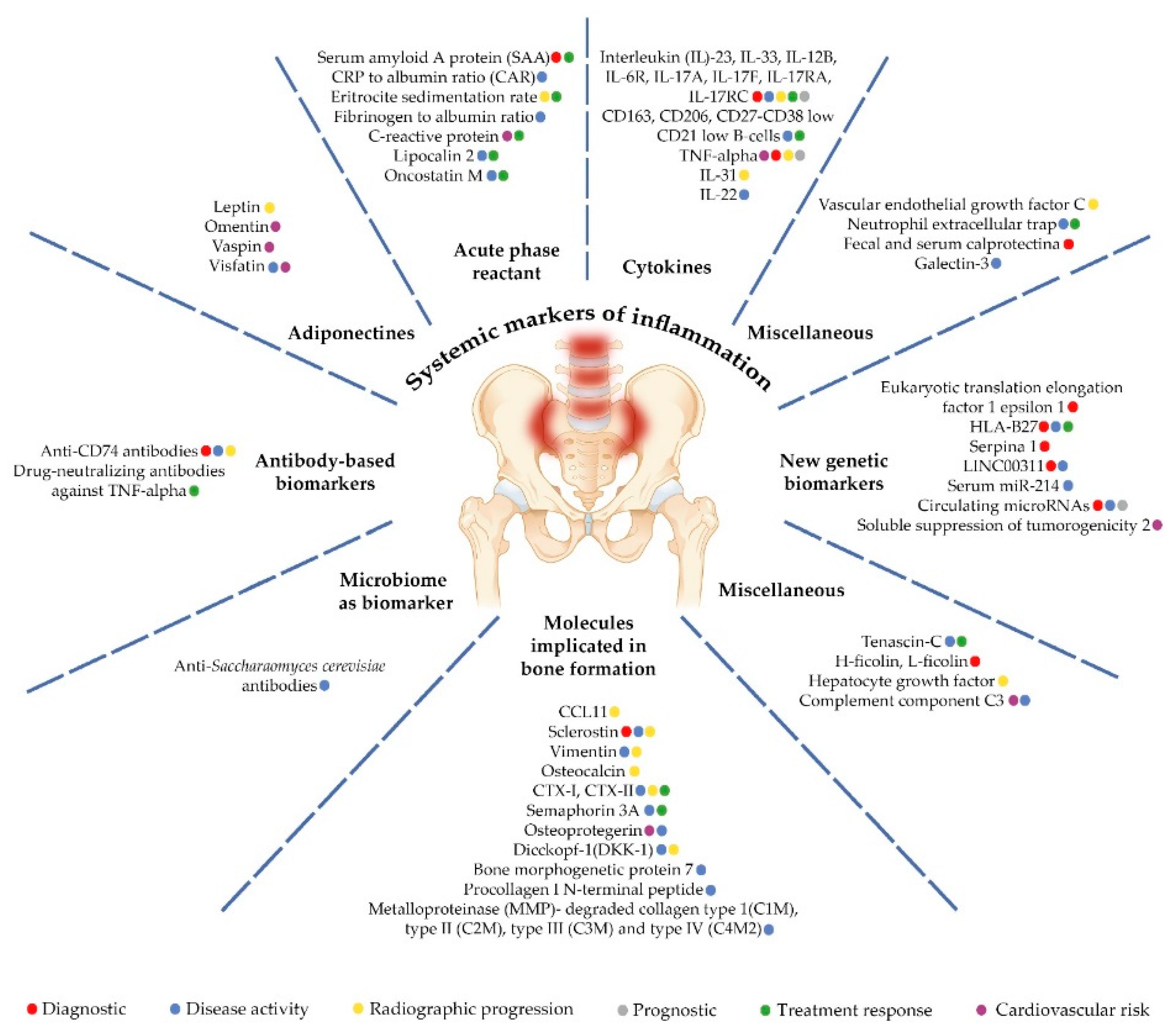
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that can lead to ankylosis by secondary ossification of inflammatory lesions, with progressive disability and a significant impact on quality of life. It is also a risk factor for the occurrence of comorbidities, especially cardiovascular diseases (CVDs), mood disorders, osteoporosis, and malignancies. Early diagnosis and treatment are needed to prevent or decrease functional decline and to improve the patient’s prognosis. In respect of axSpA, there is an unmet need for biomarkers that can help to diagnose the disease, define disease activity and prognosis, and establish personalized treatment approaches. The aim of this review was to summarize the available information regarding the most promising biomarkers for axSpA. It can be classified and identified six core categories of biomarkers: (i) systemic markers of inflammation; (ii) molecules involved in bone homeostasis; (iii) HLA-B27 and newer genetic biomarkers; (iv) antibody-based biomarkers; (v) microbiome biomarkers; and (vi) miscellaneous biomarkers. Unfortunately, despite efforts to validate new biomarkers, few of them are used in clinical practice; however, we believe that these studies provide useful data that could aid in better disease management.
- axial spondyloarthritis
- diagnosis
- disease activity
- prognosis
1. Axial Spondyloarthritis: Past, Present, and Future
2. AxSpA and Biomarkers
| Biomarker | Predictability | Study type | Reference |
|---|---|---|---|
| Anti-CD74 antibodies | Positive association with diagnosis and disease activity. Positive association with radiographic progression. Positive association with diagnosis. Negative association with diagnosis. Positive association with diagnosis. |
Cross-sectional study Prospective study Prospective study Prospective study Prospective study |
2021, Marwa Mahmoud Abdelaziz [7] 2021, Lan Do [8] 2019, Nelly R. Ziade [9] 2018, Janneke J. de Winter [10] 2020, Chao-Jun Hu [11] |
| Bone morphogenetic protein 7 (BMP-7), sclerostin, dicckopf-1 (DKK-1) | Positive association with disease association |
Prospective multicenter study | 2021, Elise Descamps [12] |
| C-reactive protein (CRP) | Positive association with cardiovascular risk Positive association with treatment response Positive association with treatment response Positive association with treatment response |
Retrospective analysis Prospective study Case-control study Cross-sectional study |
2022, Luca Navarini [13] 2019, Xenofon Baraliakos [14] 2020, Xu Yuansheng [15] 2018, Björn Sundström [16] |
| C-C motif chemokine ligand 11 (CCL11) | Positive association with radiographic progression |
Cross-sectional study | 2018, Dong Hyun Sohn [17] |
| CD163, CD206 | Positive association with disease activity, treatment response | Prospective study | 2018, Line Dam Heftdal [18] |
| CD27-CD38lowCD21low B cells | Positive association with disease activity |
Cross-sectional study | 2021, Rick Wilbrink [19] |
| circRNA hsa_circ_0079787 | Positive association with diagnosis, disease activity |
Cross-sectional study | 2020, Qing Luo [20] |
| Circulating microRNAs | Positive association with disease activity Positive association with diagnosis and disease activity |
Prospective study Cross-sectional study |
2020, Qing Luo [20] 2018, Carlos Perez-Sanchez [21] |
| Complement component C3 | Positive association with disease activity and cardiovascular risk |
Cross-sectional study | 2020, Ivan Arias de la Rosa [22] |
| CRP to albumin ratio (CAR) | Positive association with disease activity Positive association with disease activity |
Retrospective study Cross-sectional study |
2021, Zheng Zhong [23] 2021, Melih Pamukcu [24] |
| Dickkopf homologue-1 (Dkk-1) | Positive association with radiographic progression |
Prospective study | 2019, Zhonghai Zhao [25] |
| Bone morphogenetic proteins (BMPs) and Dkk-1 | Positive association with disease activity |
Prospective study | 2018, Hsien-Tzung Liao [26] |
| Drug-neutralizing Antibodies against TNF-α | Positive association with treatment response | Cross-sectional study | 2020, Krasimir Kraev [27] |
| ERAP1 (rs2287987, rs30187, rs27044), ERAP2 (rs2248374) | Positive association with diagnosis |
Case-control study | 2019, Andrzej Wiśniewski [28] |
| Erythrocyte sedimentation rate (ESR) ESR + Interleukin (IL)-6 |
Positive association with radiographic progression Positive association with treatment response |
Retrospective study Prospective study |
2018, Kwi Young Kang [29] 2019, Yidian Dong [30] |
| Eukaryotic translation elongation factor 1 epsilon 1 (EEF1E1) | Positive association with diagnosis |
Prospective study | 2019, Xutao Fan [31] |
| Fecal calprotectina and anti-Saccharaomyces cerevisiae antibodies (ASCA) | Positive association with disease activity |
Cross-sectional study | 2019, Tor Olofsson [32] |
| Fibrinogen-to-albumin ratio (FAR) | Positive association with disease activity |
Retrospective study | 2020, Meng Liu [33] |
| Galectin-3 | Positive association with disease activity |
Prospective study | 2019, Ming-Yu Cao [34] |
| Galectin-3, soluble suppression-of-tumorogenity-2 (sST2) (cardiac fibrosis), hsCRP | Positive association with cardiovascular risk |
Prospective study | 2018, Demet Ozkaramanli Gur [35] |
| H-ficolin, L-ficolin | Positive association with diagnosis |
Cross-sectional cohort | 2020, Anne Troldborg [36] |
| Hepatocyte growth factor (s-HGF) |
Positive association with radiographic progression Positive association with radiographic progression |
Prospective cohort study Prospective study |
2021, Anna Deminger [37] 2019, Liset Torres [38] |
| HLA B-27 antigen | Positive association with diagnosis Positive association with diagnosis Positive association with diagnosis |
Prospective study Prospective study Prospective study |
2019, Nelly Ziade [39] 2018, Chong Seng Edwin Lim [40] 2021, Henriëtte M Y de Jong [41] |
| Interleukin (IL)-31 | Positive association with radiographic progression |
Longitudinal prospective cohort study | 2018, Nicolas Rosine [42] |
| IL-33 | Positive association with disease activity and treatment response | Prospective study | 2021, Milena Iwaszko [43] |
| IL-12B and IL-6R | Positive association with diagnosis and prognosis | Prospective cohort study | 2018, Wen-Feng Ruan [44] |
| IL-22 | Positive association with diagnosis |
Retrospective study | 2022, Michal Sagiv [45] |
| IL-17 IL-23 and IL-17 |
Positive association with diagnosis Positive association with diagnosis |
Cross-sectional study Case-control study |
2020, Dalia S. Saif [46] 2018, Jacob Sode [47] |
| Leptin | Positive association with radiographic progression Positive association with radiographic progression |
Longitudinal prospective study Prospective study |
2017, Ji-Heg Park [48] 2019, Judith Rademacher [49] |
| Leptin + high molecular weight adiponectin + VEGF | Positive association with radiographic progression |
Cross-sectional study | 2019, Judith Rademacher [49] |
| LINC00311 | Positive association with diagnosis and disease activity |
Prospective study | 2019, Hongfa Zhong [50] |
| Lipocalin 2 (LCN 2), Oncostatin M (OSM) | Positive association with disease activity and treatment response | Longitudinal observational study | 2021, Florence W.L Tsui [51] |
| Metalloproteinase (MMP)-degraded collagen type I (C1M), type II (C2M), type III (C3M), and type IV (C4M2) | Positive association with disease activity |
Prospective cohort study | 2019, Markéta Husšáková [52] |
| Neutrophil extracellular trap (NET) | Positive association with disease activity and treatment response | Cross-sectional study, case report | 2020, Patricia Ruiz-Limon [53] |
| Omentin-1 | Positive association with cardiovascular risk |
Prospective study | 2020, Fernanda Genre [54] |
| Osteoprotegerin (OPG) and sclerostin (SCL) | Positive association with cardiovascular risk |
Prospective study | 2018, Fernanda Genre [55] |
| Pentraxin 3 | Positive association with disease activity |
Cross-sectional study | 2018, Renato Nisihara [56] |
| Procollagen I N-terminal peptide | Positive association with disease activity |
Cross-sectional study | 2022, Xuegang Li [57] |
| Sclerostin and antisclerostin antibody | Positive association with gastrointestinal risk Positive association with diagnosis Positive association with radiographic progression |
Prospective study Case-control study Prospective study |
2018, Michele Maria Luchetti [58] 2018, Perrota Fabio Massimo [59] 2019, Judith Rademacher [49] |
| Semaphorin 3A (Sema 3A) | Positive association with disease activity Positive association with treatment response |
Prospective study Prospective study |
2019, Hsien-Tzung Liao [60] 2017, Fabio Massimo Perrotta [61] |
| Serum amyloid A1 (SAA1) | Positive association with diagnosis |
Prospective study | 2020, Shijia Liu [62] |
| Serum calprotectin | Positive association with disease activity Positive association with treatment response Positive association with cardiovascular risk Positive association with radiographic progression |
Prospective study Prospective study Prospective study Prospective study |
2020, Matthias Jarlborg [63] 2019, Hua Hu [64] 2018, Fernanda Genre [65] 2022, Judith Rademacher [66] |
| Serum miR-214 | Positive association with disease activity |
Prospective study | 2019, Hyun Yi Kook [67] |
| Tenascin-C (TNC) | Positive association with disease activity Positive association with disease activity |
Prospective study Prospective study |
2020, Kristyna Bubova [68] 2018, Latika Gupta [69] |
| Vaspin | Positive association with cardiovascular risk |
Prospective study | 2021, Javier Rueda-Gotor [70] |
| Vimentin | Positive association with radiographic progression |
Prospective study | 2019, Anne Sofie Siebuhr [71] |
| Visfatin | Positive association with cardiovascular risk Positive association with disease activity |
Prospective study Prospective study |
2021, Rabia Aydogan Baykara [72] 2022, Judith Rademacher [66] |
References
- Firestein, G.S.; Budd, R.C.; Koretzky, G.; Gabriel, S.E.; McInnes, I.B.; O’Dell, J.R. Firestein & Kelley’s Textbook of Rheumatology, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, p. 1307.
- Cardelli, C.; Monti, S.; Terenzi, R.; Carli, L. One year in review 2021: Axial spondyloarthritis. Clin. Exp. Rheumatol. 2021, 39, 1272–1281.
- Walsh, J.A.; Magrey, M. Clinical Manifestations and Diagnosis of Axial Spondyloarthritis. J. Clin. Rheumatol. 2021, 27, e547–e560.
- Ancuta, C.; Pomirleanu, C. Principii de Diagnostic si Tratament in Reumatologie, 2nd ed.; Universitatea de Medicină și Farmacie „Grigore T. Popa” din Iași: Iasi, Romania, 2019; p. 177.
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221.
- Abdal, S.J.; Yesmin, S.; Shazzad, M.N.; Azad, M.A.K.; Shahin, M.A.; Choudhury, M.R.; Islam, M.N.; Haq, S.A. Development of a Bangla version of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Bath Ankylosing Spondylitis Functional Index (BASFI). Int. J. Rheum. Dis. 2021, 24, 74–80.
- Abdelaziz, M.M.; Gamal, R.M.; Ismail, N.M.; Lafy, R.A.; Hetta, H.F. Diagnostic value of anti-CD74 antibodies in early and late axial spondyloarthritis and its relationship to disease activity. Rheumatology 2021, 60, 263–268.
- Do, L.; Granåsen, G.; Hellman, U.; Lejon, K.; Geijer, M.; Baraliakos, X.; Witte, T.; Forsblad-d’Elia, H. Anti-CD74 IgA autoantibodies in radiographic axial spondyloarthritis: A longitudinal Swedish study. Rheumatology 2021, 60, 4085–4093.
- Ziade, N.R.; Mallak, I.; Merheb, G.; Ghorra, P.; Baerlecken, N.; Witte, T.; Baraliakos, X. Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population with Low HLA-B27 Prevalence. Front. Immunol. 2019, 10, 574.
- de Winter, J.J.; van de Sande, M.G.; Baerlecken, N.; Berg, I.; Ramonda, R.; van der Heijde, D.; van Gaalen, F.A.; Witte, T.; Baeten, D.L. Anti-CD74 antibodies have no diagnostic value in early axial spondyloarthritis: Data from the spondyloarthritis caught early (SPACE) cohort. Arthritis Res. Ther. 2018, 20, 38.
- Hu, C.J.; Li, M.T.; Li, X.; Peng, L.Y.; Zhang, S.Z.; Leng, X.M.; Su, J.M.; Zeng, X.F. CD74 auto-antibodies display little clinical value in Chinese Han population with axial spondyloarthritis. Medicine 2020, 99, 23433.
- Descamps, E.; Molto, A.; Borderie, D.; Lories, R.; Richard, C.M.; Pons, M.; Roux, C.; Briot, K. Changes in bone formation regulator biomarkers in early axial spondyloarthritis. Rheumatology 2021, 60, 1185–1194.
- Navarini, L.; Currado, D.; Marino, A.; Di Donato, S.; Biaggi, A.; Caso, F.; Costa, L.; Tasso, M.; Ruscitti, P.; Pavlych, V.; et al. Persistence of C-reactive protein increased levels and high disease activity are predictors of cardiovascular disease in patients with axial spondyloarthritis. Sci. Rep. 2022, 12, 7498.
- Baraliakos, X.; Szumski, A.; Koenig, A.S.; Jones, H. The role of C-reactive protein as a predictor of treatment response in patients with ankylosing spondylitis. Semin. Arthritis Rheum. 2019, 48, 997–1004.
- Xu, Y.; Jiang, W.; Zhang, H. Association between C-reactive protein gene variant and treatment efficacy of etanercept in ankylosing spondylitis patients receiving hip arthroplasty. J. Clin. Lab. Anal. 2020, 34, e23343.
- Sundström, B.; Ljung, L.; Wållberg-Jonsson, S. Exercise habits and C-reactive protein may predict development of spinal immobility in patients with ankylosing spondylitis. Clin. Rheumatol. 2018, 37, 2881–2885.
- Sohn, D.H.; Jeong, H.; Roh, J.S.; Lee, H.N.; Kim, E.; Koh, J.H.; Lee, S.G. Serum CCL11 level is associated with radiographic spinal damage in patients with ankylosing spondylitis. Rheumatol. Int. 2018, 38, 1455–1464.
- Heftdal, L.D.; Loft, A.G.; Hendricks, O.; Ashouri Christiansen, A.; Schiøttz-Christensen, B.; Arnbak, B.; Jurik, A.G.; Østgård, R.; Winding Deleuran, B.; Møller, H.J.; et al. Divergent effects on macrophage biomarkers soluble CD163 and CD206 in axial spondyloarthritis. Scand. J. Clin. Lab. Investig. 2018, 78, 483–489.
- Wilbrink, R.; Spoorenberg, A.; Arends, S.; van der Geest, K.S.M.; Brouwer, E.; Bootsma, H.; Kroese, F.G.M.; Verstappen, G.M. CD27-CD38lowCD21low B-Cells Are Increased in Axial Spondyloarthritis. Front. Immunol. 2021, 12, 686273.
- Luo, Q.; Fu, B.; Zhang, L.; Guo, Y.; Huang, Z.; Li, J. Expression and clinical significance of circular RNA hsa_circ_0079787 in the peripheral blood of patients with axial spondyloarthritis. Mol. Med. Rep. 2020, 22, 4197–4206.
- Perez-Sanchez, C.; Font-Ugalde, P.; Ruiz-Limon, P.; Lopez-Pedrera, C.; Castro-Villegas, M.C.; Abalos-Aguilera, M.C.; Barbarroja, N.; Arias-de la Rosa, I.; Lopez-Montilla, M.D.; Escudero-Contreras, A.; et al. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Hum. Mol. Genet. 2018, 27, 875–890.
- Arias de la Rosa, I.; Font, P.; Escudero-Contreras, A.; López-Montilla, M.D.; Pérez-Sánchez, C.; Ábalos-Aguilera, M.C.; Ladehesa-Pineda, L.; Ibáñez-Costa, A.; Torres-Granados, C.; Jimenez-Gomez, Y.; et al. Complement component 3 as biomarker of disease activity and cardiometabolic risk factor in rheumatoid arthritis and spondyloarthritis. Ther. Adv. Chronic. Dis. 2020, 11, 2040622320965067.
- Zhong, Z.; Huang, Y.; Liu, Y.; Chen, J.; Liu, M.; Huang, Q.; Zheng, S.; Guo, X.; Deng, W.; Li, T. Correlation between C-Reactive Protein to Albumin Ratio and Disease Activity in Patients with Axial Spondyloarthritis. Dis. Markers 2021, 2021, 6642486.
- Pamukcu, M.; Duran, T.I. Could C-Reactive Protein/Albumin Ratio be an Indicator of Activation in Axial Spondyloarthritis? J. Coll. Physicians Surg. Pak. 2021, 30, 537–541.
- Zhao, Z.; Wang, G.; Wang, Y.; Yang, J.; Wang, Y.; Zhu, J.; Huang, F. Correlation between magnetic resonance imaging (MRI) findings and the new bone formation factor Dkk-1 in patients with spondyloarthritis. Clin. Rheumatol. 2019, 38, 465–475.
- Liao, H.T.; Lin, Y.F.; Tsai, C.Y.; Chou, T.C. Bone morphogenetic proteins and Dickkopf-1 in ankylosing spondylitis. Scand. J. Rheumatol. 2018, 47, 56–61.
- Kraev, K.; Geneva-Popova, M.; Popova, V.; Popova, S.; Maneva, A.; Batalov, A.; Stankova, T.; Delcheva, G.; Stefanova, K. Drug-neutralizing Antibodies against TNF-α blockers as Biomarkers of Therapy Effect Evaluation. Folia Med. 2020, 62, 282–289.
- Wiśniewski, A.; Kasprzyk, S.; Majorczyk, E.; Nowak, I.; Wilczyńska, K.; Chlebicki, A.; Zoń-Giebel, A.; Kuśnierczyk, P. ERAP1-ERAP2 haplotypes are associated with ankylosing spondylitis in Polish patients. Hum. Immunol. 2019, 80, 339–343.
- Kang, K.Y.; Chung, M.K.; Kim, H.N.; Hong, Y.S.; Ju, J.H.; Park, S.H. Severity of Sacroiliitis and Erythrocyte Sedimentation Rate are Associated with a Low Trabecular Bone Score in Young Male Patients with Ankylosing Spondylitis. J. Rheumatol. 2018, 45, 349–356.
- Dong, Y.; Guo, J.; Bi, L. Baseline Interleukin-6 and Erythrocyte Sedimentation Rate Can Predict Clinical Response of TNF Inhibitor Treatment in Patients with Ankylosing Spondylitis. Ann. Clin. Lab. Sci. 2019, 49, 611–618.
- Fan, X.; Qi, B.; Ma, L.; Ma, F. Screening of underlying genetic biomarkers for ankylosing spondylitis. Mol. Med. Rep. 2019, 19, 5263–5274.
- Olofsson, T.; Lindqvist, E.; Mogard, E.; Andréasson, K.; Marsal, J.; Geijer, M.; Kristensen, L.E.; Wallman, J.K. Elevated faecal calprotectin is linked to worse disease status in axial spondyloarthritis: Results from the SPARTAKUS cohort. Rheumatology 2019, 58, 1176–1187.
- Liu, M.; Huang, Y.; Huang, Z.; Zhong, Z.; Deng, W.; Huang, Z.; Huang, Q.; Li, T. The role of fibrinogen to albumin ratio in ankylosing spondylitis: Correlation with disease activity. Clin. Chim. Acta 2020, 505, 136–140.
- Cao, M.Y.; Wang, J.; Gao, X.L.; Hu, Y.B. Serum galectin-3 concentrations in patients with ankylosing spondylitis. J. Clin. Lab. Anal. 2019, 33, e22914.
- Ozkaramanli Gur, D.; Ozaltun, D.N.; Guzel, S.; Sarifakioglu, B.; Akyuz, A.; Alpsoy, S.; Aycicek, O.; Baykiz, D. Novel imaging modalities in detection of cardiovascular involvement in ankylosing spondylitis. Scand. Cardiovasc. J. 2018, 52, 320–327.
- Troldborg, A.; Thiel, S.; Mistegaard, C.E.; Hansen, A.; Korsholm, T.L.; Stengaard-Pedersen, K.; Loft, A.G. Plasma levels of H- and L-ficolin are increased in axial spondyloarthritis: Improvement of disease identification. Clin. Exp. Immunol. 2020, 199, 79–87.
- Deminger, A.; Klingberg, E.; Nurkkala, M.; Geijer, M.; Carlsten, H.; Jacobsson, L.T.H.; Forsblad-d’Elia, H. Elevated serum level of hepatocyte growth factor predicts development of new syndesmophytes in men with ankylosing spondylitis. Rheumatology 2021, 60, 1804–1813.
- Torres, L.; Klingberg, E.; Nurkkala, M.; Carlsten, H.; Forsblad-d’Elia, H. Hepatocyte growth factor is a potential biomarker for osteoproliferation and osteoporosis in ankylosing spondylitis. Osteoporos. Int. 2019, 30, 441–449.
- Ziade, N.; Abi Karam, G.; Merheb, G.; Mallak, I.; Irani, L.; Alam, E.; Messaykeh, J.; Menassa, J.; Mroue’, K.; Uthman, I.; et al. HLA-B27 prevalence in axial spondyloarthritis patients and in blood donors in a Lebanese population: Results from a nationwide study. Int. J. Rheum. Dis. 2019, 22, 708–714.
- Lim, C.S.E.; Sengupta, R.; Gaffney, K. The clinical utility of human leucocyte antigen B27 in axial spondyloarthritis. Rheumatology 2018, 57, 959–968.
- de Jong, H.M.Y.; de Winter, J.J.H.; van der Horst-Bruinsma, I.E.; van Schaardenburg, D.J.; van Gaalen, F.A.; van Tubergen, A.M.; Weel, A.E.A.M.; Landewé, R.B.M.; Baeten, D.L.P.; van de Sande, M.G.H. Progression from subclinical inflammation to overt SpA in first degree relatives of SpA patients is associated with HLA-B27: The Pre-SpA cohort. Arthritis Care Res. 2021, 0, 1–9.
- Rosine, N.; Etcheto, A.; Hendel-Chavez, H.; Seror, R.; Briot, K.; Molto, A.; Chanson, P.; Taoufik, Y.; Wendling, D.; Lories, R.; et al. Increase In Il-31 Serum Levels Is Associated With Reduced Structural Damage In Early Axial Spondyloarthritis. Sci. Rep. 2018, 8, 7731.
- Iwaszko, M.; Wielińska, J.; Świerkot, J.; Kolossa, K.; Sokolik, R.; Bugaj, B.; Chaszczewska-Markowska, M.; Jeka, S.; Bogunia-Kubik, K. IL-33 Gene Polymorphisms as Potential Biomarkers of Disease Susceptibility and Response to TNF Inhibitors in Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients. Front. Immunol. 2021, 12, 631603.
- Ruan, W.F.; Xie, J.T.; Jin, Q.; Wang, W.D.; Ping, A.S. The Diagnostic and Prognostic Role of Interleukin 12B and Interleukin 6R Gene Polymorphism in Patients With Ankylosing Spondylitis. J. Clin. Rheumatol. 2018, 24, 18–24.
- Sagiv, M.; Adawi, M.; Awisat, A.; Shouval, A.; Peri, R.; Sabbah, F.; Rosner, I.; Kessel, A.; Slobodin, G. The association between elevated serum interleukin-22 and the clinical diagnosis of axial spondyloarthritis: A retrospective study. Int. J. Rheum. Dis. 2022, 25, 56–60.
- Saif, D.S.; El Tabl, M.A.; Afifi, N.; Abdallah, M.S.; El Hefnawy, S.M.; Hassanein, S.A. Interleukin-17A biomarker as a predictor for detection of early axial spondyloarthritis changes in patients with psoriasis. Int. J. Rheum. Dis. 2020, 23, 1664–1669.
- Sode, J.; Bank, S.; Vogel, U.; Andersen, P.S.; Sørensen, S.B.; Bojesen, A.B.; Andersen, M.R.; Brandslund, I.; Dessau, R.B.; Hoffmann, H.J.; et al. Genetically determined high activities of the TNF-alpha, IL23/IL17, and NFkB pathways were associated with increased risk of ankylosing spondylitis. BMC Med. Genet. 2018, 19, 165.
- Park, J.H.; Lee, S.G.; Jeon, Y.K.; Park, E.K.; Suh, Y.S.; Kim, H.O. Relationship between serum adipokine levels and radiographic progression in patients with ankylosing spondylitis: A preliminary 2-year longitudinal study. Medicine 2017, 96, e7854.
- Rademacher, J.; Tietz, L.M. Added value of biomarkers compared with clinical parameters for the prediction of radiographic spinal progression in axial spondyloarthritis. Rheumatology 2019, 58, 1556–1564.
- Zhong, H.; Zhong, M. LINC00311 is overexpressed in ankylosing spondylitis and predict treatment outcomes and recurrence. BMC Musculoskelet. Disord. 2019, 20, 278.
- Tsui, F.W.L.; Lin, A.; Sari, I.; Zhang, Z.; Tsui, H.W.; Inman, R.D. Serial Lipocalin 2 and Oncostatin M levels reflect inflammation status and treatment response in axial spondyloarthritis. Arthritis Res. Ther. 2021, 23, 141.
- Hušáková, M.; Bay-Jensen, A.C.; Forejtová, Š.; Zegzulková, K.; Tomčík, M.; Gregová, M.; Bubová, K.; Hořínková, J.; Gatterová, J.; Pavelka, K.; et al. Metabolites of type I, II, III, and IV collagen may serve as markers of disease activity in axial spondyloarthritis. Sci. Rep. 2019, 9, 11218.
- Ruiz-Limon, P.; Ladehesa-Pineda, M.L.; Castro-Villegas, M.D.C.; Abalos-Aguilera, M.D.C.; Lopez-Medina, C.; Lopez-Pedrera, C.; Barbarroja, N.; Espejo-Peralbo, D.; Gonzalez-Reyes, J.A.; Villalba, J.M.; et al. Enhanced NETosis generation in radiographic axial spondyloarthritis: Utility as biomarker for disease activity and anti-TNF-α therapy effectiveness. J. Biomed. Sci. 2020, 27, 54.
- Genre, F.; Rueda-Gotor, J.; Remuzgo-Martínez, S.; Pulito-Cueto, V.; Corrales, A.; Mijares, V.; Lera-Gómez, L.; Portilla, V.; Expósito, R.; Mata, C.; et al. Omentin: A biomarker of cardiovascular risk in individuals with axial spondyloarthritis. Sci. Rep. 2020, 10, 9636.
- Genre, F.; Rueda-Gotor, J.; Remuzgo-Martínez, S.; Corrales, A.; Ubilla, B.; Mijares, V.; Fernández-Díaz, C.; Portilla, V.; Blanco, R.; Hernández, J.L.; et al. Implication of osteoprotegerin and sclerostin in axial spondyloarthritis cardiovascular disease: Study of 163 Spanish patients. Clin. Exp. Rheumatol. 2018, 36, 302–309.
- Nisihara, R.; Skare, T.L.; Zeni, J.O.; Rasera, H.; Lidani, K.; Messias-Reason, I. Plasma levels of pentraxin 3 in patients with spondyloarthritis. Biomarkers 2018, 23, 14–17.
- Li, X.; Liang, A.; Chen, Y.; Lam, N.S.; Long, X.; Xu, X.; Zhong, S. Procollagen I N-terminal peptide correlates with inflammation on sacroiliac joint magnetic resonance imaging in ankylosing spondylitis but not in non-radiographic axial spondyloarthritis: A cross-sectional study. Mod. Rheumatol. 2022, 32, 770–775.
- Luchetti, M.M.; Ciccia, F.; Avellini, C.; Benfaremo, D.; Guggino, G.; Farinelli, A.; Ciferri, M.; Rossini, M.; Svegliati, S.; Spadoni, T.; et al. Sclerostin and Antisclerostin Antibody Serum Levels Predict the Presence of Axial Spondyloarthritis in Patients with Inflammatory Bowel Disease. J. Rheumatol. 2018, 45, 630–637.
- Perrotta, F.M.; Ceccarelli, F.; Barbati, C.; Colasanti, T.; De Socio, A.; Scriffignano, S.; Alessandri, C.; Lubrano, E. Serum Sclerostin as a Possible Biomarker in Ankylosing Spondylitis: A Case-Control Study. J. Immunol. Res. 2018, 2018, 9101964.
- Liao, H.T.; Lin, Y.F.; Chou, C.T.; Tsai, C.Y. Semaphorin 3A in Ankylosing Spondylitis. J. Microbiol. Immunol. Infect. 2019, 52, 151–157.
- Perrotta, F.M.; Ceccarelli, F.; Barbati, C.; Colasanti, T.; Montepaone, M.; Alessandri, C.; Valesini, G.; Lubrano, E. Assessment of semaphorin 3A and its role in bone remodelling in a group of ankylosing spondylitis patients. Clin. Exp. Rheumatol. 2017, 35, 313–316.
- Liu, S.; Ji, W.; Lu, J.; Tang, X.; Guo, Y.; Ji, M.; Xu, T.; Gu, W.; Kong, D.; Shen, Q. Discovery of Potential Serum Protein Biomarkers in Ankylosing Spondylitis Using Tandem Mass Tag-Based Quantitative Proteomics. J. Proteome Res. 2020, 19, 864–872.
- Jarlborg, M.; Courvoisier, D.S.; Lamacchia, C.; Martinez Prat, L.; Mahler, M.; Bentow, C.; Finckh, A.; Gabay, C.; Nissen, M.J. Serum calprotectin: A promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res. Ther. 2020, 22, 105.
- Hu, H.; Du, F.; Zhang, S.; Zhang, W. Serum calprotectin correlates with risk and disease severity of ankylosing spondylitis and its change during first month might predict favorable response to treatment. Mod. Rheumatol. 2019, 29, 836–842.
- Genre, F.; Rueda-Gotor, J.; Remuzgo-Martínez, S.; Corrales, A.; Mijares, V.; Expósito, R.; Mata, C.; Portilla, V.; Blanco, R.; Hernández, J.L.; et al. Association of circulating calprotectin with lipid profile in axial spondyloarthritis. Sci. Rep. 2018, 8, 13728.
- Rademacher, J.; Siderius, M.; Gellert, L.; Wink, F.R.; Verba, M.; Maas, F.; Tietz, L.M.; Poddubnyy, D.; Spoorenberg, A.; Arends, S. Baseline serum biomarkers of inflammation, bone turnover and adipokines predict spinal radiographic progression in ankylosing spondylitis patients on TNF inhibitor therapy. Semin. Arthritis Rheum. 2022, 53, 151974.
- Kook, H.Y.; Jin, S.H.; Park, P.R.; Lee, S.J.; Shin, H.J.; Kim, T.J. Serum miR-214 as a novel biomarker for ankylosing spondylitis. Int. J. Rheum. Dis. 2019, 22, 1196–1201.
- Bubová, K.; Prajzlerová, K.; Hulejová, H.; Gregová, M.; Mintálová, K.; Hušáková, M.; Forejtová, Š.; Filková, M.; Tomčík, M.; Vencovský, J.; et al. Elevated Tenascin-C Serum Levels in Patients with Axial Spondyloarthritis. Physiol. Res. 2020, 69, 653–660.
- Gupta, L.; Bhattacharya, S.; Aggarwal, A. Tenascin-C, a biomarker of disease activity in early ankylosing spondylitis. Clin. Rheumatol. 2018, 37, 1401–1405.
- Rueda-Gotor, J.; López-Mejías, R.; Remuzgo-Martínez, S.; Pulito-Cueto, V.; Corrales, A.; Lera-Gómez, L.; Portilla, V.; González-Mazón, Í.; Blanco, R.; Expósito, R.; et al. Vaspin in atherosclerotic disease and cardiovascular risk in axial spondyloarthritis: A genetic and serological study. Arthritis Res. Ther. 2021, 23, 111.
- Siebuhr, A.S.; Hušaková, M.; Forejtová, S.; Zegzulková, K.; Tomčik, M.; Urbanová, M.; Grobelná, K.; Gatterová, J.; Bay-Jensen, A.C.; Pavelka, K. Metabolites of C-reactive protein and vimentin are associated with disease activity of axial spondyloarthritis. Clin. Exp. Rheumatol. 2019, 37, 358–366.
- Baykara, A.R.; Küçük, A.; Tuzcu, A.; Tuzcu, G.; Cüre, E.; Uslu, A.U.; Omma, A. The relationship of serum visfatin levels with clinical parameters, flow-mediated dilation, and carotid intima-media thickness in patients with ankylosing spondylitis. Turk. J. Med. Sci. 2021, 51, 1865–1874.
