Leukocyte-associated immunoglobulin (Ig)-like receptor 1 (LAIR1, CD305) belongs to the family of immune-inhibitory receptors and is widely expressed on hematopoietic mature cells, particularly on immune cells. Four different types of ligands of LAIR1 have been described, including collagens, suggesting a potential immune-regulatory function on the extracellular matrix. By modulating cytokine secretion and cellular functions, LAIR1 displays distinct patterns of expression among NK cell and T/B lymphocyte subsets during their differentiation and cellular activation and plays a major negative immunoregulatory role. Beyond its implications in physiology, the activity of LAIR1 can be inappropriately involved in various autoimmune or inflammatory disorders and has been implicated in cancer physiopathology, including hematological neoplasms. Its action as an inhibitory receptor can result in the dysregulation of immune cellular responses and in immune escape within the tumor microenvironment.
- LAIR1
- autoimmunity
- inflammation
- inhibitory receptor
- collagen
- hematological neoplasms
- immunoregulatory
1. Introduction
2. Expression Patterns of LAIR1
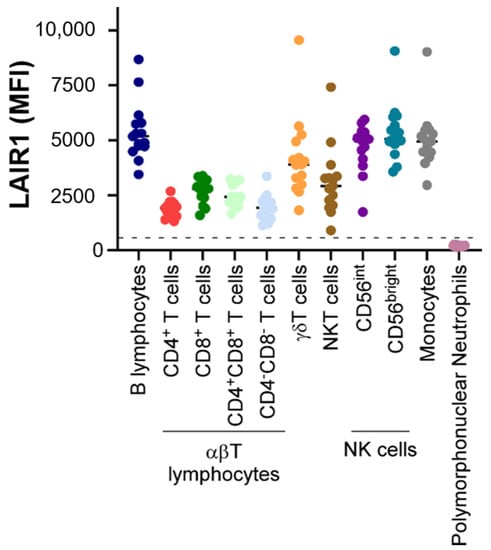
LAIR1 is widely expressed on almost all hematopoietic mature cells (Figure 1), including NK cells, T and B lymphocytes, monocytes [7], macrophages, basophils, mastocytes [8], eosinophils [9], dendritic cells [10], and a fraction of circulating plasmocytes [11].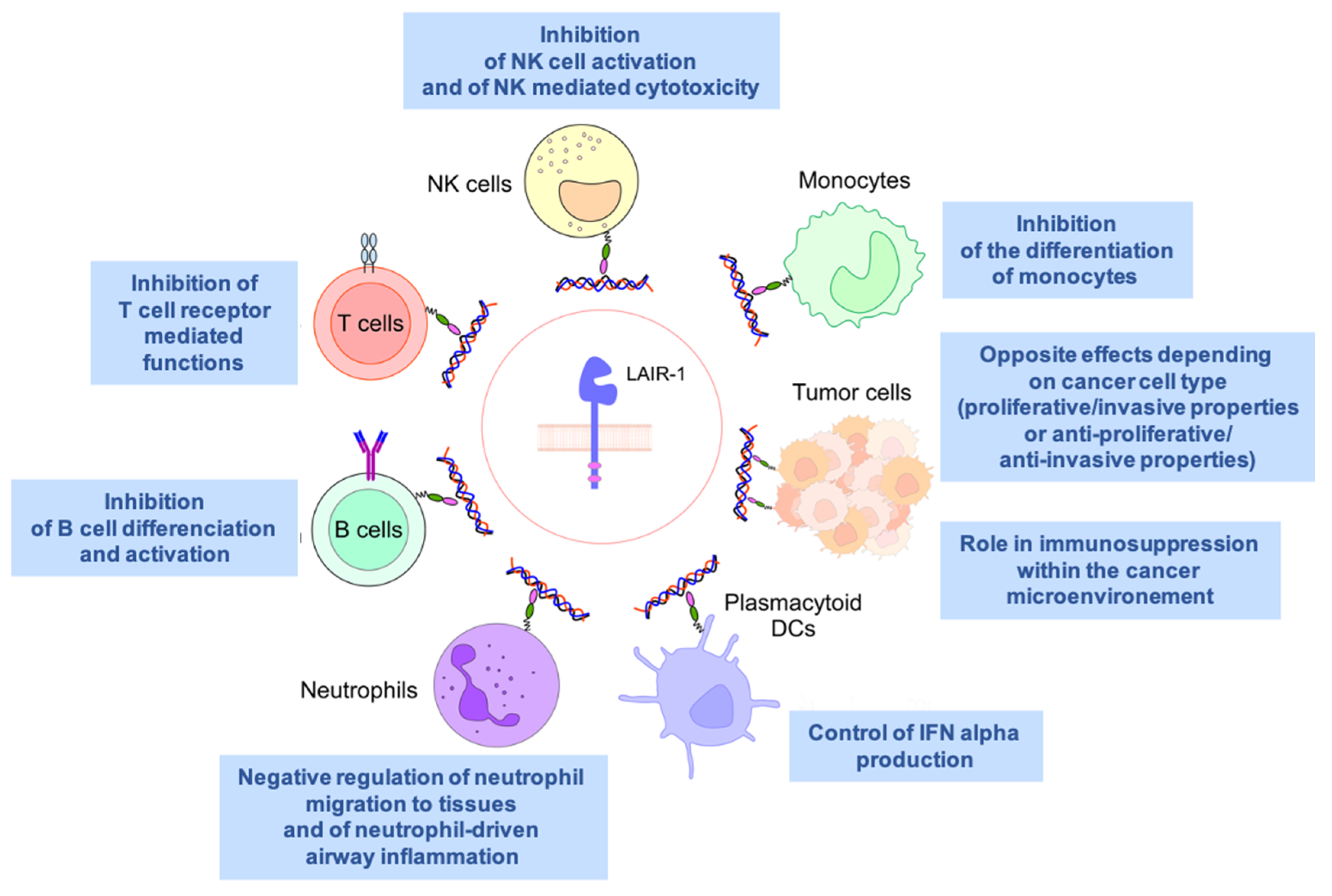
3. LAIR1 in Autoimmunity and Inflammatory Diseases
Protection against self-damage due to the loss of self-tolerance, hyperimmune activation, or autoimmunity relies on immune-inhibitory receptors expressed by both innate and adaptive immune cells. It is therefore not surprising that LAIR1 has a critical role in autoimmunity, given its widespread pattern of expression and its ability to suppress cell activation in a number of hematopoietic cells. It is thought that the inhibitory signals created through LAIR1 provide a threshold for immune activation, especially in collagen-rich tissues, thereby preventing uncontrolled immune responses and allowing the maintenance of immune cell homeostasis. In this section, wresearchers will discuss some instances in which LAIR1 has been shown to be involved in autoimmunity and inflammatory diseases.4. LAIR1 and Allergy
To address its role in allergic responses, Omiya and colleagues generated a transgenic mouse system expressing a chimeric version of LAIR1. This chimeric protein contained its extracellular domain fused with an immunoglobulin tag and acted as a decoy by competing with endogenous LAIR1 [38][21]. Using a murine experimental model of allergic dermatitis, the authors showed that the decoy LAIR1 transgenic mice had an increased susceptibility to contact hypersensibility (CHS). This increased susceptibility to CHS happened both at the sensitization and elicitation phases and implicated the repression of cytokine production (IL-6 and IL-12) by dendritic cells and the inhibition of proliferation and cytokine of both naïve and memory T cells. Similarly, the role of LAIR1 in airway hyper-reactivity and type 2 asthma was recently addressed by Helou and colleagues [39][22]. They were able to demonstrate that LAIR1 can be induced on activated pulmonary ILC2, leading to decreased cytokine secretion and effector functions. Airway hyper-reactivity was indeed exacerbated in the absence of LAIR1 in both mouse and human models, highlighting its normal protective anti-inflammatory role. Interestingly, circulating human ILC2 shows surface LAIR1 expression, which can be further increased upon IL-33 stimulation. This contrasts with mouse ILC2, which only expresses LAIR1 upon cytokine stimulation [39][22]. It still remains to be seen how LAIR1 can be targeted clinically for therapeutic purposes in the case of asthma.5. LAIR1 and Lupus
The complement system is involved in both innate and adaptive immune systems and has been shown to be important in the pathogenesis of systemic lupus erythematosus (SLE). Deficiencies in some members of the classical pathway, such as C1q, C4, and C2, can predispose individuals to the development of autoimmune diseases, explained in part by their important roles in promoting the clearance of immune complexes and apoptotic cells [40][23]. Furthermore, C1q has been shown to regulate, via LAIR1, myeloid cell activity and prevent the unwarranted activation of circulating monocytes and the development of mono-derived dendritic cells [23,41][24][25]. At the protein level, the collagen tail of C1q interacts with LAIR1, whereas C1q’s globular head interacts with the transmembrane receptor CD33 (Siglec-3), inducing the phosphorylation of the ITIM motifs in the tail of CD33, and altogether forms a C1q/CD33/LAIR1 inhibitory ternary complex [42][26]. Disruption of this inhibitory complex is frequently found in SLE, consistent with abnormalities related to C1q or a lack of LAIR1 and CD33 expression on circulating SLE myelomonocytes observed in SLE patients [42,43][26][27]. Aberrant C1q function may be linked to impaired LAIR1 expression and may be CD33-independent, since LAIR1 has been shown to be abnormally expressed on B cells and plasmacytoid DCs (pDCs) in SLE patients [44][28]. Defective LAIR1 expression in both B cells and pDCs, as well as the subsequent decreased inhibitory signals, lead to abnormal cell activation and may contribute to SLE development, highlighting the critical role of complement-mediated inhibition through LAIR1 in controlling immune homeostasis in normal and anomalous physiology.6. LAIR1 and Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a common autoimmune disease associated with progressive disability and systemic complications, characterized by events such as synovial inflammation, autoantibody production, and cartilage and bone destruction [45][29]. Patients with RA have lower surface expression of LAIR1 on their fibroblast-like synoviocytes (FLS) but significantly higher levels of soluble LAIR1 and its secreted homolog LAIR2 in the serum and synovial fluid [46][30]. Its expression is used as a biomarker when analyzing synovial fluid for evidence of RA [34][31]. Although FLS expressed low levels of surface LAIR1, treatment of these cells with TNF-alpha induced LAIR1′s shedding. A reduction in FLS invasion could be achieved by LAIR1 overexpression, as well as a decrease in inflammatory cytokines such as IL-6 and IL-8. All these data suggest that LAIR1 serves as an anti-inflammatory molecule in RA. LAIR1 expression is also affected on T cells from RA patients. As similarly observed with FLC cells, reduced surface LAIR1 expression on CD4+ T cells was seen in RA patients, leading to the decreased inhibition of T cell activation and hyperactivation. Mice rendered deficient for LAIR1 show more severe arthritis than their wildtype counterparts [47][32]. The mechanisms responsible for the inhibition of TCR signaling by LAIR1 engagement have recently been addressed by Park and colleagues. They were able to show that LAIR1 stimulation by collagen inhibits TCR signaling by decreasing the phosphorylation of key components of the TCR signaling pathway, such as Lck, ZAP-70, c-Jun, and p38 [48][33]. The kinase Csk, known to bind the intracellular tail of LAIR1 after phosphorylation of the ITIM motifs [1[1][34],36], was shown to be essential for the LAIR1-dependent inhibition of the TCR signaling cascade [48][33]. In conclusion, LAIR1 engagement by collagen-like domains could be an interesting therapeutic strategy to control inflammation in autoimmune diseases such as RA, SLE, and many other inflammatory states. Interestingly, it was recently observed that the effect of the vitamin D could be at least partially mediated by the upregulation of LAIR1 in a model of arthritis [49][35].References
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290.
- Billadeau, D.D.; Leibson, P.J. ITAMs versus ITIMs: Striking a balance during cell regulation. J. Clin. Investig. 2002, 109, 161–168.
- Verbrugge, A.; Ruiter Td, T.; Clevers, H.; Meyaard, L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int. Immunol. 2003, 15, 1349–1358.
- Ravetch, J.V.; Lanier, L.L. Immune inhibitory receptors. Science 2000, 290, 84–89.
- Lebbink, R.J.; van den Berg, M.C.; de Ruiter, T.; Raynal, N.; van Roon, J.A.; Lenting, P.J.; Jin, B.; Meyaard, L. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR1 inhibitory immune interaction. J. Immunol. 2008, 180, 1662–1669.
- Meyaard, L. The inhibitory collagen receptor LAIR1 (CD305). J. Leukoc. Biol. 2008, 83, 799–803.
- Meyaard, L. LAIR1, a widely distributed human ITIM-bearing receptor on hematopoietic cells. Curr. Top. Microbiol. Immunol. 1999, 244, 151–157.
- Florian, S.; Sonneck, K.; Czerny, M.; Hennersdorf, F.; Hauswirth, A.W.; Buhring, H.J.; Valent, P. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy 2006, 61, 1054–1062.
- Verbrugge, A.; de Ruiter, T.; Geest, C.; Coffer, P.J.; Meyaard, L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J. Leukoc. Biol. 2006, 79, 828–836.
- Bonaccorsi, I.; Cantoni, C.; Carrega, P.; Oliveri, D.; Lui, G.; Conte, R.; Navarra, M.; Cavaliere, R.; Traggiai, E.; Gattorno, M.; et al. The immune inhibitory receptor LAIR1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNalpha production. PLoS ONE 2010, 5, e15080.
- Rodriguez-Bayona, B.; Ramos-Amaya, A.; Brieva, J.A. Differential expression of SLAMS and other modulatory molecules by human plasma cells during normal maturation. Immunol. Lett. 2011, 134, 122–128.
- Maasho, K.; Masilamani, M.; Valas, R.; Basu, S.; Coligan, J.E.; Borrego, F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol. Immunol. 2005, 42, 1521–1530.
- van der Vuurst de Vries, A.R.; Clevers, H.; Logtenberg, T.; Meyaard, L. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 1999, 29, 3160–3167.
- Devin, J.; Kassambara, A.; Bruyer, A.; Moreaux, J.; Bret, C. Phenotypic Characterization of Diffuse Large B-Cell Lymphoma Cells and Prognostic Impact. J. Clin. Med. 2019, 8, 1074.
- Jin, J.; Wang, Y.; Ma, Q.; Wang, N.; Guo, W.; Jin, B.; Fang, L.; Chen, L. LAIR1 activation inhibits inflammatory macrophage phenotype in vitro. Cell. Immunol. 2018, 331, 78–84.
- Poggi, A.; Tomasello, E.; Ferrero, E.; Zocchi, M.R.; Moretta, L. p40/LAIR1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur. J. Immunol. 1998, 28, 2086–2091.
- Ouyang, W.; Ma, D.; Lin, D.; Sun, Y.; Liu, X.; Li, Q.; Jia, W.; Cao, Y.; Zhu, Y.; Jin, B. 9.1C3 is identical to LAIR1, which is expressed on hematopoietic progenitors. Biochem. Biophys. Res. Commun. 2003, 310, 1236–1240.
- Xue, J.; Zhang, X.; Zhao, H.; Fu, Q.; Cao, Y.; Wang, Y.; Feng, X.; Fu, A. Leukocyte-associated immunoglobulin-like receptor-1 is expressed on human megakaryocytes and negatively regulates the maturation of primary megakaryocytic progenitors and cell line. Biochem. Biophys. Res. Commun. 2011, 405, 128–133.
- Smith, C.W.; Thomas, S.G.; Raslan, Z.; Patel, P.; Byrne, M.; Lordkipanidze, M.; Bem, D.; Meyaard, L.; Senis, Y.A.; Watson, S.P.; et al. Mice Lacking the Inhibitory Collagen Receptor LAIR1 Exhibit a Mild Thrombocytosis and Hyperactive Platelets. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 823–835.
- Zhang, Y.; Ding, Y.; Huang, Y.; Zhang, C.; Boquan, J.; Ran, Z. Expression of leukocyte-associated immunoglobulin-like receptor-1 (LAIR1) on osteoclasts and its potential role in rheumatoid arthritis. Clinics 2013, 68, 475–481.
- Omiya, R.; Tsushima, F.; Narazaki, H.; Sakoda, Y.; Kuramasu, A.; Kim, Y.; Xu, H.; Tamura, H.; Zhu, G.; Chen, L.; et al. Leucocyte-associated immunoglobulin-like receptor-1 is an inhibitory regulator of contact hypersensitivity. Immunology 2009, 128, 543–555.
- Helou, D.G.; Shafiei-Jahani, P.; Hurrell, B.P.; Painter, J.D.; Quach, C.; Howard, E.; Akbari, O. LAIR1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. J. Allergy Clin. Immunol. 2021, 149, 223–236.e6.
- Walport, M.J. Complement and systemic lupus erythematosus. Arthritis Res. 2002, 4 (Suppl. 3), S279–S293.
- Son, M.; Santiago-Schwarz, F.; Al-Abed, Y.; Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR1. Proc. Natl. Acad. Sci. USA 2012, 109, E3160–E3167.
- Hosszu, K.K.; Santiago-Schwarz, F.; Peerschke, E.I.; Ghebrehiwet, B. Evidence that a C1q/C1qR system regulates monocyte-derived dendritic cell differentiation at the interface of innate and acquired immunity. Innate Immun. 2010, 16, 115–127.
- Son, M.; Diamond, B.; Volpe, B.T.; Aranow, C.B.; Mackay, M.C.; Santiago-Schwarz, F. Evidence for C1q-mediated crosslinking of CD33/LAIR1 inhibitory immunoreceptors and biological control of CD33/LAIR1 expression. Sci. Rep. 2017, 7, 270.
- Leffler, J.; Bengtsson, A.A.; Blom, A.M. The complement system in systemic lupus erythematosus: An update. Ann. Rheum. Dis. 2014, 73, 1601–1606.
- Colombo, B.M.; Canevali, P.; Magnani, O.; Rossi, E.; Puppo, F.; Zocchi, M.R.; Poggi, A. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS ONE 2012, 7, e31903.
- Chang, M.H.; Nigrovic, P.A. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight 2019, 4, e125278.
- Zhang, Y.; Wang, S.; Dong, H.; Yi, X.; Zhang, J.; Liu, X.; Zhuang, R.; Ding, Y. LAIR1 shedding from human fibroblast-like synoviocytes in rheumatoid arthritis following TNF-alpha stimulation. Clin. Exp. Immunol. 2018, 192, 193–205.
- Zhang, Y.; Lv, K.; Zhang, C.M.; Jin, B.Q.; Zhuang, R.; Ding, Y. The role of LAIR1 (CD305) in T cells and monocytes/macrophages in patients with rheumatoid arthritis. Cell. Immunol. 2014, 287, 46–52.
- Kim, S.; Easterling, E.R.; Price, L.C.; Smith, S.L.; Coligan, J.E.; Park, J.E.; Brand, D.D.; Rosloniec, E.F.; Stuart, J.M.; Kang, A.H.; et al. The Role of Leukocyte-Associated Ig-like Receptor-1 in Suppressing Collagen-Induced Arthritis. J. Immunol. 2017, 199, 2692–2700.
- Park, J.E.; Brand, D.D.; Rosloniec, E.F.; Yi, A.K.; Stuart, J.M.; Kang, A.H.; Myers, L.K. Leukocyte-associated immunoglobulin-like receptor 1 inhibits T-cell signaling by decreasing protein phosphorylation in the T-cell signaling pathway. J. Biol. Chem. 2020, 295, 2239–2247.
- Verbrugge, A.; Rijkers, E.S.; de Ruiter, T.; Meyaard, L. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur. J. Immunol. 2006, 36, 190–198.
- Myers, L.K.; Winstead, M.; Kee, J.D.; Park, J.J.; Zhang, S.; Li, W.; Yi, A.K.; Stuart, J.M.; Rosloniec, E.F.; Brand, D.D.; et al. 1,25-Dihydroxyvitamin D3 and 20-Hydroxyvitamin D3 Upregulate LAIR1 and Attenuate Collagen Induced Arthritis. Int. J. Mol. Sci. 2021, 22, 13342.
