Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Catherine Yang and Version 2 by Catherine Yang.
The epicardial adipose tissue (EAT) is a metabolically active organ recently associated with heart failure and atrial fibrillation and classified as an independent risk factor for subclinical coronary artery disease. Some evidence suggests as the assessment of EAT using coronary artery calcium (CCTAAC) might represent an additional tool to quantify patients’ cardiovascular risk.
- cardiac computed tomography (CCT)
- cardiovascular (CV) risk
- coronary artery calcium (CAC)
1. Anatomy and Functions of Epicardial Adipose Tissue
The intrathoracic adipose tissue surrounding the heart can be divided into epicardial adipose tissue (EAT) and pericardial fat (PF). The PF is located between the pericardial visceral and parietal layers; it derives from the primitive thoracic mesenchyme and is supplied by branches of the internal thoracic artery [1]. On the other hand, EAT is placed between the pericardial visceral layer and the myocardial muscle covering about 80% of the cardiac surface; it originates from the splanchnopleuric mesoderm and is vascularized by branches of coronary arteries. Lastly, the portion of the EAT immediately contiguous with the adventitial layer of coronary arteries is called pericoronary adipose tissue (PCAT) [2].
The EAT is microscopically composed of different cells, including adipocytes, resident monocytes, immune cells, and nerve cells [3]. Interestingly, no muscle fascia between EAT and myocardium is present, allowing direct communication between these two organs through paracrine and vasocrine mechanisms [1]. Physiologically, EAT plays a protective role for the heart. It acts as a local energy store and provides free fatty acids to the myocardial tissue in times of high demand [4]. It also releases adipokines with anti-inflammatory, antioxidant, and vasodilator functions (adiponectin and adrenomedullin) [5]. In addition, the EAT offers mechanical support preventing coronary artery torsion during cardiac contraction, exerts a thermoregulatory function [4] and acts as an immune organ [6].
However, in pathological conditions, EAT becomes pro-arrhythmogenic and pro-atherogenic. Indeed, in patients who have CAD, EAT shows pro-inflammatory features with an increased concentration of inflammatory M1 macrophages than anti-inflammatory M2 macrophages [7], higher production of interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-alpha (TNF-a) associated with reduced production of adiponectin and adrenomedullin, and an elevated concentration of reactive oxygen species (ROS) [8].
The mechanisms of EAT dysfunction might translate into atherosclerotic plaque development are not entirely known. However, it has been proposed that macrophages, together with T- and B-lymphocytes, are triggered by the Toll-like receptor (TLR) binding that stimulates nuclear factor-κB (NF-κΒ) and JUNN-terminal kinase (JNK) [9][10]. The activation of these two molecules leads to increased interleukin production, such as IL-6, facilitating endothelial cell permeability and monocyte adhesion [11]. The entire process is powered by the increased ROS levels that further favor endothelial dysfunction and interleukin secretion [8].
2. Quantification of Epicardial Adipose Tissue Using CCTA
A trustworthy quantification of EAT can be obtained using different non-invasive techniques. EAT thickness could be measured by transthoracic echocardiography on the free wall of the right ventricle [12]. However, CCTA is considered the most validated and reproducible technique for its quantification, given the higher spatial resolution compared to magnetic resonance (MR) and allowing for simultaneous evaluation of CAD [13].
In detail, quantification of EAT by CCTA envisages the measure of density (Hounsfield units), volume (cm3), and thickness (mm). Epicardial fat density ranges from −190 to −30 Hounsfield units (HU) [14]. Measurements of thickness may be achieved in the horizontal long-axis plane, in the basal short-axis plane, and over the right ventricular free wall [15]. Regarding volume quantification, manual segmentation of EAT is the most widespread method. The operator manually draws EAT contours every 15 mm within a region of interest that usually includes the anterosuperior mediastinum at the pulmonary trunk level, the left main trunk, the left anterior descending and circumflex arteries in proximal segments, the posterior mediastinum, and the inferior diaphragmatic surface. (Figure 1a–c) Different software can be used to optimize and speed up this process [16]. The advantages of CT volumetric quantification are the high definition and reproducibility, whereas the main limitation is the need for a long segmentation time for an accurate measurement [17]. EAT can also be measured during coronary CT in the interval between contrast administration and angiographic acquisition and does not require specific acquisition [16].
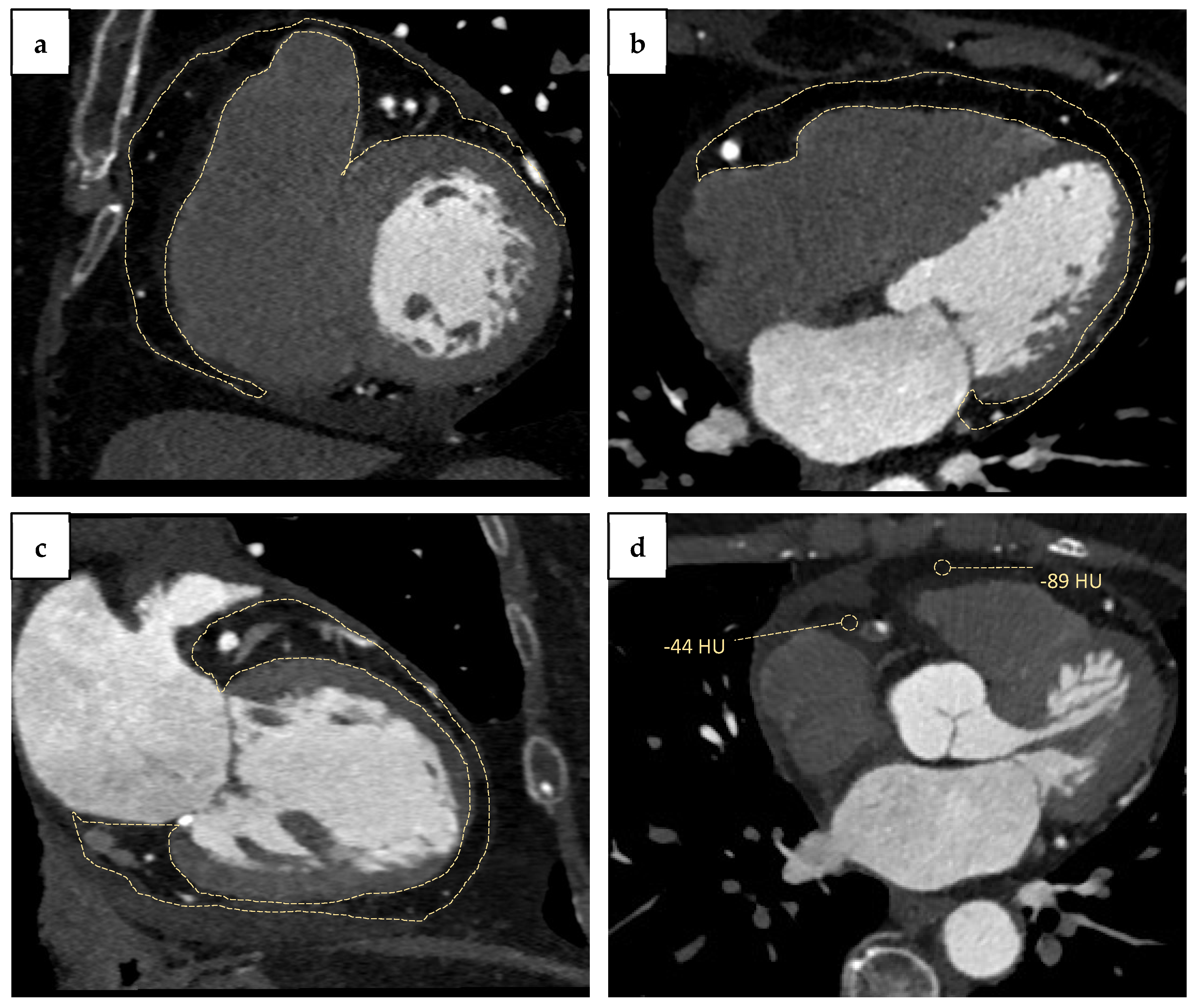
Figure 1. Cardiac computed tomography angiography (CCTA) assessment of epicardial adipose tissue (EAT) and peri-coronary adipose tissue (PCAT): (a) EAT in short-axis view; (b) EAT in four-chamber view; (c) EAT in two-chamber view (dashed line); (d) peri-coronary adipose tissue (PCAT) radiodensity (−44 HU) compared with radiodensity of EAT surrounding free left ventricle wall (−89 HU).
3. Epicardial Fat Volume and Atherosclerosis Progression
Recent studies have demonstrated that EAT volume is associated with the development and progression of atherosclerotic plaques. Therefore, previous studies reported greater EAT volumes in patients with angiographic evidence of coronary stenoses than those without [18][19]. Similarly, Gitsioudis et al., enrolling patients with intermediate CAD risk undergoing cardiac CT with contextual EAT volume quantification, showed a higher EAT volume in patients with coronary stenosis >50% compared with those with <50%. Moreover, the multivariate regression analysis revealed an independent association between coronary stenosis severity, plaque burden, and EAT volume [20].
Notably, EAT volume is also associated with atherosclerosis progression and, more specifically, with the development of high-risk plaques. In this regard, Alexopoulos et al. demonstrated that EAT was higher in patients with mixed/non-calcified plaques than those with calcified lesions [21]. The same evidence was reported by Tsuyoshi et al., that quantified epicardial fat volume in 1308 patients with symptomatic CAD and a zero-calcium score. EAT was greater in patients with obstructive atherosclerotic plaques than no plaque and even more in those with vulnerable plaque than no plaque [22]. Similarly, a recent meta-analysis by Nerlekar et al. confirmed the association between EAT volume and high-risk plaques [23]. Otsuka et al. found that EAT volume in ACS patients was significantly higher than in those suffering from CCS [24]. Finally, Yamashita et al. demonstrated a significant positive correlation between EAT volume and necrotic plaque detected using intravascular ultrasound imaging (IVUS) and a negative correlation between EAT volume and fibrous plaque [25].
Several studies also reported a direct relationship between EAT and CAC score. Iwasaki et al. demonstrated increased CAC in patients with EAT volume >100 cm3 than in those with EAT volume <100 cm3, with a higher incidence of CAD in the same group [26]. These results were confirmed in the study conducted by Cosson et al., where EAT volume was independently associated with CAC ≥ 100 AU (per 10 cm3 increase: OR 1.11 (1.02–1.20)) [27].
Another measurable parameter derived from EAT assessment and associated with a worse prognosis is fat radiodensity. Franssens et al. demonstrated in 140 patients undergoing CT-scan that one standard deviation lower EAT attenuation (5 HU) was associated with 1.9 and 1.07 higher odds (for men and women respectively) of being in higher CAC class (0, 1 to 100, 101 to 400, and >400) [28]. Similarly, Goeller et al. quantified both EAT volume and density in 456 asymptomatic patients; EAT volume resulted lowest in patients without coronary calcium and higher in patients with severe atherosclerosis, while EAT density resulted lower in patients with high coronary calcium and increased occurrence of adverse clinical events [29].
Finally, all this evidence allows identifying EAT volume as a predictor of MACEs. For example, Eisenberg et al. quantify EAT volume in asymptomatic patients from the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) trial, showing its association with increased risk of MACE after 14 ± 3 years of follow-up. It was also demonstrated that MACE risk progressively increased with EAT volume ≥ 113 cm3 and CAC ≥ 100 AU [30]. Thus, Fuller et al. revealed that patients with CAD who died from sudden cardiac death had significantly higher EAT thickness [31]. Moreover, in the Heinz Nixdorf Recall Study, the incidence of coronary events increased directly with EAT quartiles, with a doubling of EAT volume associated with a 1.5-fold risk of coronary events independently from other traditional CV risk factors [32].
4. Pericoronary Fat: Marker of Coronary Artery Stenosis Severity
Another CT-measurable parameter associated with CAD severity is the pericoronary fat (Figure 1d). In this regard, a study conducted by Gorter et al. evaluated in 128 symptomatic patients undergoing percutaneous coronary intervention (PCI) the EAT volume and the pericoronary fat. Patients with low BMI and multivessel coronary disease had increased values of both CT parameters compared to patients without CAD [33]. Likewise, Balcer et al. analyzed PCAT volume in 46 patients with acute MI undergoing coronary-CT angiography, demonstrating higher values around culprit lesions than non-culprit ones [34]. Furthermore, Ma et al. reported a raised pericoronary adipose tissue mean attenuation (PCATMA) in arteries with CT-measured plaques, particularly in non-calcified/mixed plaques [35]. Interestingly, PCATMA was also associated with in-stent restenosis. Thus, Nogic et al. enrolled 151 patients undergoing CT-scan for suspected CAD, treated with stent implantation within three months and that repeated coronary angiography after a maximum of five years for any reason. The study demonstrated that patients with ISR had a significantly increased lesion-specific PCATMA at baseline compared with patients without stent hyperplasia [36].
5. Effective Therapies in the Reduction of Epicardial Fat
Recent studies have shown several drugs' efficacy in reducing EAT thickness and volume. Thanks to their pleiotropic effects, statins have been reported to reduce epicardial fat metabolic activity, thickness, and attenuation [37]. In this setting, atorvastatin provided better results than pravastatin [38]. Furthermore, metformin also reduced EAT thickness after three months of treatment in diabetic patients [39]; similar results were reported for liraglutide, semaglutide, and dulaglutide [40][41]. Correspondingly, sodium-glucose cotransporter 2 (SGLT-2) inhibitors (dapagliflozin and empagliflozin) demonstrated a reduced EAT volume in CAD patients after six months of treatment [42]. Notwithstanding, further studies are needed to evaluate how the well-known cardiovascular benefit of all these drugs might be related to the attenuation of EAT thickness and volume.
References
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial Adipose Tissue: Anatomic, Biomolecular and Clinical Relationships with the Heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543.
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting Human Coronary Inflammation by Imaging Perivascular Fat. Sci. Transl. Med. 2017, 9, eaal2658.
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466.
- Rabkin, S.W. Epicardial Fat: Properties, Function and Relationship to Obesity. Obes. Rev. 2007, 8, 253–261.
- Lin, A.; Dey, D.; Wong, D.T.L.; Nerlekar, N. Perivascular Adipose Tissue and Coronary Atherosclerosis: From Biology to Imaging Phenotyping. Curr. Atheroscler. Rep. 2019, 21, 47.
- Chechi, K.; Richard, D. Thermogenic Potential and Physiological Relevance of Human Epicardial Adipose Tissue. Int. J. Obes. Suppl. 2015, 5, S28–S34.
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255.
- Demir, B.; Demir, E.; Acıksarı, G.; Uygun, T.; Utku, I.K.; Gedikbasi, A.; Caglar, I.M.; Pirhan, O.; Tureli, H.O.; Oflar, E.; et al. The Association between the Epicardial Adipose Tissue Thickness and Oxidative Stress Parameters in Isolated Metabolic Syndrome Patients: A Multimarker Approach. Int. J. Endocrinol. 2014, 2014, 954045.
- Baker, A.R.; Harte, A.L.; Howell, N.; Pritlove, D.C.; Ranasinghe, A.M.; da Silva, N.F.; Youssef, E.M.; Khunti, K.; Davies, M.J.; Bonser, R.S.; et al. Epicardial Adipose Tissue as a Source of Nuclear Factor-ΚB and c-Jun N-Terminal Kinase Mediated Inflammation in Patients with Coronary Artery Disease. J. Clin. Endocrinol. Metab. 2009, 94, 261–267.
- Nusca, A.; Piccirillo, F.; Bernardini, F.; De Filippis, A.; Coletti, F.; Mangiacapra, F.; Ricottini, E.; Melfi, R.; Gallo, P.; Cammalleri, V.; et al. Glycaemic Control in Patients Undergoing Percutaneous Coronary Intervention: What Is the Role for the Novel Antidiabetic Agents? A Comprehensive Review of Basic Science and Clinical Data. Int. J. Mol. Sci. 2022, 23, 7261.
- Montazerifar, F.; Bolouri, A.; Paghalea, R.S.; Mahani, M.K.; Karajibani, M. Obesity, Serum Resistin and Leptin Levels Linked to Coronary Artery Disease. Arq. Bras. Cardiol. 2016, 107, 348–353.
- Yafei, S.; Elsewy, F.; Youssef, E.; Ayman, M.; Elshafei, M.; Abayazeed, R. Echocardiographic Association of Epicardial Fat with Carotid Intima–Media Thickness in Patients with Type 2 Diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 378–384.
- Militello, C.; Rundo, L.; Toia, P.; Conti, V.; Russo, G.; Filorizzo, C.; Maffei, E.; Cademartiri, F.; La Grutta, L.; Midiri, M.; et al. A Semi-Automatic Approach for Epicardial Adipose Tissue Segmentation and Quantification on Cardiac CT Scans. Comput. Biol. Med. 2019, 114, 103424.
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial Adipose Tissue Volume Assessed by Computed Tomography and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Eur. Heart J.—Cardiovasc. Imaging 2018, 19, 490–497.
- Wang, T.-D.; Lee, W.-J.; Shih, F.-Y.; Huang, C.-H.; Chang, Y.-C.; Chen, W.-J.; Lee, Y.-T.; Chen, M.-F. Relations of Epicardial Adipose Tissue Measured by Multidetector Computed Tomography to Components of the Metabolic Syndrome Are Region-Specific and Independent of Anthropometric Indexes and Intraabdominal Visceral Fat. J. Clin. Endocrinol. Metab. 2009, 94, 662–669.
- La Grutta, L.; Toia, P.; Farruggia, A.; Albano, D.; Grassedonio, E.; Palmeri, A.; Maffei, E.; Galia, M.; Vitabile, S.; Cademartiri, F.; et al. Quantification of Epicardial Adipose Tissue in Coronary Calcium Score and CT Coronary Angiography Image Data Sets: Comparison of Attenuation Values, Thickness and Volumes. Br. J. Radiol. 2016, 89, 20150773.
- Spearman, J.V.; Renker, M.; Schoepf, U.J.; Krazinski, A.W.; Herbert, T.L.; De Cecco, C.N.; Nietert, P.J.; Meinel, F.G. Prognostic Value of Epicardial Fat Volume Measurements by Computed Tomography: A Systematic Review of the Literature. Eur. Radiol. 2015, 25, 3372–3381.
- Bastarrika, G.; Broncano, J.; Schoepf, U.J.; Schwarz, F.; Lee, Y.S.; Abro, J.A.; Costello, P.; Zwerner, P.L. Relationship between Coronary Artery Disease and Epicardial Adipose Tissue Quantification at Cardiac CT. Acad. Radiol. 2010, 17, 727–734.
- Yu, W.; Liu, B.; Zhang, F.; Wang, J.; Shao, X.; Yang, X.; Shi, Y.; Wang, B.; Xu, Y.; Wang, Y. Association of Epicardial Fat Volume with Increased Risk of Obstructive Coronary Artery Disease in Chinese Patients with Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2021, 10, e018080.
- Gitsioudis, G.; Schmahl, C.; Missiou, A.; Voss, A.; Schüssler, A.; Abdel-Aty, H.; Buss, S.J.; Mueller, D.; Vembar, M.; Bryant, M.; et al. Epicardial Adipose Tissue Is Associated with Plaque Burden and Composition and Provides Incremental Value for the Prediction of Cardiac Outcome. A Clinical Cardiac Computed Tomography Angiography Study. PLoS ONE 2016, 11, e0155120.
- Alexopoulos, N.; McLean, D.S.; Janik, M.; Arepalli, C.D.; Stillman, A.E.; Raggi, P. Epicardial Adipose Tissue and Coronary Artery Plaque Characteristics. Atherosclerosis 2010, 210, 150–154.
- Ito, T.; Suzuki, Y.; Ehara, M.; Matsuo, H.; Teramoto, T.; Terashima, M.; Nasu, K.; Kinoshita, Y.; Tsuchikane, E.; Suzuki, T.; et al. Impact of Epicardial Fat Volume on Coronary Artery Disease in Symptomatic Patients with a Zero Calcium Score. Int. J. Cardiol. 2013, 167, 2852–2858.
- Nerlekar, N.; Brown, A.J.; Muthalaly, R.G.; Talman, A.; Hettige, T.; Cameron, J.D.; Wong, D.T.L. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006379.
- Otsuka, K.; Ishikawa, H.; Yamaura, H.; Shirasawa, K.; Kasayuki, N. Epicardial Adipose Tissue Volume Is Associated with Low-Attenuation Plaque Volume in Subjects with or without Increased Visceral Fat: A 3-Vessel Coronary Artery Analysis with CT Angiography. Eur. Heart J. 2021, 42.
- Yamashita, K.; Yamamoto, M.H.; Igawa, W.; Ono, M.; Kido, T.; Ebara, S.; Okabe, T.; Saito, S.; Amemiya, K.; Isomura, N.; et al. Association of Epicardial Adipose Tissue Volume and Total Coronary Plaque Burden in Patients with Coronary Artery Disease. Int. Heart J. 2018, 59, 1219–1226.
- Iwasaki, K.; Matsumoto, T.; Aono, H.; Furukawa, H.; Samukawa, M. Relationship between Epicardial Fat Measured by 64-Multidetector Computed Tomography and Coronary Artery Disease. Clin. Cardiol. 2011, 34, 166–171.
- Cosson, E.; Nguyen, M.T.; Rezgani, I.; Berkane, N.; Pinto, S.; Bihan, H.; Tatulashvili, S.; Taher, M.; Sal, M.; Soussan, M.; et al. Epicardial Adipose Tissue Volume and Myocardial Ischemia in Asymptomatic People Living with Diabetes: A Cross-Sectional Study. Cardiovasc. Diabetol. 2021, 20, 224.
- Franssens, B.T.; Nathoe, H.M.; Visseren, F.L.J.; van der Graaf, Y.; Leiner, T.; Algra, A.; van der Graaf, Y.; Grobbee, D.E.; Rutten, G.E.H.M.; Visseren, F.L.J.; et al. Relation of Epicardial Adipose Tissue Radiodensity to Coronary Artery Calcium on Cardiac Computed Tomography in Patients at High Risk for Cardiovascular Disease. Am. J. Cardiol. 2017, 119, 1359–1365.
- Goeller, M.; Achenbach, S.; Marwan, M.; Doris, M.K.; Cadet, S.; Commandeur, F.; Chen, X.; Slomka, P.J.; Gransar, H.; Cao, J.J.; et al. Epicardial Adipose Tissue Density and Volume Are Related to Subclinical Atherosclerosis, Inflammation and Major Adverse Cardiac Events in Asymptomatic Subjects. J. Cardiovasc. Comput. Tomogr. 2018, 12, 67–73.
- Eisenberg, E.; McElhinney, P.A.; Commandeur, F.; Chen, X.; Cadet, S.; Goeller, M.; Razipour, A.; Gransar, H.; Cantu, S.; Miller, R.J.H.; et al. Deep Learning–Based Quantification of Epicardial Adipose Tissue Volume and Attenuation Predicts Major Adverse Cardiovascular Events in Asymptomatic Subjects. Circ. Cardiovasc. Imaging 2020, 13, e009829.
- Fuller, B.; Garland, J.; Anne, S.; Beh, R.; McNevin, D.; Tse, R. Increased Epicardial Fat Thickness in Sudden Death From Stable Coronary Artery Atherosclerosis. Am. J. Forensic Med. Pathol. 2017, 38, 162–166.
- Mahabadi, A.A.; Berg, M.H.; Lehmann, N.; Kälsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jöckel, K.-H.; Erbel, R.; et al. Association of Epicardial Fat with Cardiovascular Risk Factors and Incident Myocardial Infarction in the General Population. J. Am. Coll. Cardiol. 2013, 61, 1388–1395.
- Gorter, P.M.; de Vos, A.M.; van der Graaf, Y.; Stella, P.R.; Doevendans, P.A.; Meijs, M.F.L.; Prokop, M.; Visseren, F.L.J. Relation of Epicardial and Pericoronary Fat to Coronary Atherosclerosis and Coronary Artery Calcium in Patients Undergoing Coronary Angiography. Am. J. Cardiol. 2008, 102, 380–385.
- Balcer, B.; Dykun, I.; Schlosser, T.; Forsting, M.; Rassaf, T.; Mahabadi, A.A. Pericoronary Fat Volume but Not Attenuation Differentiates Culprit Lesions in Patients with Myocardial Infarction. Atherosclerosis 2018, 276, 182–188.
- Ma, R.; van Assen, M.; Ties, D.; Pelgrim, G.J.; van Dijk, R.; Sidorenkov, G.; van Ooijen, P.M.A.; van der Harst, P.; Vliegenthart, R. Focal Pericoronary Adipose Tissue Attenuation Is Related to Plaque Presence, Plaque Type, and Stenosis Severity in Coronary CTA. Eur. Radiol. 2021, 31, 7251–7261.
- Nogic, J.; Kim, J.; Layland, J.; Chan, J.; Cheng, K.; Wong, D.; Brown, A. TCT-241 Pericoronary Adipose Tissue Is a Predictor of In-Stent Restenosis and Stent Failure in Patients Undergoing Coronary Artery Stent Insertion. J. Am. Coll. Cardiol. 2021, 78, B98.
- Kang, J.; Kim, Y.-C.; Park, J.J.; Kim, S.; Kang, S.-H.; Cho, Y.J.; Yoon, Y.E.; Oh, I.-Y.; Yoon, C.-H.; Suh, J.-W.; et al. Increased Epicardial Adipose Tissue Thickness Is a Predictor of New-Onset Diabetes Mellitus in Patients with Coronary Artery Disease Treated with High-Intensity Statins. Cardiovasc. Diabetol. 2018, 17, 10.
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104.
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin Monotherapy Significantly Decreases Epicardial Adipose Tissue Thickness in Newly Diagnosed Type 2 Diabetes Patients. Rev. Port. Cardiol. 2019, 38, 419–423.
- Iacobellis, G.; Villasante Fricke, A.C. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J. Endocr. Soc. 2020, 4, bvz042.
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide Causes Large and Rapid Epicardial Fat Reduction. Obesity 2017, 25, 311–316.
- Sato, T.; Aizawa, Y.; Yuasa, S.; Kishi, S.; Fuse, K.; Fujita, S.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Sato, M.; et al. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume. Cardiovasc. Diabetol. 2018, 17, 6.
More
