Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by José S. Câmara.
In the upcoming years, the world will face societal challenges arising, in particular, from the impact of climate change and the inefficient use of natural resources, in addition to an exponential growth of the world population, which according to the United Nations (UN) estimations will be 9.8 billion in 2050. This increasing trend requires optimized management of natural resources with the use of value-added waste and a significant reduction in food loss and food waste. Moreover, the recent pandemic situation, COVID-19, has contributed indisputably. Along with the agri-food supply chain, several amounts of waste or by-products are generated.
- agri-food waste
- valorisation
- food loss
- food waste
1. Introduction
In recent years, the valorisation of agri-food wastes migrated from a trending ecological movement to an urgent need. The destructive effects of unstable and extreme climate variations on agriculture, soil exhaustion, and water scarcity, among other concerns, lead to a decrease in agri-food production. In contrast, the continuous exponential growth of the human population requires more food to feed everyone, and currently, around 700 million people are estimated to be suffering from hunger [1]. Paradoxically, nutrient loss due to agri-food waste is estimated to provide a diet for 2000 million people. On top of this, agri-food waste disposal in landfills is responsible for greenhouse gas (GHG) emissions and air pollution (e.g., dioxins, ash), as well as groundwater contamination. Overall, the impact on the world economy is very high, affecting different features, such as water and land management, energy production, transport, or storage [2] (Figure 1). These enormous societal challenges have been already addressed by the European Commission which included the mitigation of food waste as a priority area of the Action Plan for the European Circular Economy Strategy [3]. To make such a strategy economically viable, the valorisation of agri-food wastes can be achieved by the extraction of valuable compounds for different industrial sectors, like the nutraceutical, cosmetic and pharmaceutical industries [4,5][4][5]. A myriad of phytochemicals is available in diverse agri-food wastes which are mostly from plant origin and less animal-based (Figure 1), such as peels, leaves, seeds, pomace, meat derivatives, egg products, and food industry rejects, constituting promising raw materials for other industries. However, there are other challenges and obstacles to overcome. Overall, most food waste is generated in five different stages in the food value chain (Figure 1). During production, losses of fruits, vegetables and cereals occur mostly during harvesting on the farm. Edible crops, for instance, are rejected due to their non-standardized measures or defects, inadequate harvesting time, or even due to mechanical damage. Another considerable fraction of food loss happens during the transportation, handling, and storage of the products. These losses are often due to the degradation of edible products by fungi, diseases, handling, or even by poor transportation infrastructure. Processing and packing make the lowest contribution to food loss, which can occur through inappropriate packaging or contaminations. During the distribution and market, some products might be lost due to spoilage during transportation or lack of cooling storage, which is a common situation in the distribution of fruits and vegetables. Human consumption is responsible for the highest amount of waste in the food chain, often due to excessive buying, exceeding use dates, and wrong storage [4,5,6][4][5][6].
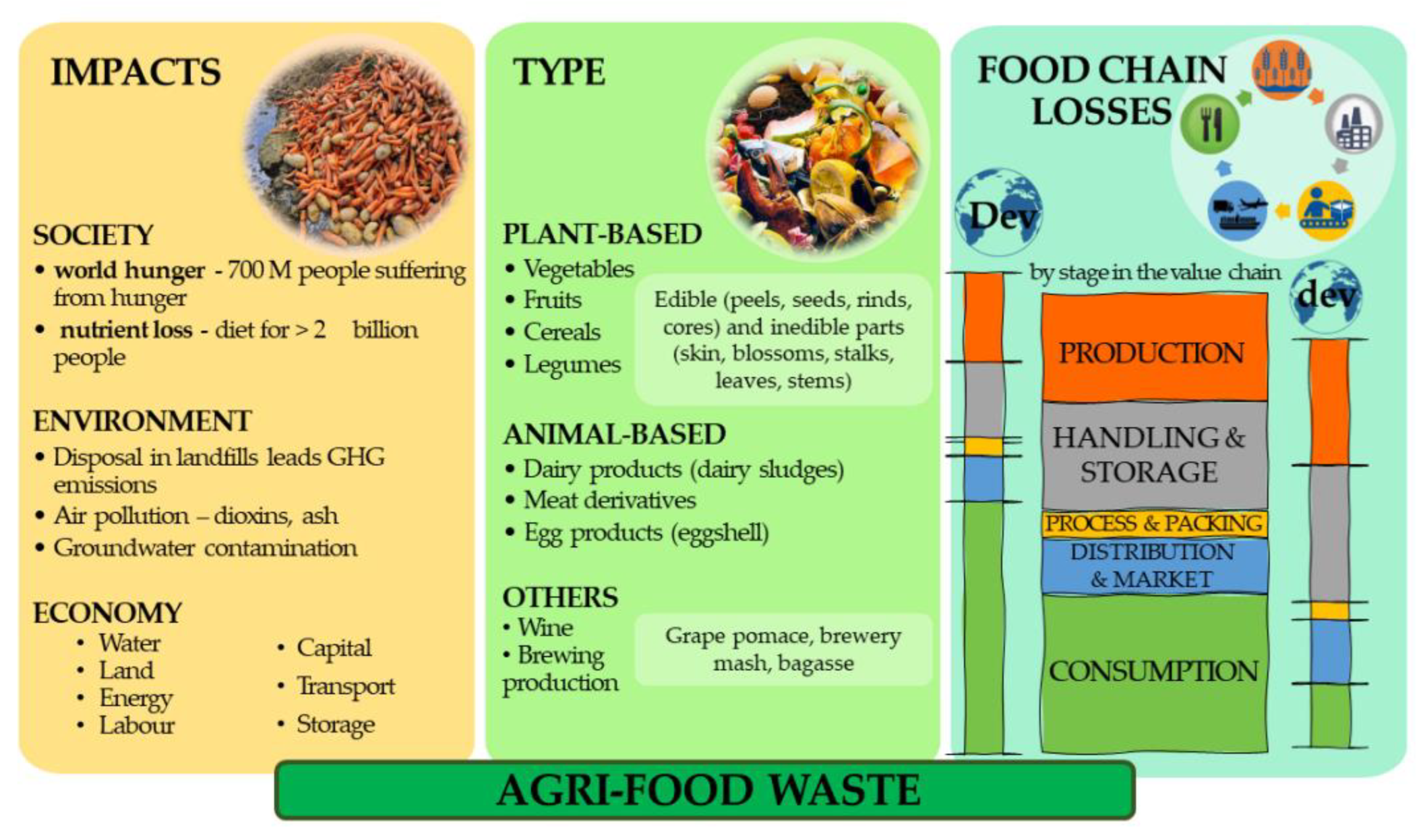
Figure 1. Overview of food waste impact, type, and food chain losses by stage in the value chain in developed (Dev) and developing (dev) countries [2].
In this respect, however, there are significant differences between the performance of developed and developing countries. By far, most of the food waste generated in developed countries occurs during the consumption step. One of the reasons for this paradox is correlated with the fact that proper separation and management of agri-food wastes is still very incipient in many fields, making their valorisation expensive and technologically very demanding for smaller industries [2]. Consequently, it is cheaper to pay to deposit agri-foods in landfills than develop a zero-residue strategy for the value chain of specific food products [2]. In turn, food wastes produced in developing countries are mainly associated with the production, handling and storage stages. This fact is certainly explained by the poor agri-food systems devoted to these stages in developing countries [2]. Irrespective of the stage where agri-food wastes are generated and their respective causes, there is great potential in the extraction of phytochemicals from agri-food wastes, particularly those obtained from plants, such as fruits and vegetables. These agri-food wastes include edible (peels, seeds, rinds, and cores) and inedible parts (skin, blossoms, stalks, leaves, and stems) which are rich in many bioactive compounds, such as probiotics, dietary fibres, fat-soluble vitamins, essential omega-3 fatty acids, phytoestrogens, and several phytochemicals, namely carotenoids, flavonoids, and phenolic acids, known to exhibit antioxidant, anti-microbial or anti-inflammatory activities [6]. Hence, these compounds can provide the most diverse applications in food, health, pharmaceutical, cosmetic, and environmental fields, as substitutes for synthetic preservatives, pigments, fragrances, and antioxidants in both food and cosmetical products or the addition of health protective effects to ourthe diet [4,5,7,8][4][5][7][8]. This strategy would allow to obtain better food with less waste, and consequently a better environmental footprint.
2. Extraction Techniques for Bioactive Recovery from Agri-Food Wastes
The extraction of bioactive compounds from agri-food wastes using green extraction procedures (e.g., supercritical fluid extraction, pulsed electric fields, ultrasound-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, pressurized liquid extraction) (Figure 2) has gained special attention due to their exceptional practices focused on economic, environmental, and safety concerns [9]. Moreover, green extraction procedures comprise six principles of green chemistry, namely: (i) the use of renewable and sustainable bio-resources, (ii) use of water or green solvents, (iii) lower energy input, (iv) co-products production from waste, (v) a minimal number of unit operations, and (iv) resulting non-denatured and biodegradable extract [9]. The following sub-sections present the most common green extraction procedures used for the recovery of bioactive compounds from agri-food wastes.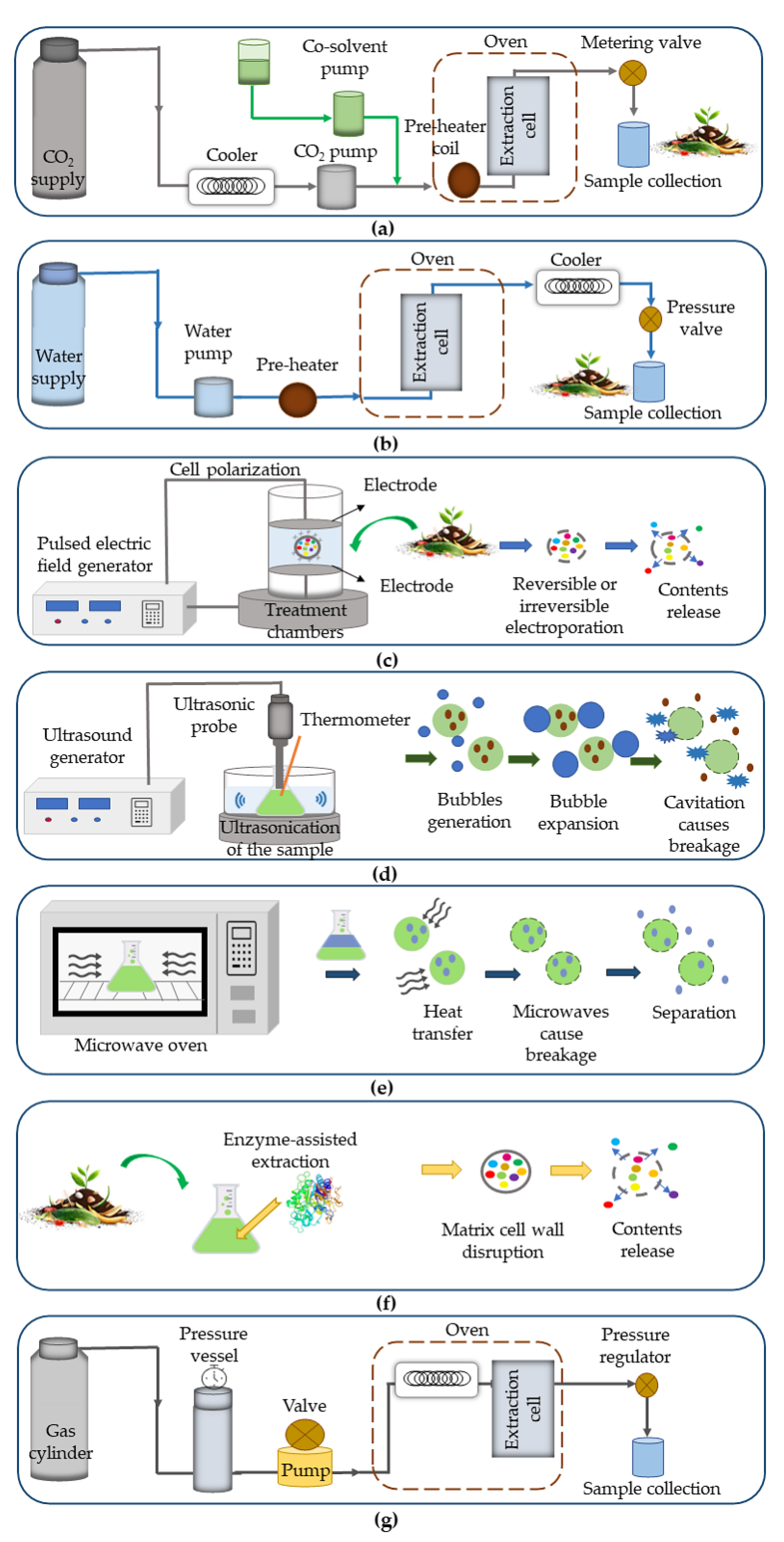
Figure 2. Simplified representation of the most used green extraction procedures, (a) supercritical fluid extraction, (b) subcritical water extraction, (c) pulsed electric fields, (d) ultrasound-assisted extraction, (e) microwave-assisted extraction, (f) enzyme-assisted extraction, and (g) pressurized liquid extraction.
2.1. Supercritical Fluid Extraction
Supercritical fluid extraction (SFE) using carbon dioxide (CO2) has been proposed as a green extraction procedure, since it requires low volumes of organic solvent to recover the value-added bioactive compounds (e.g., carotenoids, phenolic compounds) from agri-food wastes [10,11,12,13][10][11][12][13]. CO2 is the most used supercritical fluid due to its mild critical temperature (31.2 °C) and pressure (73.8 bar), which allows for operation at moderate conditions, generally ranging from 40 to 60 °C and 200–400 bar pressure [9]. Additionally, CO2 is non-carcinogenic, non-toxic, non-mutagenic, non-flammable, thermodynamically stable, and generally identified as safe [14]. The main benefit of this green extraction technique is that the solvent physicochemical properties can be changed by adjusting the pressure and temperature conditions within the system, consequently improving the extraction selectivity and extraction yields due to the fast diffusion of fluid through the solids [12,14][12][14]. However, the low polarity of supercritical CO2 represents the major drawback of this procedure. This problem can be minimized by adding small percentages of co-solvents (e.g., ethanol, methanol, water) or modifiers that change the polarity of the solvent. Consequently, this results in an enhancement of the extraction yield by improving the solubility of the solute or the swelling of the solid matrix that facilitates the solute–solvent contact [15]. This versatility makes SFEs very appealing for several applications in different fields (e.g., industry, pharmaceutical). Table 1 shows the potential of SFE in the extraction of important value-added compounds from agri-food wastes [10,11,12,13][10][11][12][13]. The bioactive compounds extracted by SFE include a wide diversity, namely, phenolic compounds from onion peels [16], antioxidants and saponins from Agave salmiana bagasse [12], and carotenoids from carrot peels [10], among others. The effect of pressure, temperature, and the addition of a co-solvent in the extraction of bioactive compounds by SFE are evaluated in some studies in Table 1. Generally, the extraction was performed by applying pressures, temperatures and co-solvents ranging from 30–400 bar, 33–230 °C and 5–15 % v/v, respectively, while the extraction time and flux ranged from 30–180 min and 1.7–133 g/min, respectively. Some studies compared the extraction efficiency of SFE with other conventional extraction procedures (e.g., Soxhlet extraction). Soldan et al. [13] compared the extraction efficiency of SFE and Soxhlet on the recovery of the bioactive compounds phenolics, flavonoids, fatty acids, and carotenoids, from Capsicum annuum waste. The results showed that the total mass yields obtained by SFE ranged from 9.38 to 10.08%, while for Soxhlet the yields ranged from 8.45 to 15.5% (w/w). Despite revealing bioactive compounds, the extracts did not show significant antioxidant activity. Natolino and collaborators [17] also performed a comparison between SFE and Soxhlet on the recovery efficiency of seed oils from pomegranate, which showed no significant difference between these two extraction procedures, as the extraction yield from SFE (0.18 ± 0.01 g/g) was similar to Soxhlet (0.19 ± 0.01 g/g). Nevertheless, SFE was faster than Soxhlet (8 h vs. 2 h of SFE) to achieve the asymptotic extraction yield and presented more oxidation stability than Soxhlet. Santos–Zea et al. [12] evaluated the effect of ultrasound on SFE for the recovery of antioxidants and saponins from agave bagasse. The data obtained showed that the use of ultrasound-assisted SFE improved the extraction yield of antioxidants and saponins from agave bagasse when a low mass load (0.043 g/cm3) was applied. Since the CO2-SFE demonstrated low extraction efficiency of more polar compounds in some studies (e.g., phenolic compounds), a few reseauthorchers proposed the use of co-solvents to enhance the extraction yields of polar and medium polar bioactive compounds [13]. Soldan et al. [13] verified that the temperature variation and the addition of a co-solvent (ethanol) were significant in increasing the total extracted mass of oleoresin, although the pressure did not have a significant effect. In sum, this green extraction technique can be easily transferred to an industrial scale to extract large quantities of matrix and obtain a great amount of extract in a single step. However, despite the exceptional extraction properties and outstanding versatility, the high processing costs and the complex industrial equipment are restricting factors [14].Table 1. Extraction techniques for bioactive recovery from agri-food waste.
| Agri-Food Waste (Amount) | Targets | Extraction Conditions | Extraction Efficiency | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Supercritical fluid extraction | |||||||||||
| Agave salmiana | bagasse (10 g) |
Antioxidants and saponins | CO | 2 | , 60 °C, 300 bar, 10 % | v | / | v | ethanol, 1.7 g/min, 60 min | Increase in the antioxidant activity in the US-assisted extraction from 11.54 ± 0.06 to 17.61 ± 0.75 μmol of Trolox equivalents/g | [12] |
| Avocado peel and seeds (-) |
Catechin, quercetin | CO | 2 | , 80 °C, 250 bar, ethanol ratio of 1:1.5 S:L, 30 min | Integral biorefineries of avocado seed and peel allow profit margins of 47% and 43%, respectively | [18] | |||||
| Capsicum annuum | waste (20 g) | Phenolics, flavonoids, fatty acids, and carotenoids | CO | 2 | , 40 and 60 °C, 200 and 250 bar, with and without ethanol, 3 g/min, 30 min | Yield 9.38–10.08%, phenols (12.30–23.94 mg/g), flavonoids (0.6–1.52 mg/g), and carotenoids (0.27–2.01 mg/g) | [13] | ||||
| Carrot peels (50 g) |
Carotenoid | CO | 2 | , 59 °C, 349 bar, 15% | v | / | v | ethanol, 15 g/min, 80 min | Carotenoid recovery was (86.1%) with 97 % purity | [10] | |
| Grape seeds (17 g) |
Triacylglycerols | CO | 2 | , 40–60 °C, up 400 bar, 1.8–2.8 g/min | Oil yield in the range of 12.0–12.7%, as compared to 12.3% obtained by a conventional | n | -hexane extraction | [19] | |||
| Mandarin peel (100 g) |
Limonene, hesperidin | CO | 2 | , 130–220 °C, 100–300 bar, 33 g/min, 90 min | Limonene (13.16 and 30.65% at 100 and 300 bar), hesperidin (0.16–15.07 mg/g) | [20] | |||||
| Mango peel (5 g) |
Carotenoids | CO | 2 | , 60 °C, 250 bar, 15% | w | / | w | ethanol, 6.7 g/min, 180 min | Carotenoids (1.9 mg all-trans-β-carotene equivalent/g dried mango peel) | [11] | |
| Melon seeds (5 g) |
Phytosterols | CO | 2 | , 33 °C, 200 bar, 11 g/min, 3 h | β-sitosterol (304 mg/kg) and stigmasterol (121 mg/kg) | [21] | |||||
| Pomegranate seed (100 g) |
Seed oil | CO | 2 | , 60 °C, 320 bar 133 g/min, 180 min | Oil (85.4% of punicic acid) | [17] | |||||
| Subcritical water extraction | |||||||||||
| Citrus peel (3 g) |
Hesperidin, narirutin | Water, 110–190 °C, 10 MPa, 3–15 min | Hesperidin (6.96 mg/g peel dw), narirutin (8.76 mg/g peel dw) | [22] | |||||||
| Grape pomace (50–60 g) | Phenolic compounds | Water, 130–190 °C, 100 bar, 10 mL/min | 29 g of phenolic compounds ( | p | -hydroxyphenyl, guaiacyl, and syringyl)/100 g extract | [23] | |||||
| Kiwifruit peel (2% S:S ratio) | Phenolic compounds | Water, 160 °C, S:S ratio (2%), pH 2, 20 min | TPC (51.2 mg GAE/g dw), TFC (22.5 mg QE/g dw) | [24] | |||||||
| Onion peel (2 wt % onion skins into 600 mL of H | 2 | O) | Phenolic compounds | Water, 170–230 °C, 30 bar, 400 rpm/30 min | 63–75 mg GAE/g, 23–26 QE/g | [16] | |||||
| Onion skin (4 g) |
Phenolic compounds | Water, 105–180 °C, 5 MPa, 2.5 mL/min | Quercetin (15 mg/g onion skin) and quercetin-4-glucoside (8 mg/g onion skin) | [25] | |||||||
| Peach palm (4 g) |
Phenolic compounds, sugars | Water, 130 °C, 100 bar, 1 mL/min, 90 min | Soluble sugar (15 g/100 g), TPC (921 mg/100 g) | [26] | |||||||
| Shellfish waste (1 g) | Protein hydrolysates | Water, 200 °C, heating rate of 6 °C/min | 8.5 g protein/100 g dw (improved extraction yield of up to 65%) | [27] | |||||||
| Vine-canes (40 g) |
Phenolic compounds | Water, 250 °C, 50 min | 181 mg GAE/g dw, 203 mg TE/g dw | [28] | |||||||
| Vine co-products: cane, wood, and root (5 g) | Stilbenes | Water, 160 °C, 100 bar, 5 min | Cane (3.62 g/kg dw), wood (9.32 g/kg dw), and root (12.1 g/kg dw) | [29] | |||||||
| Pulsed electric fields | |||||||||||
| Apple pomace (28.7 g) | Phenolic compounds | E = 2, 3 kV/cm, U = 17, 100 kJ/kg, 40 °C |
PEF performed with EtOH:H | 2 | O (70:30, | v | / | v | ) showed the highest content of phlorizin (753.84 ± 26.38 µg/g fresh apple pomace) | [30] | |
| Banana peels (-) |
Phenolic compounds | E = 1.3–6.45 kV/cm | Increase the TPC and antioxidant activity | [31] | |||||||
| Jackfruit waste (1:20 w/v solid-to-solvent ratio) | Pectin polysaccharide | E = 5–15 kV/cm | No significant effect on pectin yield | [32] | |||||||
| Lemon peels (30 g) | Phenolic compounds | E = 7 kV/cm, U = 7.6 kJ/kg | Increase the efficiency of phenolic compounds (hesperidin and eriocitrin) extraction by 300% | [33] | |||||||
| Potato peels (5 g) |
Phenolic compounds | E = 0.25–3 kV/ cm, U = 1–20 kJ/kg | PEF showed higher TPC yield (10%) and antioxidant activity (9%) compared to conventional solid–liquid extraction with same extraction protocol but without the application of the PEF pre-treatment) | [34] | |||||||
| Pomegranate peels (30 g) | Ellagic acid | E = 10 kV/cm | PEF selectively extracted and enhanced the recovery of ellagic acid (≈740 μg/g dm) | [35] | |||||||
| Pomelo peels (1 g) | Naringenin | E = 2–10 kV/cm | Increase the extraction yield of naringenin | [36] | |||||||
| Olive pomace (850 g) | Phenolic compounds | E = 1–6.5 kV/cm, U = 0.9–51.1 kJ/kg, 50 pulses spaced at 3 s, 20–27.5 °C | PEF allowed a 28.8% increased recovery yield of polyphenols (~3 mg GAE/L) compared to untreated | [37] | |||||||
| Tomato peels (10 g) | Lycopene | E = 5 kV/cm, U = 5 kJ/kg, 20 ± 2 °C | Enhance the extraction rate (27–37%), the lycopene yields (12–18%) and the antioxidant power (18–18.2%) | [38] | |||||||
| Ultrasound-assisted extraction | |||||||||||
| Apple leaves (10 g) | Phloretin | 400 W, 20 kHz, 14.4 min, <25 °C | The phloretin concentration ranged from 292 to 726 µg/g | [39] | |||||||
| Apple pomace (1:10 ( | w | / | v | ) S:L ratio) | Phenolic compounds | 45 min, 45 °C | Increase the TPC, antioxidant activity, and recovery of interesting antioxidant compounds (quercetin derivatives, chlorogenic acid, phloridzin) | [40] | |||
| Beet leaves (1:20 ( | w | / | v | ) S:L ratio) | Bioactive compounds | 90 W, 16 min | Yields were 14.9 mg/g polyphenols, 949.1 µg/g betaxanthins, and 562.2 µg/g beta-cyanins | [41] | |||
| Brewers’ spent grains (1:30 ( | w | / | v | ) S:L ratio) | Proanthocyanidins | 400 w, 75 % acetone, 55 min, 25 °C | High recovery of proanthocyanidins (1023 µg/g dw) | [42] | |||
| Citrus peel (1 g) |
Citric acid | 119–141 W, 5.8–35.5 min, 0–7 % ( | v | / | v | ) ethanol | Recovery of 6.4 g and 3.4 g of citric acid per 100 g of dry orange and lime peels, respectively | [43] | |||
| Grape pomace (280 g) | Phenolic compounds | 450 W, 15 min, 20 °C | Increased the TPC (6.68 ± 0.05 mg of gallic acid) and antioxidant activity (ABTS: 23.84 ± 0.57 μmol of Trolox equivalents/g and DPPH: 33.27 ± 2.00 μmol of Trolox equivalents/g) | [44] | |||||||
| Kiwi peel (1.5 g) |
Flavonoids | 5–500 W, 20 kHz, 1–45 min, 25 °C | 46% extract weight and 1.51 mg/g dw of flavonoids | [45] | |||||||
| Orange peels (10 g) | Bioactive compounds | 40 kHz,85 min, 55 °C, 61% methanol | The spectra of extracts showed a similar fingerprint of hesperidin | [46] | |||||||
| Tomato peels (72 mL/g, L:S ratio) | Lycopene | 20 kHz, 20 min, 65 °C, | Lycopene recovery of 1536 µg/g | [47] | |||||||
| Microwave-assisted extraction | |||||||||||
| Carrot juice waste (flaxseed oil + waste ~ 20 g) | Carotenoids | 170 W, 9.46 min, 8:1 g/g oil-to-waste ratio | Carotenoid recovery of 77.48%. The enriched flaxseed oil showed high phenolic content (214.05 ± 1.61 μg GAE/g oil) and antioxidant activity (inhibition % of DPPH = 70.67 ± 0.85) | [48] | |||||||
| Coffee pulp (-) |
Phenolic compounds, flavonoids, chlorogenic acid, and caffeine | 1000 W, 85 min, 1:100 g/100 mL sample-to-solvent ratio, 42.5 % ( | v | / | v | ) aqueous ethanol solution | Extraction yields of TPC, flavonoids, chlorogenic acid, and caffeine were 38.68, 27.00, 6.95, and 5.47 (mg/g dw), respectively. The extract showed high antioxidant capacities (ABTS, DPPH, and FRAP assays as 87.95, 9.3, 65.31 (mg TE/g DW), respectively) | [49] | |||
| Peach waste (1000 mg) |
Phenolic compounds and anthocyanins | 500 W, 90 s, 80 % ethanol ( | v | / | v | ) | TPC of 19.35 mg GAE/g fresh plant matter and total anthocyanin 1.12 mg cyn-3-glu/g fresh plant matter) yields | [50] | |||
| Cocoa shell waste (100 g) | β-Sitosterol | 500 W, 10 min, 70 °C | The maximum yield obtained was 13% higher than the yield of conventional maceration (3546.1 mg/ 100 g) | [51] | |||||||
| Eggplant peel (-) |
Phenolic compounds, flavonoids, anthocyanins | 269.82 W, 7.98 min, 5.01 mL/g L:S ratio | The maximum extraction yield (3.27%), TPC (1,049.84 µg GAE/mL), TFC (130.40 µg QE/mL), and total anthocyanin content (6.99 mg/L) | [52] | |||||||
| Lemon peel waste (-) |
Essential oil (limonene, β-pinene, and γ-terpinene) and pigment | 500 W, 50 min, 80 °C, 80% ( | v | / | v | ) ethanol, 1:10 L:S ratio | The extraction yields of lemon essential oil and pigment were around 2 wt.% and 6 wt.%, respectively | [53] | |||
| Spent sweet potato leaves (0.1 g) | Flavonoids | 470 W, 21 min, 54 °C, 70 mg/mL S:L ratio | The yield of TFC was 40.21 ± 0.23 mg rutin equivalents/g | [54] | |||||||
| Broccoli stems, leaves and florets (2.5 g) | Phenolic compounds (vanillic, sinapic, caffeic, chlorogenic, ferulic, gallic, neochlorogenic, and | p | -coumaric acids) | Stems: 2.45 GHz, 74.54% methanol, 15.9 min, 74.45 °C Leaves: 2.45 GHz, 80% methanol, 10 min, 73.27 °C Florets: 2.45 GHz, 80% methanol, 18.9 min, 75 °C |
MAE increased the phenolic yield up to 45.70% (1940.35 ± 0.794 µg GAE/g dw), for broccoli leaves, 133.57% (657.062 ± 0.771 µg GAE/g dw) for broccoli florets, and 65.30% for broccoli stems (225.273 ± 0.897 µg GAE/g dw), in less time compared with maceration extraction | [55] | |||||
| Spent onion skins (-) | Flavonoids (quercetin, kaempferol, luteolin, and quercetin-3-O-β-D-glucoside) | 554 W, 16 min, 76 °C, 14 mg/mL S:L ratio | TFC extraction yields of 47.83 ± 0.21 mg/g | [56] | |||||||
| Enzyme-assisted extraction | |||||||||||
| Unsold tomato (-) | Carotenoids and carotenoid-containing chromoplasts | Enzymatic mix: polygalacturonase, pectin lyase, cellulose, xylanase, 25 U/g for 180 min, 45−55 °C at pH 5–5.5 | Recovery yield of 4.30 ± 0.08 mg lycopene/ kg tomato)/U as carotenoid-containing chromoplasts and 5.43 ± 0.04 mg lycopene/ kg tomato)/U as total carotenoids | [57] | |||||||
| Apricot pulp (-) | Polysaccharides (sodium glycocholate and sodium taurocholate) | 5 mL/mg liquid-material ratio, 3% enzyme dosage and incubation time 1.5 h, pH 4.5 | The yield, sodium glycocholate and sodium taurocholate binding rates were 21.90%, 39.08% and 43.80%, respectively | [58] | |||||||
| Tomato peel and seed (4 g) | Lycopene-rich oleoresins | Enzymatic reaction: 40 °C, 5 h, 0.2 mL/g enzyme:substrate ratio, 5 mL/g solvent:substrate ratio, extraction time 1 h, 1 enzyme:enzyme ratio | Celluclast:Pectinex-ethyl acetate combination yielded the highest content of phenolic compounds (oleoresin with a concentration of 11.5 mg) | [59] | |||||||
| Beetroot cell wall (200 g) | Betalains | Enzymatic mix (cellulase 37%, xylanase 35%, pectinase 28%), 25 U/g total dose of enzymatic mix, 25 °C, 240 min, pH 5.5 | Betaxanthins and betacyanins yield 10 and 15 mg/mL U, respectively | [60] | |||||||
| Sweet cherry pomace (15 g) | Non-extractable polyphenols | 0.38 g/mL S:L ratio, 70 °C, pH 10, 40 min for Depol (90 µL/g of sample) and Promod (140 µL/g of sample) enzymes and 18.4 min for Pectinase enzyme (2 µL/g of sample) | The extracts obtained by acid hydrolysis (1.87 ± 0.05 mg GAE/g of extraction residue) and Promod enzyme (1.75 ± 0.20 mg GAE/g of extraction residue) followed by alkaline hydrolysis (1.46 ± 0.20 mg GAE/g of extraction residue) and enzymatic hydrolysis with Depol enzyme (1.33 ± 0.13 mg GAE/g of extraction residue) were the richest in terms of phenolic content | [61] | |||||||
| Sugar beet leaves | Protein | 54.25 °C, 81.35 min, 27.65 mL/g solvent/solid ratio | EAE increased the protein yield by 43.27% and reached a 79.01% yield | [62] | |||||||
| Raspberry pomace (9 g) | Lipophilic compounds (phytosterols) and polyphenols | 1.2 units of thermostable alkaline protease/100 g pomace press-cake, 60 °C, 2 h hydrolysis, pH 9 | The recovery of polyphenols and antioxidant activity was, respectively, 48% and 25% higher than the obtained by extraction with methanol/acetone/water mixture | [63] | |||||||
| Pressurized liquid extraction | |||||||||||
| Pomegranate peel and carpelar (6 g) | Phenolic compounds (α, β punicalagin, and ellagic acid) | 60 °C, 80 bar, flow rate of 1 mL/min, 76 min, 10 solvent-to-feed ratio | The highest content of α, β-punicalagins, and ellagic acid obtained was 194.96 mg/100 g and 24.91 mg/100 g, respectively, representing 45% of TPC | [64] | |||||||
| Beetroot leaves and stems (5 g) | Phenolic compounds (ferulic acid, vitexin and sinapaldehyde) | 40 °C, 7.5, 10 and 12.5 MPa, flow rate of 3 mL/min | The highest TPC was obtained for beetroot leaves and varied from 7 ± 1 to 252 ± 2 mg GAE/g extract | [65] | |||||||
| Vitis vinifera | L. cv. negra criolla pomace (5 g) | Phenolic compounds (flavanols and phenolic acids) | 10 atm, 5 min with 250 s of nitrogen purge Flavanols: 20% ethanol, 160 °C Phenolic acids: 60% ethanol, 160 °C |
PLE recovered ~2.5 and ~1.5 more polyphenols from skins (6.93 µg/g dw) and seeds (45.34 µg/g dw), respectively, compared to conventional extraction | [66] | ||||||
| Olive pomace (5 g) | Phenolic compounds (phenolic alcohols, secoiridoids, flavonoids, and lignans) | Clean-step with n-hexane as the solvent and 1500 psi at room temperature to remove the lipophilic fraction from the olive pomace. Ethanol (0 to 100%), 40 to 176 °C, 1500 psi, 20 min | PLE showed higher TPC than conventional extraction (1659 mg/kg dw and 281.7 mg/kg dw, respectively) | [67] | |||||||
| Pomegranate peel (3.75 g) | Phenolic compounds (phenolic acids, flavonoids, and hydrolysable tannins) | 200 °C, ethanol 77%, 1500 psi, 20 min | TPC of 164.3 ± 10.7 mg GAE/g dw | [68] | |||||||
| Pomegranate seed (1.75 g of waste and 7 g of sand, 1:4 ratio) | Protein and phenolic compounds | Ethanol (0 to 100 %), 28 to 170 °C, 1 to 5 cycles, 3 to 12 min, pH 6.5 to 11, 103 bar | Higher extraction yield by PLE (15.3 ± 0.9 g proteins/100 g pomegranate seed waste) at a cost of a longer extraction time and the co-extraction of phenolic compounds | [69] | |||||||
2.2. Subcritical Water Extraction
Subcritical water extraction (SCWE) is an environmentally friendly and techno-economically feasible alternative to conventional extraction procedures, such as solvent extraction. This green extraction technique uses water as an extractant, which is economic, non-flammable, and renewable. Compared to conventional extraction procedures, such as solid–liquid extraction (Soxhlet) using organic solvents, maceration, and hydrodistillation, SCWE shows higher yield and purity while applying lower extraction time [16,24][16][24]. This green extraction procedure is also distinguished by the demand for a downstream solid–liquid separation step which increases the energetic requirement of the process [70]. However, and despite large research efforts, corrosion problems have not been completely solved for the application of SCWE at an industrial scale [14]. During SCWE, the feedstock is heated in the aqueous phase at a sub-critical temperature (∼150–320 °C) and pressure (∼20–150 bar). Under these conditions, the dielectric constant (ε), surface tension, and viscosity, among other properties of the water change, enhance mass transfer and the extractability of barely water-soluble bioactive compounds, since subcritical water promotes the hydrolysis of the bonds between phenolic compounds and agri-food waste matrix [25]. Moreover, the mass transfer ratio also rises due to the reduced viscosity and surface tension of the water as well as its diffusivity [24]. A diversity of SCWE applications on the extraction of bioactive compounds from agri-food wastes has been performed, namely, stilbenes from vine co-products (e.g., cane, wood, and root) [29], phenolic compounds from onion peel [16], and protein hydrolysates from shellfish waste [27], among others. Nevertheless, the main drawback of SCWE is the risk of hydrolysis and other degradation reactions during extraction [14]. In this sense, Rodrigues et al. [27] used SCWE to recover antioxidant protein hydrolysates from shellfish waste streams. In tThis study, the authoe researchers assessed the impact of operating temperature (150, 200, and 250 °C), solid/liquid ratio (1:5, 1:10, and 1:15 g/mL), and heating rate (3 and 6 °C/min) on SCWE performance. It was verified that higher temperatures enabled the production of extracts with a higher antioxidant potential, possibly due to an increase of smaller peptides/free amino acids and Maillard reaction products. On the other hand, Hwang and collaborators [22] recovered hesperidin and narirutin from Citrus unshiu peel waste using SCWE combined with pulsed electric field (PEF) treatment. The data obtained demonstrated that the concentrations of hesperidin and narirutin increased with PEF treatment time, with increased yields of hesperidin and narirutin by 22.1% and 33.6%, respectively, in PEF pretreatment combined with SCWE.2.3. Pulsed Electric Fields
Pulsed electric field (PEF) is a nonthermal agri-food processing method that applies high-intensity electric field pulses to agri-food passing through electrodes. This extraction process causes the electroporation of membranes (permeabilization) that enables the release of intracellular bioactive compounds from the matrix investigated [22,71][22][71]. The extraction efficiency of PEF treatment depends on numerous factors, involving electric field strength, total specific energy input, treatment time, and temperature. Previous studies have demonstrated that the PEF pre-treatment of moderate electric field intensity (0.5–10 kV/cm) and relatively low energy input (1–10 kJ/kg) has advantageous effects on the permeabilization of membranes of plant cells, enabling high recovery yields of intracellular compounds of interest from a wide range of food processing wastes and by-products [38]. Furthermore, PFE treatments have shown several advantages, including low solvent consumption, shorter treatment time, energy efficiency, continuous operability, ease of scale-up, non-destructive nature, and high selectivity. However, its dependence on medium composition (conductivity) and the high cost of the equipment represents the main disadvantages of PFE treatments [35,72][35][72]. Table 1 presents a diversity of PFE applications for the extraction of bioactive compounds from agri-food wastes, such as lycopene from tomato peels [38], ellagic acid from pomegranate peels [35], and phenolic compounds from lemon peels [33], among others. Pollini et al. [30] compared different extraction techniques, such as ultrasound-assisted extraction (UAE), ultraturrax extraction (UTE), accelerated solvent extraction (ASE), and PEF extraction pre-treatment to identify the most efficient method to recover phenolic compounds from apple pomace. The extraction efficiency of phloridzin, the main phenolic compound in apples, increased by applying PFE at a low intensity and for a long duration (2 kV/cm and 100 kJ/kg), using EtOH:H2O (70:30, v/v). In another study, Lal et al. [32] combined PFE with microwave-assisted extraction to recover pectin polysaccharide from jackfruit waste, but the pectin yield obtained was not significant when compared to conventional processes. Radjha et al. [35] compared the aqueous extraction efficiency and biological activities of phenolic compounds from pomegranate peels assisted by infrared (IR), ultrasound (US), PFE, and high-voltage electrical discharges (HVED). The data showed that the PFE selectively extracted and enhanced the recovery of ellagic acid (≈740 μg/g dm), whereas HVED (≈345 μg/g dm) intensified the gallic acid extraction compared to US, IR, PFE and WB. Peiró and collaborators [33] evaluated the influence of PEF of different intensities (3–9 kV/cm and 0–300 μs) on the extraction of phenolic compounds from lemon peel residues, which increased by around 300%, giving maximum values of 84 mg of hesperidin in 100 g FW and 176 mg of eriocitrin in 100 g FW.2.4. Ultrasound-Assisted Extraction
Ultrasound-assisted extraction (UAE) is a green extraction procedure and a techno-economically feasible alternative to conventional extraction procedures. This technique has gained attention in recent years, due to its excellent advantages compared to traditional extraction procedures, such as reduced solvent volumes, shorter extraction time, and use of common laboratory equipment (e.g., ultrasonic bath), making it an environmentally sustainable and economical extraction procedure [73,74][73][74]. Yet, the solid–liquid separation and drying are certainly the main disadvantages of the UAE process. This extraction procedure is based on the cavitation process induced by compression and expansion cycles associated with the passage of ultrasounds (20 kHz–100 MHz frequency) through the sample. The acoustic waves promote the distance between molecules and consequently generate spaces among them, forming bubbles. The implosion of the cavitation bubbles causes inter-particle collisions resulting in particle disruption and enhanced diffusion of extractable bioactive compounds into the solvent [70,75][70][75]. A large amount of energy is released by bubble implosions, causing significant changes in the local temperature and pressure, liquid circulation, and turbulence, consequently increasing the mass transfer rate [47]. Moreover, the extraction efficiency of UAE can be significantly influenced by the sample properties (e.g., consistency, rheology, particle mobility) which affect ultrasound energy dispersion [75]. The UAE has been extensively applied at the lab scale in diverse food fields [70]. Ben-Othman and collaborators [39] used the response surface method (RSM) with a Box–Behnken design to select the best extraction efficiency of UAE for the recovery of phloretin and other phenolic compounds from apple tree leaves (Malus domestica Borkh.) from different cultivars from Estonia. The optimal extraction conditions were 14.4 min of extraction time, 10% sonication amplitude, and 10 g of sample per 100 mL solvent (70% ethanol, w/w). By applying the ideal conditions, the phloretin concentration ranged from 292 to 726 µg/g and the antioxidant activity from 6.06 to 11.42 mg GAE/g in the local winter cultivars “Paide taliõun” and “Tellissaare”, respectively. Martín-García et al. [42] used RSM to evaluate the effect of solvent composition, extraction time, and ultrasound power on the recovery of proanthocyanidins from brewers’ spent grains. The highest content of proanthocyanidins was obtained using 80/20 acetone/water (v/v), 55 min, and 400 W, which resulted in 1.01 mg/g dw of proanthocyanidins from brewers’ spent grains. In another study, da Rocha et al. [44] compared the extraction efficiency of microwave-assisted extraction and UAE of bioactive compounds from grape pomace. The results showed that both extraction procedures allowed the recovery of 45% of the anthocyanins when compared to the exhaustive extraction with methanol acidified solution.2.5. Microwave-Assisted Extraction
Microwave-assisted extraction (MAE) is a green and cost-effective extraction technique that has gained a lot of attention recently, due to its enhanced productivity, reduced extraction time, less solvent requirement, simplicity, and low set-up costs [4]. MAE involves electromagnetic radiations, transmitted as waves in the frequency range from 300 MHz to 300 GHz [4]. This technique is based on the principle that the energy absorbed during the passage of microwaves through the medium is converted into thermal energy, which facilitates the processing, due to higher extraction temperature and resultant faster mass transfer rate [4,76][4][76]. The heating effect of microwaves depends on the dielectric properties of the mixture of the solvent. When a solvent placed in contact with the sample is heated, MAE leads to the disruption of the hydrogen bonds, which results in the dipole rotation of the molecules and migration of the ions. Consequently, this process allows for the diffusion of the solvent, and thus the dissolution of the components [4]. MAE can be influenced by a wide range of parameters, namely microwave power, frequency, irradiation time, the particle size of the sample matrix, the composition of the solvent, extraction temperature, pressure, and the number of cycles. The choice of a suitable solvent for extraction is important and depends on the solubility, dielectric constant, and dissipation factors. Solvents with both high dielectric constant and dissipation factor can lead to a better extraction, which can be accomplished by mixtures of water with other solvents, such as ethanol or methanol [76]. MAE has been frequently used in the extraction of bioactive compounds, especially for plant materials [76]. Tran, Akanbi, Kirkman, Nguyen and Vuong [49] provided a method for the recovery of total phenolics, flavonoids, chlorogenic acid, and caffeine from coffee pulp using an MAE system. The results of this study showed that the sample-to-solvent ratio and ethanol concentration significantly affected the recovery yields of the bioactive compounds and the antioxidant capacity. Under the optimal conditions (Table 1), the extraction yields of total phenolic compounds, flavonoids, chlorogenic acid, and caffeine were 38.68, 27.00, 6.95, and 5.47 (mg/g dw), respectively. The extracts showed high antioxidant capacities, with values measured by ABTS, DPPH, and FRAP assays as 87.95, 9.3, and 65.31 (mg Trolox equivalents/g dw), respectively. In another study, Kurtulbaş, Sevgen, Samli and Şahin [50] extracted phenolic compounds and anthocyanins from peach peels, with the highest total phenolic content (TPC) being 19.35 mg of gallic acid equivalents/g of fresh plant matter and a total anthocyanin of 1.12 mg of cyn-3-glu/g of fresh plant matter, under the optimal MAE conditions (Table 1). After the extract was obtained, the samples were exposed to several storage media, such as −20 °C, 4 °C, and 25 °C in dark and 25 °C in light and the storage stability was monitored in terms of 4 bioactive properties (TPC and total anthocyanin contents, p-hydroxybenzoic acid and p-coumaric acid). In a general way, the degradation rate rose with storage temperature. The longest shelf life in terms of total phenols, anthocyanins, and major phenolic compounds (p-hydroxybenzoic acid and p-coumaric acid) was calculated as 111, 107, 88, and 83 days under deep freezer conditions at −20 °C. Zhang and collaborators [54] extracted flavonoid compounds from spent sweet potato leaves with natural deep eutectic solvents (NADESs) coupled with MAE. The highest extraction yield (40.21 ± 0.23 mg of rutin equivalents/g of sweet potato leaves) was obtained with NADES-2 synthesized by choline chloride and malic acid (molar ratio 1:2). The extracts were recovered by macroporous resin for the biological activity detection of flavonoid compounds, in which the AB-8 macroporous resin provided a recovery yield of 85.46% ± 2.33%. Additionally, the in vitro bioactivity experiments confirmed that the flavonoid compounds had good DPPH and O2− radical-scavenging activity, as well as inhibitory effects on E. coli, S. aureus, E. carotovora, and B. subtilis. Rodríguez García and Raghavan [55] evaluated the potential of MAE as a green technique to obtain phenolics. The reseauthorchers extracted phenolic compounds (vanillic, sinapic, caffeic, chlorogenic, ferulic, gallic, neochlorogenic, and p-coumaric acids, identified by HPLC) from broccoli by-products (stems, leaves, and florets). MAE was found to increase the phenolic yield up to 45.70% for broccoli leaves, 133.57% for broccoli florets, and 65.30% for broccoli stems, in less time compared with maceration extraction. Despite the advantages of MAE over conventional extraction methods, the high dependency on the solvent nature and the extraction temperature limits the application of MAE [4,76][4][76].References
- United Nations Department of Economic and Social Affairs. World Population Prospects 2019: Highlights. United Nations Dep. Public Inf. 2019. (ST/ESA/SER.A/423).
- FAO. FAO Statistical Yearbook 2021-World Food and Agriculture; FAO: Rome, Italy, 2021.
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; European Commission: Luxembourg, 2018.
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26.
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2061–2108.
- Pereira, J.A.M.; Berenguer, C.V.; Andrade, C.F.P.; Câmara, J.S. Unveiling the Bioactive Potential of Fresh Fruit and Vegetable Waste in Human Health from a Consumer Perspective. Appl. Sci. 2022, 12, 2747.
- Genkinger, J.M.; Platz, E.A.; Hoffman, S.C.; Comstock, G.W.; Helzlsouer, K.J. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am. J. Epidemiol. 2004, 160, 1223–1233.
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C.C. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int. J. Cancer 2008, 122, 2330–2336.
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315.
- de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102.
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574.
- Santos-Zea, L.; Gutierrez-Uribe, J.A.; Benedito, J. Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J. Supercrit. Fluids 2019, 144, 98–107.
- Fornereto Soldan, A.C.; Arvelos, S.; Watanabe, É.O.; Hori, C.E. Supercritical fluid extraction of oleoresin from Capsicum annuum industrial waste. J. Clean. Prod. 2021, 297, 126593.
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-food Waste. Food Eng. Rev. 2019, 12, 83–100.
- Manna, L.; Bugnone, C.A.; Banchero, M. Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. J. Supercrit. Fluids 2015, 104, 204–211.
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494.
- Natolino, A.; Da Porto, C. Supercritical carbon dioxide extraction of pomegranate (Punica granatum L.) seed oil: Kinetic modelling and solubility evaluation. J. Supercrit. Fluids 2019, 151, 30–39.
- Restrepo-Serna, D.L.; Solarte-Toro, J.C.; Cardona-Alzate, C.A. A Biorefinery Approach for an Integral Valorisation of Avocado Peel and Seeds Through Supercritical Fluids. Waste Biomass Valorization 2022, 13, 3973–3988.
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Stateva, R.P. Recovering value from organic waste materials: Supercritical fluid extraction of oil from industrial grape seeds. J. Supercrit. Fluids 2018, 141, 68–77.
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu var. Kuno): Phytochemical Analysis and Process Optimization. Foods 2021, 10, 1043.
- Ekinci, M.S.; Gürü, M. Extraction of phytosterols from melon (Cucumis melo) seeds by supercritical CO2 as a clean technology. Green Process. Synth. 2019, 8, 677–682.
- Hwang, H.J.; Kim, H.J.; Ko, M.J.; Chung, M.S. Recovery of hesperidin and narirutin from waste Citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226.
- Pedras, B.M.; Regalin, G.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Fractionation of red wine grape pomace by subcritical water extraction/hydrolysis. J. Supercrit. Fluids 2020, 160, 104793.
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144.
- Benito-román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa cv. Horcal): Effect of Temperature and Solvent Properties. Antioxid 2020, 9, 1233.
- Giombelli, C.; Iwassa, I.J.; da Silva, C.; Bolanho Barros, B.C. Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. J. Supercrit. Fluids 2020, 165, 104985.
- Rodrigues, L.A.; Matias, A.A.; Paiva, A. Recovery of antioxidant protein hydrolysates from shellfish waste streams using subcritical water extraction. Food Bioprod. Process. 2021, 130, 154–163.
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis vinifera) Subcritical Water Extracts. Foods 2020, 9, 872.
- Gabaston, J.; Leborgne, C.; Valls, J.; Renouf, E.; Richard, T.; Waffo-Teguo, P.; Mérillon, J.M. Subcritical water extraction of stilbenes from grapevine by-products: A new green chemistry approach. Ind. Crops Prod. 2018, 126, 272–279.
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272.
- Hendrawan, Y.; Larasati, R.; Wibisono, Y.; Umam, C.; Sutan, S.M.; Choviya Hawa, L. Extraction of Phenol and Antioxidant Compounds from Kepok Banana Skin with PEF Pre-Treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 012065.
- Lal, A.M.N.; Prince, M.V.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74, 102844.
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897.
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720.
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212.
- Niu, D.; Ren, E.F.; Li, J.; Zeng, X.A.; Li, S.L. Effects of pulsed electric field-assisted treatment on the extraction, antioxidant activity and structure of naringin. Sep. Purif. Technol. 2021, 265, 118480.
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of pulsed electric fields and high pressure on improved recovery of high-added-value compounds from olive pomace. J. Food Sci. 2020, 85, 1500–1512.
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369.
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of Ultrasound-Assisted Extraction of Phloretin and Other Phenolic Compounds from Apple Tree Leaves (Malus domestica Borkh.) and Comparison of Different Cultivars from Estonia. Antioxidants 2021, 10, 189.
- Pollini, L.; Blasi, F.; Ianni, F.; Grispoldi, L.; Moretti, S.; Di Veroli, A.; Cossignani, L.; Cenci-goga, B.T. Ultrasound-Assisted Extraction and Characterization of Polyphenols from Apple Pomace, Functional Ingredients for Beef Burger Fortification. Molecules 2022, 27, 1933.
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2021, 101, 1989–1997.
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-De-cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of Sonotrode Ultrasonic-Assisted Extraction of Proanthocyanidins from Brewers’ Spent Grains. Antioxidants 2019, 8, 282.
- Fernandes, F.A.; Heleno, S.A.; Pinela, J.; Carocho, M.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Recovery of Citric Acid from Citrus Peels: Ultrasound-Assisted Extraction Optimized by Response Surface Methodology. Chemosensors 2022, 10, 257.
- Da Rocha, C.B.; Noreña, C.P.Z. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace. Int. J. Food Eng. 2020, 16, 20190191.
- Giordano, M.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Stojković, D.; Soković, M.; Tavares, D.; Cánepa, A.L.; Ferreira, I.C.F.R.; Caleja, C.; et al. Ultrasound-Assisted Extraction of Flavonoids from Kiwi Peel: Process Optimization and Bioactivity Assessment. Appl. Sci. 2021, 11, 6416.
- Jimenez, A.; Feng, C.-H. Optimizing Procedures of Ultrasound-Assisted Extraction of Waste Orange Peels by Response Surface Methodology. Molecules 2022, 27, 2268.
- Li, J.; Pettinato, M.; Casazza, A.A.; Perego, P. A Comprehensive Optimization of Ultrasound-Assisted Extraction for Lycopene Recovery from Tomato Waste and Encapsulation by Spray Drying. Processes 2022, 10, 308.
- Elik, A.; Yanik, D.K.; Gogus, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. Lwt-Food Sci. Technol. 2020, 123, 109100.
- Tran, T.M.K.; Akanbi, T.; Kirkman, T.; Nguyen, M.H.; Vuong, Q.V. Maximising Recovery of Bioactive Compounds from Coffee Pulp Waste Using Microwave-assisted Extraction. Eur. J. Eng. Technol. Res. 2022, 7, 1–6.
- Kurtulbaş, E.; Sevgen, S.; Samli, R.; Şahin, S. Microwave-assisted extraction of bioactive components from peach waste: Describing the bioactivity degradation by polynomial regression. Biomass Convers. Biorefinery 2022.
- Ibrahim, N.H.; Mahmud, M.S.; Nurdin, S. Microwave-assisted extraction of β-sitosterol from cocoa shell waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012106.
- Doulabi, M.; Golmakani, M.T.; Ansari, S. Evaluation and optimization of microwave-assisted extraction of bioactive compounds from eggplant peel by-product. J. Food Process. Preserv. 2020, 44, e14853.
- Martínez-Abad, A.; Ramos, M.; Hamzaoui, M.; Kohnen, S.; Jiménez, A.; Garrigós, M.C. Optimisation of Sequential Microwave-Assisted Extraction of Essential Oil and Pigment from Lemon Peels Waste. Foods 2020, 9, 1493.
- Zhang, Y.; Bian, S.; Hu, J.; Liu, G.; Peng, S.; Chen, H.; Jiang, Z.; Wang, T.; Ye, Q.; Zhu, H. Natural Deep Eutectic Solvent-Based Microwave-Assisted Extraction of Total Flavonoid Compounds from Spent Sweet Potato (Ipomoea batatas L.) Leaves: Optimization and Antioxidant and Bacteriostatic Activity. Molecules 2022, 27, 5985.
- Rodríguez García, S.L.; Raghavan, V. Microwave-Assisted Extraction of Phenolic Compounds from Broccoli (Brassica oleracea) Stems, Leaves, and Florets: Optimization, Characterization, and Comparison with Maceration Extraction. Recent Prog. Nutr. 2022, 2, 11.
- Shang, X.-c.; Zhang, Y.-q.; Zheng, Y.-f.; Li, Y. Temperature-responsive deep eutectic solvents as eco-friendly and recyclable media for microwave extraction of flavonoid compounds from waste onion (Allium cepa L.) skins. Biomass Convers. Biorefinery 2022.
- Lombardelli, C.; Liburdi, K.; Benucci, I.; Esti, M. Tailored and synergistic enzyme-assisted extraction of carotenoid-containing chromoplasts from tomatoes. Food Bioprod. Process. 2020, 121, 43–53.
- Xu, K.; Wu, C.; Kou, X.; Fan, G.; Li, T.; Sun, W.; Suo, A. Enzyme-assisted extraction of apricot polysaccharides: Process optimization, structural characterization, rheological properties and hypolipidemic activity. J. Food Meas. Charact. 2022, 16, 2699–2709.
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63.
- Lombardelli, C.; Benucci, I.; Mazzocchi, C.; Esti, M. A Novel Process for the Recovery of Betalains from Unsold Red Beets by Low-Temperature Enzyme-Assisted Extraction. Foods 2021, 10, 236.
- Dominguez-Rodriguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086.
- Akyuz, A.; Ersus, S. Optimization of enzyme assisted extraction of protein from the sugar beet (Beta vulgaris L.) leaves for alternative plant protein concentrate production. Food Chem. 2021, 335, 127673.
- Saad, N.; Louvet, F.; Tarrade, S.; Meudec, E.; Grenier, K.; Landolt, C.; Ouk, T.S.; Bressollier, P. Enzyme-Assisted Extraction of Bioactive Compounds from Raspberry (Rubus idaeus L.) Pomace. J. Food Sci. 2019, 84, 1371–1381.
- Toledo-Merma, P.R.; Cornejo-Figueroa, M.H.; Crisosto-Fuster, A.D.R.; Strieder, M.M.; Chañi-Paucar, L.O.; Náthia-Neves, G.; Rodríguez-Papuico, H.; Rostagno, M.A.; A. Meireles, M.A.; Alcázar-Alay, S.C. Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction. Foods 2022, 11, 1070.
- Battistella Lasta, H.F.; Lentz, L.; Gonçalves Rodrigues, L.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353.
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot Pressurized Liquid Extraction of Polyphenols from the Skin and Seeds of Vitis vinifera L. cv. Negra Criolla Pomace a Peruvian Native Pisco Industry Waste. Agronomy 2021, 11, 866.
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108.
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203.
- Guzmán-Lorite, M.; Marina, M.L.; García, M.C. Pressurized liquids vs. high intensity focused ultrasounds for the extraction of proteins from a pomegranate seed waste. Innov. Food Sci. Emerg. Technol. 2022, 77, 102958.
- Paini, J.; Benedetti, V.; Ail, S.S.; Castaldi, M.J.; Baratieri, M.; Patuzzi, F. Valorization of Wastes from the Food Production Industry: A Review Towards an Integrated Agri-Food Processing Biorefinery. Waste Biomass Valoriz. 2021, 13, 31–50.
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; Bekhit, A.E.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54.
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxid 2021, 10, 1417.
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Env. Res. Public Health 2021, 18, 9153.
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406.
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60.
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841.
More
