Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Ekaterina A. Kozlova.
Carbon nitride, albeit known for a long time, has been tested as a photocatalyst only since 2009. Extensive searches for reliable visible-light-activated semiconductor photocatalysts revealed the next generation photocatalyst based on a polymeric semiconductor—graphitic carbon nitride.
- g-C3N4
- hydrogen
- photocatalysis
- platinum
1. Introduction
Fossil fuels are a limited and exhaustible resource for conventional energy production that induces reasonable concerns of energy and economic crises [1]. Besides, wide use of fossil fuels causes environmental and human health impacts [2]. In this context, triggering hydrogen as an alternative energy source is a promising trend, since hydrogen is an environmentally benign fuel [3,4,5][3][4][5]. Currently, numerous industrial processes to produce hydrogen are available, but they all are energy intensive, require high temperatures, and appear economically efficient only at large scales [6]. In this regard, the photocatalytic production of hydrogen attracts particular interest as the process proceeding at ambient conditions and simulating photosynthesis, i.e., the direct conversion of solar energy into the energy of chemical bonds [7,8,9,10,11,12,13,14,15,16][7][8][9][10][11][12][13][14][15][16].
The process of photocatalytic splitting of water using TiO2 catalysts under UV radiation was pioneered by Fujishima and Honda in 1972 [17]. Since that time, a great number of research has been performed to produce hydrogen by photocatalytic splitting of water with various semiconductor photocatalysts [18,19,20,21,22][18][19][20][21][22]. However, this process has an intrinsic problem of hydrogen-oxygen recombination [23]. To improve the process’ quantum efficiency, photocatalytic reduction of water is carried out with the use of electron donor agents, such as various organic and inorganic compounds capable of donating electrons and thereby reducing the recombination of electron-hole pairs on the photocatalyst surface [24,25,26][24][25][26].
Promising photocatalysts activated by UV and visible light include TiO2 [27[27][28][29][30],28,29,30], ZnO [31], Fe2O3 [32], CdS [33], Cd1−ZnxS [34], Bi2WO6 [35], BiVO4 [36], Ta2O5 [37], Ta3N5 [38], and TaON [39]. Nowadays, the research and development of high-performance semiconductor photocatalysts for solving the problems of energy shortages and environmental safety are particularly important. Recently, visible-light-activated photocatalysts have been developed, which allow efficient use of the solar spectrum containing a large fraction of visible light (about 43%) [17]. Traditional semiconductor photocatalysts, such as TiO2, have a large band gap and, therefore, are unable to absorb visible light, and can be activated only by UV light, which constitutes a small fraction of the solar radiation spectrum [40,41,42][40][41][42]. Extensive searches for reliable visible-light-activated semiconductor photocatalysts revealed the next generation photocatalyst based on a polymeric semiconductor—graphitic carbon nitride [43,44,45,46][43][44][45][46].
2. Graphitic Carbon Nitride as a Photocatalyst
Carbon nitride, albeit known for a long time, has been tested as a photocatalyst only since 2009 [47]. Every year, the interest of researchers in this material increases, as shown in Figure 1a. It can also be seen in Figure 1b that, to date, about 10–12% of all studies on the photocatalytic H2 production devoted to the synthesis and study of photocatalysts based on g-C3N4 and their applications.
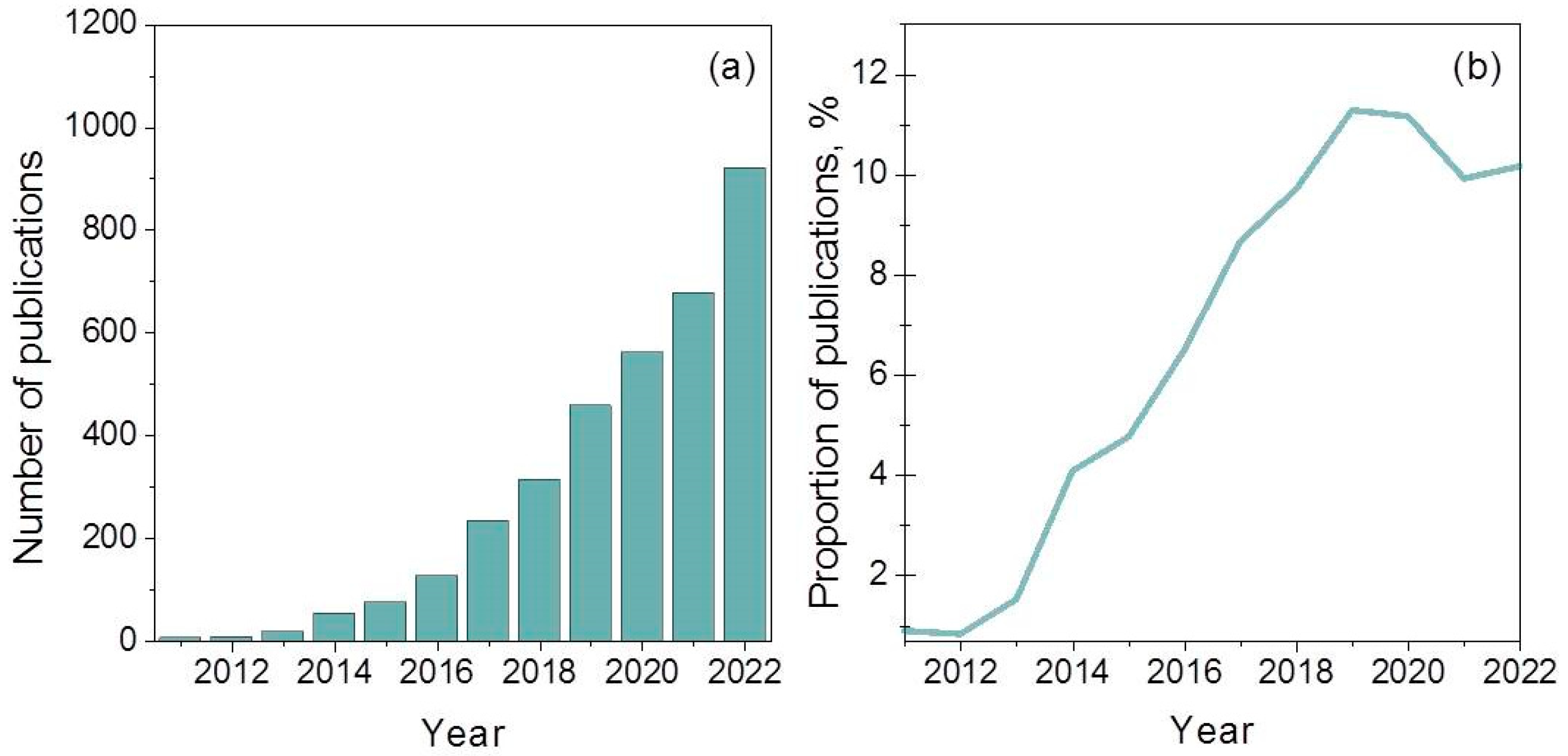
Figure 1. (a) Number of annual publications using “g-C3N4 photocatalytic H2 production” as a keyword since 2011. (b) Proportion between the number of publications with keywords “g-C3N4 photocatalytic H2 production” and “photocatalytic H2 production” based on data of Science Direct (Elsevier).
It reveals various allotropic forms, such as α-C3N4, β-C3N4, graphitic, cubic, and pseudocubic [48]. The most stable modification is a polymeric graphitic structure in which s-triazine or tri-s-triazine (s-heptazine) units are bound to each other through tertiary amines [47]. Graphitic carbon nitride g-C3N4 has the band gap of 2.7 eV and the conduction band-edge potential −1.3 V vs. NHE, which enables an efficient hydrogen production process [47]. This material is thermally and chemically stable, safe for the environment, acid and alkali resistant; its surface can be modified without infringing its composition and structure [49]. It is easily synthesized by thermal polycondensation of inexpensive nitrogen-containing precursors, such as dicyandiamide, cyanamide, melamine, urea, and thiourea [46,50,51,52][46][50][51][52]. The downside of this process is that it yields materials with low specific surface areas and high electron-hole recombination rates that provokes the loss in the catalytic activity [53]. To stabilize the photocatalytic activity of g-C3N4, the following approaches are used:
-
Alteration of textural characteristics by introducing templates, pore formers, or pretreatment method [54,55,56,57,58,59,60,61,62,63,64,65,66];
- Alteration of textural characteristics by introducing templates, pore formers, or pretreatment method [54][55][56][57][58][59][60][61][62][63][64][65][66];
- ,
- Modification with metals
- [74
- ,
- Creation of composite photocatalysts [
- Creation of composite photocatalysts
- [
- 91
The formation of graphitic carbon nitride proceeds by various routes at thermal condensation of the abovementioned precursors. At thermal pyrolysis, the cyclization of any of the above mentioned nitrogen-containing precursors yields melamine [49]. The dimerization of melamine at 350 °C yields melem and melon that transforms into polymeric g-C3N4 at temperatures above 500 °C (Figure 2).
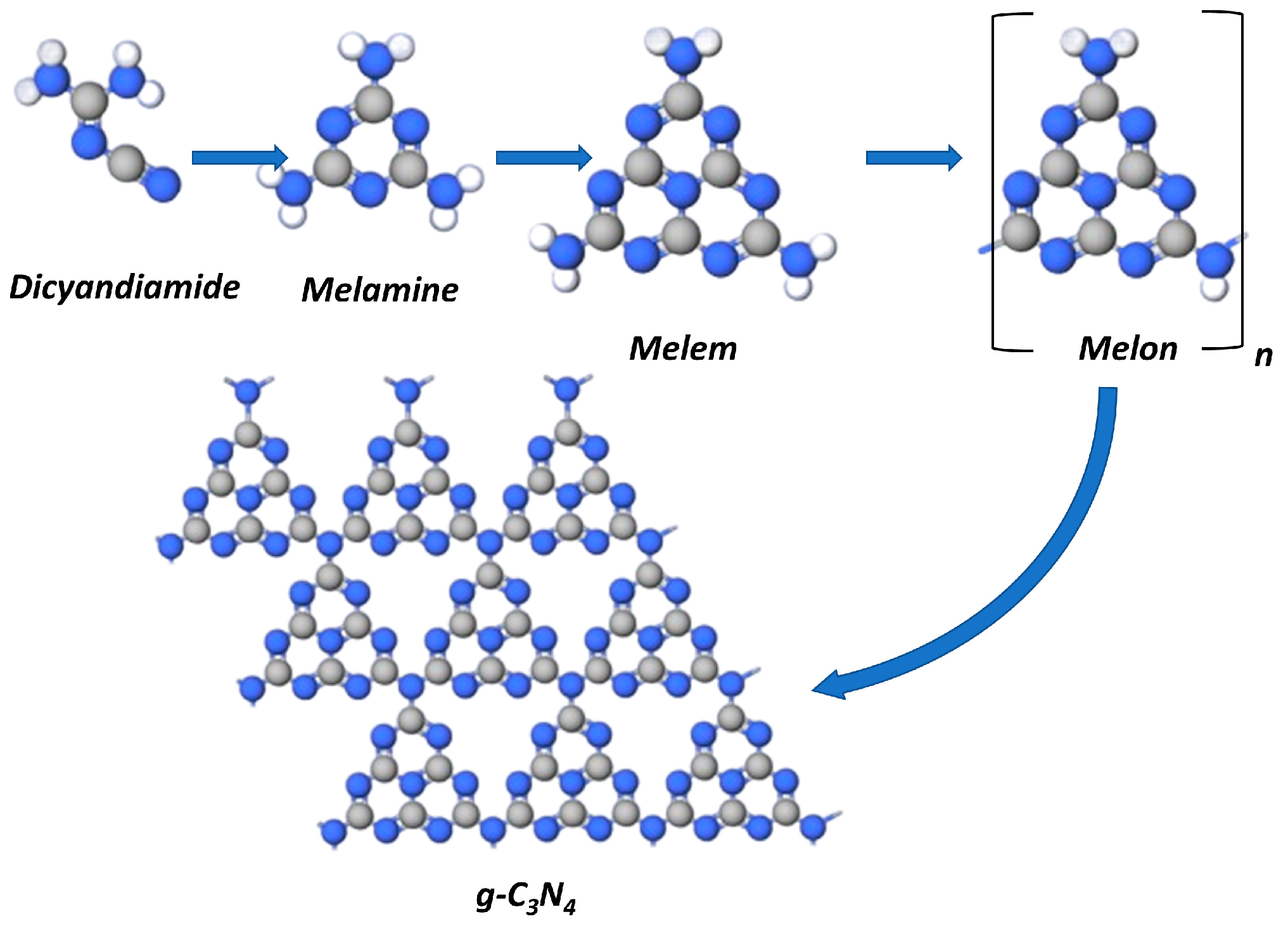
Figure 2.
Thermal condensation of dicyandiamide to form g-C
3
N
4
.
Graphitic carbon nitride is known to have a defect-rich structure, which results from incomplete deamination during thermal polycondensation [49]. For this reason, synthesizing high-crystalline g-C3N4 is a difficult task, albeit exact crystallinity (correct arrangement of atoms) is a critically important parameter affecting the band structure and charge recombination rate (eȡ/h+) of a photocatalytically active material. A large number of structural defects increases the charge recombination rate, and thus impedes photocatalyst activity. Texture controlling can improve the chemical, physical, and optical properties of g-C3N4 [101]. The relationship between the g-C3N4 morphology and photocatalytic hydrogen evolution performance is discussed below.
2.1. Textural Characteristics and Methods to Control Them
2.1.1. The Use of Templates
The textural and morphological characteristics, such as porosity, specific surface area, and pore size distribution, of a carbon nitride based material can be controlled by using appropriate templates, which are subdivided into hard and soft ones. Hard templates are, for example, SiO2 and Al2O3 [56,57][56][57]. Graphitic carbon nitrides, synthesized using these templates, possess high specific surface area and porosity, and a large number of the surface active sites. Nevertheless, soft templates, such as starch and glucose, seem much more attractive, as they are safe for the environment, require no corrosive chemicals for elimination [58[58][59],59], and provide g-C3N4 with a specific surface area of >60 m2·g−1 [58]. After elimination of solvents and templates, the g-C3N4 nanoporous structure turns quite delicate (fragile) and easily destroyable, which presents one of the main problems of creating polymeric hollow nanospheres. A green soft-template approach allows synthesizing various g-C3N4 structures through relatively simple routes, keeping these structures durable even after template removal. Amphiphilic molecules, surfactants, ionic liquids, and gas bubbles are used as soft templates [60]. However, the template polymeric materials are inclined to early decomposition that results in some carbon residue in the material, which induces pores isolating, and thus affects negatively the g-C3N4 structure and photocatalytic activity.
2.1.2. The Use of Pore-Forming Agents
Pore-forming agents can significantly improve the photocatalytic characteristics of graphitic carbon nitride due to their affecting of its textural characteristics. Ammonium chloride [61] and urea [62] are among the most commonly used pore-forming agents reported in the literature. This approach ensures the synthesis of complex porous structures, such as ultrathin nanosheets [61]. Favorably compared to hard templates, the pore-forming agents require no eliminating from the target material by using harmful compounds or procedures. For example, ammonium chloride (NH4Cl) additive in the g-C3N4 precursor decomposes at temperatures of 280–370 °C into NH3 and HCl thus facilitating the formation of a loose ultrathin nanosheet structure (thickness 1–2.4 nm or 3–7 atomic layers) with a defective matrix [61,63][61][63]. The bottleneck of this process is the cost-efficiency and ecological issues; obviously, the cheaper and environmentally benign pore-forming agents should be developed.
2.1.3. Pretreatment Method
There are many studies in which precursors are modified prior to calcination in order to obtain g-C3N4 with improved properties [64,65,66][64][65][66]. One such modification method is the pretreatment of the precursor with acids (H2SO4, HNO3), which makes it possible to obtain a much higher specific surface area of resulting g-C3N4 [65]. The strong oxidizing power of the acids separates the g-C3N4 layers into thinner nanosheets, weakening the van der Waals force between them [65]. The addition of strong alkalis (NaOH) also facilitates the creation of ultrathin nanosheets. However, despite the ability to improve textural characteristics, it is difficult to control the yields and reproducibility of the resulting material in these pretreatment methods [65].
2.2. Doping Heteroatoms
The polymeric structure of graphitic carbon nitride is suitable for doping by other atoms or molecules by means of appropriate chemical procedures. The introduction of various atoms affects the electronic structure of the polymer material. Doping with nonmetals or anions leads to the g-C3N4 band gap narrowing. It is assumed that the band gap narrowing results from the formation of localized states and elevation of the valence band maximum due to the presence of doping atoms [67]. Besides doping with mono-nonmetal heteroatoms, co-doping with several heteroatoms is applied to provide more efficient band gap width variation [68,69,70][68][69][70].
Particular attention is focused on the doping of graphitic carbon nitride with halogens: Br, I, Cl [71,72][71][72]. For example, doping with bromine increases optical absorption, conductivity, charge carrier transfer rate, and photocatalytic activity without disturbing the g-C3N4 structural stability. It is assumed that halogen doping shifts the optical absorption spectrum towards longer wavelengths in the UV-visible spectrum [72]. The hindering of the charge carrier recombination at the introduction of Br atoms is attributed to the delocalization of the Br valence electrons to π-conjugated g-C3N4 structures. Graphitic carbon nitride doped with halogen atoms (F, Cl, Br, I) exhibited enhanced optical absorption and improved photocatalytic characteristics [72]. It was found that electronegativity of the halogen atoms affects the photocatalytic properties of g-C3N4. The higher the halogen atomic number, the lower is its electronegativity, and more electrons can transfer from the halogen atom to g-C3N4, thus leading to the gradually uplifting Fermi level and decreasing work function. Doping halogen atoms facilitate the electron escape from the g-C3N4 surface to participate in photocatalytic reactions.
Doping is an effective approach to improve the photocatalyst light absorption capacity, oxidizability, and separation efficiency of photoinduced charge carriers [73].
2.3. Doping Metals
One of the most frequently used and quite simple methods to modify the surface of graphitic carbon nitride is the use of metal dopants. The nanoparticles of noble and non-noble metals improve photocatalyst ability to absorb visible light and resist recombination of photoinduced charges. Also, metal nanoparticles can act as catalysts for the formation of molecular hydrogen. Noble metals attract particular attention, as they have the most suitable electronic and optical characteristics [74,75][74][75]. The Schottky barrier, also known as the space charge separation region, is formed at the noble metal-semiconductor heterojunction; it impedes electron migration from one material to another, and reduces the charge recombination. The creation of the Schottky barrier allows for the accumulation of additional negative charges (electrons) and positive charges (holes) in the noble metal and semiconductor, respectively. Noble metals also have the ability to absorb visible light owing to the plasmon resonance effect. There are two main approaches for depositing noble metal particles on the catalyst surface: impregnation with a precursor followed by reduction with NaBH4 [76] or another reductant [83], and photodeposition under the action of ultraviolet radiation [77,84][77][84]. There is also a possibility to attach pre-synthesized nanocrystals of desired sizes and shapes on graphitic carbon nitride nanosheets through electrostatic attraction [85]. The chlorocomplexes are common precursors for the deposition of noble metals onto a g-C3N4 surface [86,87,88][86][87][88]: for example, hexachloroplatinic acid (H2PtCl6) is usually used as the platinum precursor [78,84,89][78][84][89]. The photodeposition method does not use high-temperature treatments or hazardous materials. The downside of using noble metals as cocatalysts is that they are very expensive, especially in view of the high metal loading in the catalysts (1–5 wt.%) [79]. Currently, many studies are aimed at replace noble metals with non-noble ones, such as copper or nickel, which are much less expensive and are able to absorb visible light [80,81,90][80][81][90].
Although metal doping facilitates significant acceleration of the hydrogen production reaction, an excess of cocatalyst on the surface reduces the catalyst ability to absorb light in the visible region and acts as an electron trap [82].
2.4. Development of Composite Photocatalysts
In order to provide efficient light utilization and enhanced the redox ability of a material, it is common practice to shift the energy levels of the valence and conduction bands by creating a composite of two different materials. Composite materials are used to develop photocatalysts with enhanced light absorption in the visible region, efficient charge separation retarding charge recombination, and improved redox performance. There are various composite photocatalysts differing by heterojunction types: type I [91], type II [91[91][92],92], S-scheme [93,94][93][94], Z-scheme [95,96,97][95][96][97].
The creation of effective composites between two different compounds depends on their crystal structures, electron affinities, band structures, and the strength of the interfacial interaction. The type I heterojunctions assume that the valence band (VB) of semiconductor 1 lies higher than the VB of semiconductor 2, and the conduction band (CB) lies lower; in such a system, both electrons and holes are transferred to one semiconductor, where redox reactions occur [91]. Such a transfer of photogenerated charges from one semiconductor to another does not improve the efficiency of charge separation.
In type II heterojunctions, the level of VB and CB potentials of semiconductor 1 are higher than the level of VB and CB potentials of semiconductor 2. Here, the separation of photogenerated electrons and holes occurs through double charge transfer: an electron migrates from the CB of semiconductor 1 to the CB of semiconductor 2; a hole moves from the VB of semiconductor 2 to the VB of semiconductor 1. Electrons are accumulated in the CB of semiconductor 2, while holes accumulate in the VB of semiconductor 1, which facilitates efficient charge separation and reduced recombination [91,92][91][92]. Although traditional type II heterojunctions improve charge separation, they reduce the initial redox ability of electrons and holes.
The formation of S-scheme heterojunctions facilitates efficient charge separation without affecting the intrinsic redox ability of semiconductors [93]. To achieve successful construction of the S-scheme, the position of the CB and the Fermi level of the photocatalyst on which the reduction reaction proceeds must be higher than respective characteristics of the photocatalyst which runs the oxidation process. The internal electric field caused by the band deviation due to the difference in the Fermi levels of the two semiconductors enhances the charge mobility. The synthesis of two different semiconductors for subsequent integration into an S-scheme is considered rather difficult [94]. To date, the S-scheme heterojunction approach is considered to be the best choice for the development of photocatalysts with high charge separation efficiency and redox ability. Compared to S-scheme, the Z-scheme heterojunction photocatalysts have higher charge separation efficiency and are easier to synthesize [97]. Electrons from the CB of semiconductor 1 migrate through electron mediators (metals) to the VB of semiconductor 2 to recombine with photoinduced holes.
In the case of type I heterojunction photocatalysts [98], the redox process occurs on one semiconductor. The type II [99] heterojunctions significantly promote the separation of photoinduced electrons and holes, but have a weak redox ability that is detrimental for the photocatalytic reaction. Currently, the S-scheme approach is the most promising with regard to efficient charge separation and redox reactions [100]. However, the creation of S-scheme heterojunction photocatalysts is a difficult task that requires the structural modification of both semiconductors.
References
- Krausmann, F.; Schaffartzik, A.; Mayer, A.; Eisenmenger, N.; Gingrich, S.; Haberl, H.; Fischer-Kowalski, M. Long-Term Trends in Global Material and Energy Use. In Social Ecology Human-Environment Interactions; Haberl, H., Fischer-Kowalski, M., Krausmann, F., Winiwarter, V., Eds.; Springer: Cham, Switzerland, 2016; Volume 5, pp. 199–216.
- Romanello, M.; Di Napoli, C.; Drummond, P.; Green, C.; Kennard, H.; Lampard, P.; Scamman, D.; Arnell, N.; Ayeb-Karlsson, S.; Ford, L.B.; et al. The 2022 Report of the Lancet Countdown on Health and Climate Change: Health at the Mercy of Fossil Fuels. Lancet 2022, 400, 1619–1654.
- Zhang, B.; Zhang, S.X.; Yao, R.; Wu, Y.H.; Qiu, J.S. Progress and Prospects of Hydrogen Production: Opportunities and Challenges. J. Electron. Sci. Technol. 2021, 19, 100080.
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781.
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45, 18753–18771.
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Liu, X.; Wang, S.; Li, Y.; Zhang, L.; et al. Industrial Status, Technological Progress, Challenges, and Prospects of Hydrogen Energy. Nat. Gas Ind. B 2022, 9, 427–447.
- Bie, C.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. Enhanced Solar-to-Chemical Energy Conversion of Graphitic Carbon Nitride by Two-Dimensional Cocatalysts. EnergyChem 2021, 3, 100051.
- Ning, X.; Lu, G. Photocorrosion Inhibition of CdS-Based Catalysts for Photocatalytic Overall Water Splitting. Nanoscale 2020, 12, 1213–1223.
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-Based Heterojunction Photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107.
- Tang, S.; Xia, Y.; Fan, J.; Cheng, B.; Yu, J.; Ho, W. Enhanced Photocatalytic H2 Production Performance of CdS Hollow Spheres Using C and Pt as Bi-Cocatalysts. Chin. J. Catal. 2021, 42, 743–752.
- Khan, M.; Assal, M.E.; Nawaz Tahir, M.; Khan, M.; Ashraf, M.; Rafe Hatshan, M.; Khan, M.; Varala, R.; Mohammed Badawi, N.; Farooq Adil, S. Graphene/Inorganic Nanocomposites: Evolving Photocatalysts for Solar Energy Conversion for Environmental Remediation. J. Saudi Chem. Soc. 2022, 26, 101544.
- Huang, C.W.; Nguyen, B.S.; Wu, J.C.S.; Nguyen, V.H. A Current Perspective for Photocatalysis towards the Hydrogen Production from Biomass-Derived Organic Substances and Water. Int. J. Hydrogen Energy 2020, 45, 18144–18159.
- Gao, W.; Zhang, S.; Wang, G.; Cui, J.; Lu, Y.; Rong, X.; Gao, C. A Review on Mechanism, Applications and Influencing Factors of Carbon Quantum Dots Based Photocatalysis. Ceram. Int. 2022, 48, 35986–35999.
- Li, X.; Dong, Q.; Tian, Q.; Sial, A.; Wang, H.; Wen, H.; Pan, B.; Zhang, K.; Qin, J.; Wang, C. Recent Advance in Metal- and Covalent-Organic Framework-Based Photocatalysis for Hydrogen Evolution. Mater. Today Chem. 2022, 26, 101037.
- Li, Z.; Li, K.; Du, P.; Mehmandoust, M.; Karimi, F.; Erk, N. Carbon-Based Photocatalysts for Hydrogen Production: A Review. Chemosphere 2022, 308, 135998.
- Durmus, Z.; Maijenburg, A.W. A Review on Graphitic Carbon Nitride (g-C3N4)—Metal Organic Framework (MOF) Heterostructured Photocatalyst Materials for Photo(Electro)Chemical Hydrogen Evolution. Int. J. Hydrogen Energy 2022, 47, 36784–36813.
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38.
- Kausar, F.; Varghese, A.; Pinheiro, D.; Devi, K.R.S. Recent Trends in Photocatalytic Water Splitting Using Titania Based Ternary Photocatalysts—A Review. Int. J. Hydrogen Energy 2022, 47, 22371–22402.
- Pattanayak, P.; Singh, P.; Bansal, N.K.; Paul, M.; Dixit, H.; Porwal, S.; Mishra, S.; Singh, T. Recent Progress in Perovskite Transition Metal Oxide-Based Photocatalyst and Photoelectrode Materials for Solar-Driven Water Splitting. J. Environ. Chem. Eng. 2022, 10, 108429.
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–Organic Frameworks and Derived Materials as Photocatalysts for Water Splitting and Carbon Dioxide Reduction. Coord. Chem. Rev. 2022, 469, 214664.
- Hota, P.; Das, A.; Maiti, D.K. A Short Review on Generation of Green Fuel Hydrogen through Water Splitting. Int. J. Hydrogen Energy 2022, in press.
- Jaryal, R.; Kumar, R.; Khullar, S. Mixed Metal-Metal Organic Frameworks (MM-MOFs) and Their Use as Efficient Photocatalysts for Hydrogen Evolution from Water Splitting Reactions. Coord. Chem. Rev. 2022, 464, 214542.
- Melián, E.P.; Díaz, O.G.; Méndez, A.O.; López, C.R.; Suárez, M.N.; Rodríguez, J.M.D.; Navío, J.A.; Hevia, D.F.; Peña, J.P. Efficient and Affordable Hydrogen Production by Water Photo-Splitting Using TiO2-Based Photocatalysts. Int. J. Hydrogen Energy 2013, 38, 2144–2155.
- Perumal, V.; Uthrakumar, R.; Chinnathambi, M.; Inmozhi, C.; Robert, R.; Rajasaravanan, M.E.; Raja, A.; Kaviyarasu, K. Electron-Hole Recombination Effect of SnO2-CuO Nanocomposite for Improving Methylene Blue Photocatalytic Activity in Wastewater Treatment under Visible Light. J. King Saud Univ.-Sci. 2023, 35, 102388.
- Meenakshi, G.; Sivasamy, A. Enhanced Photocatalytic Activities of CeO2@ZnO Core-Shell Nanostar Particles through Delayed Electron Hole Recombination Process. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 645, 128920.
- Deng, H.; Qin, C.; Pei, K.; Wu, G.; Wang, M.; Ni, H.; Ye, P. TiO2/Reduced Hydroxylated Graphene Nanocomposite Photocatalysts: Improved Electron–Hole Separation and Migration. Mater. Chem. Phys. 2021, 270, 124796.
- Rozman, N.; Nadrah, P.; Cornut, R.; Jousselme, B.; Bele, M.; Dražić, G.; Gaberšček, M.; Kunej, Š.; Škapin, A.S. TiO2 Photocatalyst with Single and Dual Noble Metal Co-Catalysts for Efficient Water Splitting and Organic Compound Removal. Int. J. Hydrogen Energy 2021, 46, 32871–32881.
- Berdyugin, S.; Kozlova, E.; Kurenkova, A.; Gerasimov, E.; Bukhtiyarov, A.; Kolesov, B.; Yushina, I.; Vasilchenko, D.; Korenev, S. Hydrogarnet-Derived Rh/TiO2 Catalysts with a Low Rhodium Content for a Photocatalytic Hydrogen Production. Mater. Lett. 2022, 307, 130997.
- Kurenkova, A.Y.; Kremneva, A.M.; Saraev, A.A.; Murzin, V.; Kozlova, E.A.; Kaichev, V.V. Influence of Thermal Activation of Titania on Photoreactivity of Pt/TiO2 in Hydrogen Production. Catal. Lett. 2021, 151, 748–754.
- Vasilchenko, D.; Topchiyan, P.; Tsygankova, A.; Asanova, T.; Kolesov, B.; Bukhtiyarov, A.; Kurenkova, A.; Kozlova, E. Photoinduced Deposition of Platinum from (Bu4N)2 for a Low Pt-Loading Pt/TiO2 Hydrogen Photogeneration Catalyst. ACS Appl. Mater. Interfaces 2020, 12, 48631–48641.
- Popugaeva, D.; Tian, T.; Ray, A.K. Hydrogen Production from Aqueous Triethanolamine Solution Using Eosin Y-Sensitized ZnO Photocatalyst Doped with Platinum. Int. J. Hydrogen Energy 2020, 45, 11097–11107.
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manag. 2020, 258, 110050.
- Kurenkova, A.Y.; Markovskaya, D.V.; Gerasimov, E.Y.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlova, E.A. New Insights into the Mechanism of Photocatalytic Hydrogen Evolution from Aqueous Solutions of Saccharides over CdS-Based Photocatalysts under Visible Light. Int. J. Hydrogen Energy 2020, 45, 30165–30177.
- Kozlova, E.A.; Lyulyukin, M.N.; Markovskaya, D.V.; Selishchev, D.S.; Cherepanova, S.V.; Kozlov, D.V. Synthesis of Cd1-: XZnxS Photocatalysts for Gas-Phase CO2 Reduction under Visible Light. Photochem. Photobiol. Sci. 2019, 18, 871–877.
- Liu, X.; Sayed, M.; Bie, C.; Cheng, B.; Hu, B.; Yu, J.; Zhang, L. Hollow CdS-Based Photocatalysts. J. Mater. 2021, 7, 419–439.
- Palaniswamy, V.K.; Ramasamy, B.; Manoharan, K.; Raman, K.; Sundaram, R. Enhanced Photocatalytic Degradation of Tetracycline Antibiotic Using M-BiVO4 Photocatalyst under Visible Light Irradiation. Chem. Phys. Lett. 2021, 771, 138531.
- Gurylev, V. A Review on the Development and Advancement of Ta2O5 as a Promising Photocatalyst. Mater. Today Sustain. 2022, 18, 100131.
- Lian, J.; Li, D.; Qi, Y.; Yang, N.; Zhang, R.; Xie, T.; Guan, N.; Li, L.; Zhang, F. Metal-Seed Assistant Photodeposition of Platinum over Ta3N5 Photocatalyst for Promoted Solar Hydrogen Production under Visible Light. J. Energy Chem. 2021, 55, 444–448.
- Hara, M.; Takata, T.; Kondo, J.N.; Domen, K. Photocatalytic Reduction of Water by TaON under Visible Light Irradiation. Catal. Today 2004, 90, 313–317.
- Ismael, M. Latest Progress on the Key Operating Parameters Affecting the Photocatalytic Activity of TiO2-Based Photocatalysts for Hydrogen Fuel Production: A Comprehensive Review. Fuel 2021, 303, 121207.
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the Strategies for Enhancing the Photocatalytic Activity of TiO2: Conversion from UV-Light Active to Visible-Light Active Photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700.
- Firtina-Ertis, I.; Kerkez-Kuyumcu, Ö. Synthesis of NiFe2O4/TiO2-Ag+ S-Scheme Photocatalysts by a Novel Complex-Assisted Vapor Thermal Method for Photocatalytic Hydrogen Production. J. Photochem. Photobiol. A Chem. 2022, 432, 114106.
- Gao, R.H.; Ge, Q.; Jiang, N.; Cong, H.; Liu, M.; Zhang, Y.Q. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalytic Materials for Hydrogen Evolution. Front. Chem. 2022, 10, 1048504.
- Cao, S.; Yu, J. G-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107.
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294.
- Xia, P.; Li, G.; Li, X.; Yuan, S.; Wang, K.; Huang, D.; Ji, Y.; Dong, Y.; Wu, X.; Zhu, L.; et al. Synthesis of G-C3N4 from Various Precursors for Photocatalytic H2 Evolution under the Visible Light. Crystals 2022, 12, 1719.
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80.
- Pati, S.; Acharya, R. An Overview on G-C3N4 as a Robust Photocatalyst towards the Sustainable Generation of H2 energy. Mater. Today Proc. 2021, 35, 175–178.
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic Carbon Nitride (g–C3N4)–Based Metal-Free Photocatalysts for Water Splitting: A Review. Carbon 2019, 149, 693–721.
- Hu, C.; Chiu, W.L.; Wang, C.Y.; Nguyen, V.H. Freeze-Dried Dicyandiamide-Derived g-C3N4 as an Effective Photocatalyst for H2 Generation. J. Taiwan Inst. Chem. Eng. 2021, 129, 128–134.
- Liang, L.; Cong, Y.; Wang, F.; Yao, L.; Shi, L. Hydrothermal Pre-Treatment Induced Cyanamide to Prepare Porous g-C3N4 with Boosted Photocatalytic Performance. Diam. Relat. Mater. 2019, 98, 107499.
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329.
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607.
- Wang, Y.; He, F.; Chen, L.; Shang, J.; Wang, J.; Wang, S.; Song, H.; Zhang, J.; Zhao, C.; Wang, S.; et al. Acidification and Bubble Template Derived Porous G-C3N4 for Efficient Photodegradation and Hydrogen Evolution. Chin. Chem. Lett. 2020, 31, 2668–2672.
- Wu, X.; Ma, H.; Zhong, W.; Fan, J.; Yu, H. Porous Crystalline G-C3N4: Bifunctional NaHCO3 Template-Mediated Synthesis and Improved Photocatalytic H2-Evolution Rate. Appl. Catal. B Environ. 2020, 271, 118899.
- Zhang, H.; Bao, C.; Hu, X.; Wen, Y.; Li, K.; Zhang, H. Synthesis of Tunnel Structured G-C3N4 through a Facile Vapor Deposition Method Using SBA-15 and KIT-6 as Templates and Their Photocatalytic Degradation of Tetracycline Hydrochloride and Phenol. J. Environ. Chem. Eng. 2022, 10, 107871.
- Ramesh, A.; Da, C.T.; Manigandan, R.; Bhargav, P.B.; Nguyen-Le, M.T. Selectivity Oxidation of Benzyl Alcohol Using Mesoporous G-C3N4 Catalysts Prepared by Hard Template Method. Colloids Interface Sci. Commun. 2022, 48, 100608.
- Iqbal, W.; Wang, L.; Tan, X.; Zhang, J. One-Step in Situ Green Template Mediated Porous Graphitic Carbon Nitride for Efficient Visible Light Photocatalytic Activity. J. Environ. Chem. Eng. 2017, 5, 3500–3507.
- Sun, S.; Li, J.; Song, P.; Cui, J.; Yang, Q.; Zheng, X.; Yang, Z.; Liang, S. Facile Constructing of Isotype G-C3N4(Bulk)/g-C3N4(Nanosheet) Heterojunctions through Thermal Polymerization of Single-Source Glucose-Modified Melamine: An Efficient Charge Separation System for Photocatalytic Hydrogen Production. Appl. Surf. Sci. 2020, 500, 143985.
- Yang, Z.; Zhang, Y.; Schnepp, Z. Soft and Hard Templating of Graphitic Carbon Nitride. J. Mater. Chem. A 2015, 3, 14081–14092.
- Wu, X.; Gao, D.; Wang, P.; Yu, H.; Yu, J. NH4Cl-Induced Low-Temperature Formation of Nitrogen-Rich g-C3N4 Nanosheets with Improved Photocatalytic Hydrogen Evolution. Carbon 2019, 153, 757–766.
- Li, R.; Cui, X.; Bi, J.; Ji, X.; Li, X.; Wang, N.; Huang, Y.; Huang, X.; Hao, H. Urea-Induced Supramolecular Self-Assembly Strategy to Synthesize Wrinkled Porous Carbon Nitride Nanosheets for Highly-Efficient Visible-Light Photocatalytic Degradation. RSC Adv. 2021, 11, 23459–23470.
- Babu, B.; Shim, J.; Kadam, A.N.; Yoo, K. Modification of Porous G-C3N4 Nanosheets for Enhanced Photocatalytic Activity: In-Situ Synthesis and Optimization of NH4Cl Quantity. Catal. Commun. 2019, 124, 123–127.
- Wang, Y.; Tan, G.; Dang, M.; Dong, S.; Liu, Y.; Liu, T.; Ren, H.; Xia, A.; Lv, L. Study on Surface Modification of G-C3N4 Photocatalyst. J. Alloys Compd. 2022, 908, 164507.
- Cui, J.; Qi, D.; Wang, X. Research on the Techniques of Ultrasound-Assisted Liquid-Phase Peeling, Thermal Oxidation Peeling and Acid-Base Chemical Peeling for Ultra-Thin Graphite Carbon Nitride Nanosheets. Ultrason. Sonochem. 2018, 48, 181–187.
- Ghosh, U.; Pal, A. Drastically Enhanced Tetracycline Degradation Performance of a Porous 2D G-C3N4 Nanosheet Photocatalyst in Real Water Matrix: Influencing Factors and Mechanism Insight. J. Water Process Eng. 2022, 50, 103315.
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648.
- Zhang, W.; Xu, D.; Wang, F.; Liu, H.; Chen, M. Enhanced Photocatalytic Performance of S/Cd Co-Doped g-C3N4 Nanorods for Degradation of Dyes. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 653, 130079.
- Chi, X.; Liu, F.; Gao, Y.; Song, J.; Guan, R.; Yuan, H. An Efficient B/Na Co-Doped Porous g-C3N4 Nanosheets Photocatalyst with Enhanced Photocatalytic Hydrogen Evolution and Degradation of Tetracycline under Visible Light. Appl. Surf. Sci. 2022, 576, 151837.
- Xu, J.; Chen, Y.; Chen, M.; Wang, J.; Wang, L. In Situ Growth Strategy Synthesis of Single-Atom Nickel/Sulfur Co-Doped g-C3N4 for Efficient Photocatalytic Tetracycline Degradation and CO2 Reduction. Chem. Eng. J. 2022, 442, 136208.
- Tan, Y.; Chen, W.; Liao, G.; Li, X.; Wang, J.; Tang, Y.; Li, L. Strategy for Improving Photocatalytic Ozonation Activity of G-C3N4 by Halogen Doping for Water Purification. Appl. Catal. B Environ. 2022, 306, 121133.
- Zhu, B.; Zhang, J.; Jiang, C.; Cheng, B.; Yu, J. First Principle Investigation of Halogen-Doped Monolayer g-C3N4 Photocatalyst. Appl. Catal. B Environ. 2017, 207, 27–34.
- Guo, P.; Zhao, F.; Hu, X. Boron- and Europium-Co-Doped g-C3N4 Nanosheets: Enhanced Photocatalytic Activity and Reaction Mechanism for Tetracycline Degradation. Ceram. Int. 2021, 47, 16256–16268.
- Huang, K.; Li, C.; Yang, J.; Zheng, R.; Wang, W.; Wang, L. Platinum Nanodots Modified Nitrogen-Vacancies g-C3N4 Schottky Junction for Enhancing Photocatalytic Hydrogen Evolution. Appl. Surf. Sci. 2022, 581, 152298.
- Ding, J.; Sun, X.; Wang, Q.; Li, D.S.; Li, X.; Li, X.; Chen, L.; Zhang, X.; Tian, X.; Ostrikov, K. (Ken) Plasma Synthesis of Pt/g-C3N4 Photocatalysts with Enhanced Photocatalytic Hydrogen Generation. J. Alloys Compd. 2021, 873, 159871.
- Zhurenok, A.V.; Larina, T.V.; Markovskaya, D.V.; Cherepanova, S.V.; Mel’gunova, E.A.; Kozlova, E.A. Synthesis of Graphitic Carbon Nitride-Based Photocatalysts for Hydrogen Evolution under Visible Light. Mendeleev Commun. 2021, 31, 157–159.
- Qin, Y.; Lu, J.; Meng, F.; Lin, X.; Feng, Y.; Yan, Y.; Meng, M. Rationally Constructing of a Novel 2D/2D WO3/Pt/g-C3N4 Schottky-Ohmic Junction towards Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution and Mechanism Insight. J. Colloid Interface Sci. 2021, 586, 576–587.
- Zhao, G.; Huang, X.; Fina, F.; Zhang, G.; Irvine, J.T.S. Facile Structure Design Based on C3N4 for Mediator-Free Z-Scheme Water Splitting under Visible Light. Catal. Sci. Technol. 2015, 5, 3416–3422.
- Han, C.; Du, L.; Konarova, M.; Qi, D.-C.; Phillips, D.L.; Xu, J. Beyond Hydrogen Evolution: Solar-Driven, Water-Donating Transfer Hydrogenation over Platinum/Carbon Nitride. ACS Catal. 2020, 10, 9227–9235.
- Wang, L.; Zang, L.; Shen, F.; Wang, J.; Yang, Z.; Zhang, Y.; Sun, L. Preparation of Cu Modified G-C3N4 Nanorod Bundles for Efficiently Photocatalytic CO2 Reduction. J. Colloid Interface Sci. 2022, 622, 336–346.
- Bi, L.; Xu, D.; Zhang, L.; Lin, Y.; Wang, D.; Xie, T. Metal Ni-Loaded g-C3N4 for Enhanced Photocatalytic H2 Evolution Activity: The Change in Surface Band Bending. Phys. Chem. Chem. Phys. 2015, 17, 29899–29905.
- Zhou, X.; Wang, Y.; Wang, Y.; Zhang, M.; Gao, H.; Zhang, X. Superior Uniform Carbon 3N4 Core-Shell Nanostructures Embedded by Au Nanoparticles for High-Efficiency Photocatalyst. J. Hazard. Mater. 2020, 388, 121759.
- Shiraishi, Y.; Kofuji, Y.; Kanazawa, S.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Platinum Nanoparticles Strongly Associated with Graphitic Carbon Nitride as Efficient Co-Catalysts for Photocatalytic Hydrogen Evolution under Visible Light. Chem. Commun. 2014, 50, 15255–15258.
- Liu, D.; Shen, J.; Xie, Y.; Qiu, C.; Zhang, Z.; Long, J.; Lin, H.; Wang, X. Metallic Pt and PtO2 Dual-Cocatalyst-Loaded Binary Composite RGO-CNx for the Photocatalytic Production of Hydrogen and Hydrogen Peroxide. ACS Sustain. Chem. Eng. 2021, 9, 6380–6389.
- Guo, Y.; Jia, H.; Yang, J.; Yin, H.; Yang, Z.; Wang, J.; Yang, B. Understanding the Roles of Plasmonic Au Nanocrystal Size, Shape, Aspect Ratio and Loading Amount in Au/g-C3N4 Hybrid Nanostructures for Photocatalytic Hydrogen Generation. Phys. Chem. Chem. Phys. 2018, 20, 22296–22307.
- Li, L.; Wang, X.; Gu, H.; Zhang, H.; Zhang, J.; Zhang, Q.; Dai, W.L. Which Is More Efficient in Promoting the Photocatalytic H2 Evolution Performance of G-C3N4: Monometallic Nanocrystal, Heterostructural Nanocrystal, or Bimetallic Nanocrystal? Inorg. Chem. 2022, 61, 4760–4768.
- Fei, H.; Shao, J.; Li, H.; Li, N.; Chen, D.; Xu, Q.; He, J.; Lu, J. Construction of Ultra-Thin 2D CN-Br0.12/2%RhOx Photo-Catalyst with Rapid Electron and Hole Separation for Efficient Bisphenol A Degradation. Appl. Catal. B Environ. 2021, 299, 120623.
- Alwin, E.; Wojcieszak, R.; Kočí, K.; Edelmannová, M.; Zieliński, M.; Suchora, A.; Pędziński, T.; Pietrowski, M. Reductive Modification of Carbon Nitride Structure by Metals—The Influence on Structure and Photocatalytic Hydrogen Evolution. Materials 2022, 15, 710.
- Zhang, L.; Long, R.; Zhang, Y.; Duan, D.; Xiong, Y.; Zhang, Y.; Bi, Y. Direct Observation of Dynamic Bond Evolution in Single-Atom Pt/C3N4 Catalysts. Angew. Chem. 2020, 132, 6283–6288.
- Li, H.; Zhao, J.; Geng, Y.; Li, Z.; Li, Y.; Wang, J. Construction of CoP/B Doped g-C3N4 Nanodots/g-C3N4 Nanosheets Ternary Catalysts for Enhanced Photocatalytic Hydrogen Production Performance. Appl. Surf. Sci. 2019, 496, 143738.
- Zhu, Y.; Wan, T.; Wen, X.; Chu, D.; Jiang, Y. Tunable Type I and II Heterojunction of CoOx Nanoparticles Confined in G-C3N4 Nanotubes for Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2019, 244, 814–822.
- Wang, Y.; Li, J.; Chen, S.; Xie, Y.; Ma, Y.; Luo, Y.; Huang, J.; Ling, Y.; Ye, J.; Liang, Y.; et al. In Situ Loading of ZnIn2S4 Nanosheets onto S Doped G-C3N4 Nanosheets to Construct Type II Heterojunctions for Improving Photocatalytic Hydrogen Production. J. Alloys Compd. 2022, 924, 166569.
- Van, K.N.; Huu, H.T.; Nguyen Thi, V.N.; Le Thi, T.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Tran, D.L.; Vasseghian, Y. Facile Construction of S-Scheme SnO2/g-C3N4 Photocatalyst for Improved Photoactivity. Chemosphere 2022, 289, 133120.
- Zhang, T.; Yang, Q.; Li, H.; Zhong, J.; Li, J.; Yang, H. Photocatalytic Properties of BiOBr/g-C3N4 Heterojunctions Originated from S-Scheme Separation and Transfer of Interfacial Charge Pairs. Opt. Mater. 2022, 131, 112649.
- Ma, Y.; Li, J.; Cai, J.; Zhong, L.; Lang, Y.; Ma, Q. Z-Scheme g-C3N4/ZnS Heterojunction Photocatalyst: One-Pot Synthesis, Interfacial Structure Regulation, and Improved Photocatalysis Activity for Bisphenol A. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 653, 130027.
- Bi, Z.; Guo, R.; Ji, X.; Hu, X.; Wang, J.; Chen, X.; Pan, W. Direct Z-Scheme CoS/g-C3N4 Heterojunction with NiS Co-Catalyst for Efficient Photocatalytic Hydrogen Generation. Int. J. Hydrogen Energy 2022, 47, 34430–34443.
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A Review on TiO2-Based Z-Scheme Photocatalysts. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 1936–1955.
- Li, Y.; Wang, X.; Huo, H.; Li, Z.; Shi, J. A Novel Binary Visible-Light-Driven Photocatalyst Type-I CdIn2S4/g-C3N4 Heterojunctions Coupling with H2O2: Synthesis, Characterization, Photocatalytic Activity for Reactive Blue 19 Degradation and Mechanism Analysis. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 587, 124322.
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent Advances in G-C3N4-Based Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17.
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on G-C3N4-Based S-Scheme Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144.
- Li, Y.; He, Z.; Liu, L.; Jiang, Y.; Ong, W.J.; Duan, Y.; Ho, W.; Dong, F. Inside-and-out Modification of Graphitic Carbon Nitride (g-C3N4) Photocatalysts via Defect Engineering for Energy and Environmental Science. Nano Energy 2023, 105, 108032.
More
