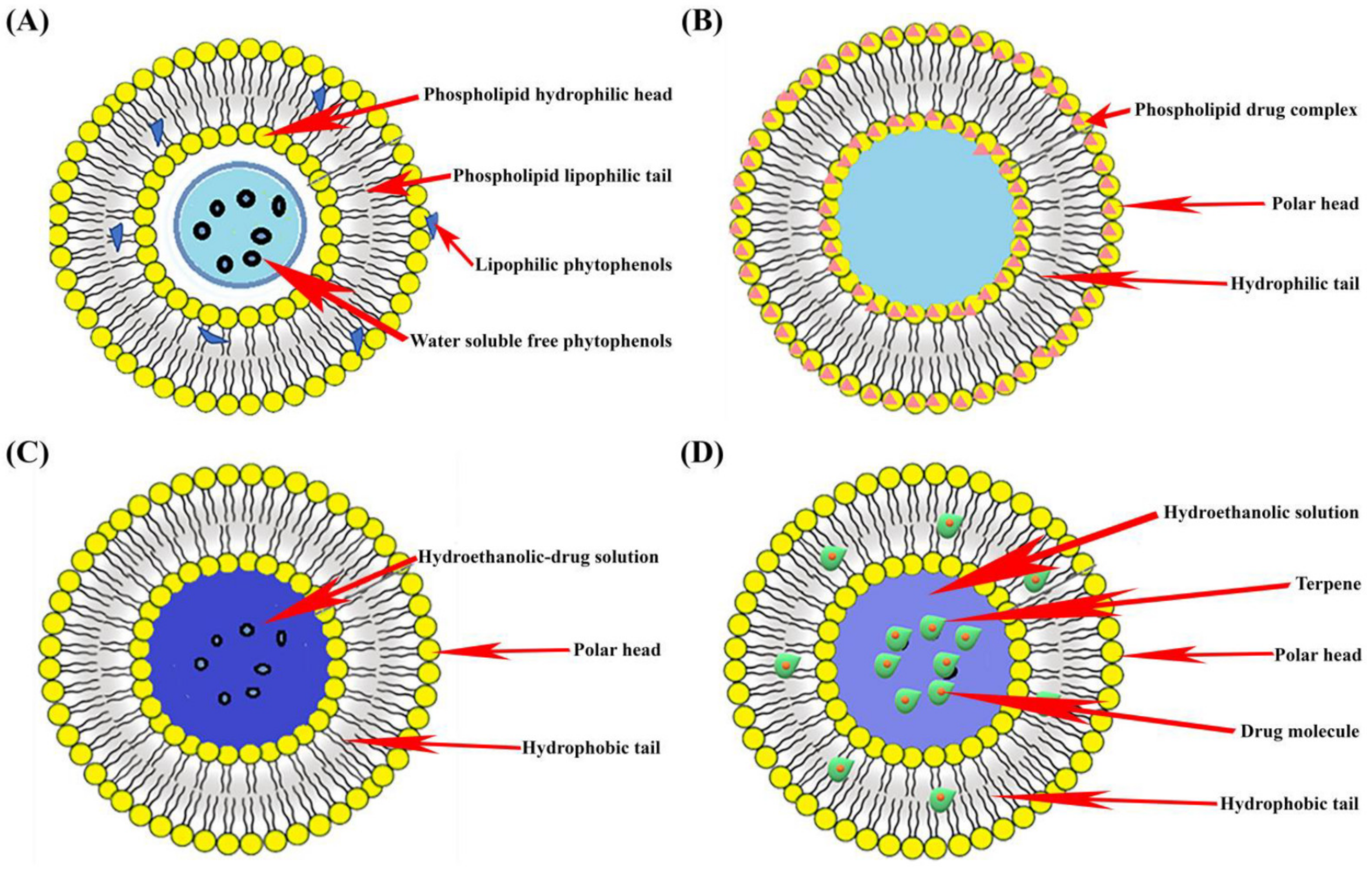
Figure 1. Graphical representation of different types of nanodelivery systems: (A) Liposome; (B) Phytosome; (C) Ethosome; and (D) Ivasome.
6. Ethosomes
Ethosomes are phospholipid-based vesicular carriers composed of a high concentration of ethanol (Figure 1). These vesicles were first developed by Touitou and co-workers in 1997 for the efficient delivery of active agents across the skin. Ethosomes are a non-invasive carrier system. The higher amount of ethanol in ethosomes provides a negative charge on the skin surface and increases its permeability, which in turn facilitates therapeutic agents to reach the deeper skin tissues for systemic circulation. Ethosomes are developed by different methods, such as the mechanical dispersion method, the cold method, the hot method, etc. [16][17].
The incorporation of phytopolyphenols into ethosomes has been considered a deliberate approach to increasing in situ stability, the permeability of polyphenols through the skin, bioavailability, and therapeutic efficacy. Researchers have encapsulated epigallocatechin3-gallate (EGCG) in ethosomes and observed an improved antioxidant activity and photostability of EGCG [18][19]. Researchers prepared apigenin-loaded ethosomes and found that ethosomal formulation showed higher in vivo skin targeting and effectiveness against ultraviolet B radiation-induced skin inflammation in comparison with its liposomal formulation [20][21].
7. Phytosomes
Phytosomes are phospholipid and herbal drug extract complex vesicles in which phytoconstituents are bound by a phospholipid layer (Figure 1). Phospholipids have the property of an active emulsifier because of their hydrophilic head and lipophilic tail. The emulsifier property of phytosomes improves phytopolyphenol’s bioavailability by facilitating them to pass from the aqueous to the lipophilic environment of the cell membrane [22]. Researchers have reported improved hepatoprotective and antioxidant effects of silybin phytosomes formulation in comparison to silybin alone [23]. Researchers have reported ginkgo phytosomes and their improved effect on the brain and vascular protection. Researchers have also prepared rutin phytosomes transdermal formulation and found its remarkable effect in rheumatoid arthritis [24][25].
8. Invasomes
Invasomes are nano-vesicular transdermal drug delivery systems, an ethanol and terpenes mixture with phospholipids (Figure 1). By breaking down lipid packing in the stratum corneum, invasomes enhance drug permeability and retention into the skin layers for localized effect [26]. Invasomes are mostly prepared by mechanical dispersion technique and film hydration technique. Invasomes, as a drug carrier for phytopolyphenols, have the advantage of enhanced solubility and therapeutic effect [27]. Researchers have developed an invasomal cream of Ocimum basilicum for the treatment of acne and observed successful drug delivery through the skin [28].
References
- Desai, J.; Thakkar, H. Effect of particle size on oral bioavailability of darunavir-loaded solid lipid nanoparticles. J. Microencapsul. 2016, 33, 669–678.
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S. Resveratrol in Solid Lipid Nanoparticles. J. Dispers. Sci. Technol. 2012, 33, 465–471.
- Ishkeh, S.R.; Shirzad, H.; Asghari, M.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Effect of chitosan nanoemulsion on enhancing the phytochemical contents, health-promoting components, and shelf life of raspberry (Rubus sanctus Schreber). Appl. Sci. 2021, 11, 2224.
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747.
- Hashemi, M.; Shamshiri, A.; Saeedi, M.; Tayebi, L.; Yazdian-Robati, R. Aptamer-conjugated PLGA nanoparticles for delivery and imaging of cancer therapeutic drugs. Arch. Biochem. Biophys. 2020, 691, 108485.
- Khan, J.; Rudrapal, M.; Bhat, E.A.; Ali, A.; Alaidarous, M.; Alshehri, B.; Banwas, S.; Ismail, R.; Egbuna, C. Perspective Insights to Bio-Nanomaterials for the Treatment of Neurological Disorders. Front. Bioeng. Biotechnol. 2021, 9, 724158.
- Rudrapal, M.; Sugumari, V.; Zothantluanga, J.H.; Chetia, D.; Chukwuebuka, E.; Walode, S.G. Nanophytomedicines: From Nature to Medicines. In Applications of Nanotechnology in Drug Discovery and Delivery; Egbuna, C., Gaman, M.-A., Jeevanandam, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 71–93.
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571.
- Karn, P.R.; Cho, W.; Hwang, S.-J. Liposomal drug products and recent advances in the synthesis of supercritical fluid-mediated liposomes. Nanomedicine 2013, 8, 1529–1548.
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 2016, 11, 737–759.
- Huang, Y.-B.; Tsai, M.-J.; Wu, P.-C.; Tsai, Y.-H.; Wu, Y.-H.; Fang, J.-Y. Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J. Drug Target. 2011, 19, 709–718.
- Hjorth Tønnesen, H.; Smistad, G.; Ågren, T.; Karlsen, J. Studies on curcumin and curcuminoids. XXIII: Effects of curcumin on liposomal lipid peroxidation. Int. J. Pharm. 1993, 90, 221–228.
- Yuan, Z.-P.; Chen, L.-J.; Fan, L.-Y.; Tang, M.-H.; Yang, G.-L.; Yang, H.-S.; Du, X.-B.; Wang, G.-Q.; Yao, W.-X.; Zhao, Q.-M.; et al. Liposomal Quercetin Efficiently Suppresses Growth of Solid Tumors in Murine Models. Clin. Cancer Res. 2006, 12, 3193–3199.
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442.
- Elsamaligy, M.; Afifi, N.; Mahmoud, E. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int. J. Pharm. 2006, 319, 121–129.
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282.
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Yadav, R.; Patel, S.; Verma, K.; Tak, K. Flurbiprofen loaded ethosomes-transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019, 18, 133.
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019, 137, 104972.
- Faisal, W.; Soliman, G.M.; Hamdan, A. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J. Liposome Res. 2016, 28, 14–21.
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288.
- Godin, B.; Touitou, E.; Rubinstein, E.; Athamna, A.; Athamna, M. A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: An in vivo study. J. Antimicrob. Chemother. 2005, 55, 989–994.
- Kumar, D.; Vats, N.; Saroha, K.; Rana, A.C. Phytosomes as Emerging Nanotechnology for Herbal Drug Delivery. In Sustainable Agriculture Reviews 43; Springer: Berlin/Heidelberg, Germany, 2020; Volume 43, pp. 217–237.
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942.
- Amin, T.; Bhat, S. A Review on Phytosome Technology as a Novel Approach to Improve the Bioavailability of Nutraceuticals. Int. J. Adv. Res. Technol. 2012, 1, 43–57.
- Das, M.K.; Kalita, B. Design and Evaluation of Phyto-Phospholipid Complexes (Phytosomes) of Rutin for Transdermal Application. J. Appl. Pharm. Sci. 2014, 4, 51–57.
- Nangare, S.; Dugam, S. Smart invasome synthesis, characterizations, pharmaceutical applications, and pharmacokinetic perspective: A review. Futur. J. Pharm. Sci. 2020, 6, 123.
- Duangjit, S. Comparison of vesicle formulations for transdermal delivery of curcumin: Liposomes, flexosomes and invasomes. Isan J. Pharm. Sci. 2017, 13, 180–188.
- Han, H.J. Development of an effective formulation for an acne treatment cream with Ocimum basilicum using invasomes. J. Cosmet. Med. 2018, 2, 69–75.
More
