Polymerase Chain Reaction (PCR) is one of the most common technologies used to produce millions of copies of targeted nucleic acid in vitro and has become an indispensable technique in molecular biology. However, it suffers from low efficiency and specificity problems, false positive results, and so on. Although many conditions can be optimized to increase PCR yield, such as the magnesium ion concentration, the DNA polymerases, the number of cycles, and so on, they are not all-purpose and the optimization can be case dependent. Nano-sized materials offer a possible solution to improve both the quality and productivity of PCR. In the last two decades, nNanoparticles (NPs) have attracted significant attention and gradually penetrated the field of life sciences because of their unique chemical and physical properties, such as their large surface area and small size effect, which have greatly promoted developments in life science and technology. Additionally, PCR technology assisted by NPs (NanoPCR) such as gold NPs (Au NPs), quantum dots (QDs), and carbon nanotubes (CNTs), etc., have been developed to significantly improve the specificity, efficiency, and sensitivity of PCR and to accelerate the PCR reaction process.
- NanoPCR
- nanomaterials
- specificity
- efficiency
- mechanisms
1. Introduction
2. Utilizing Different Nanomaterials to Enhance PCR Effects
2.1. Metal Nanomaterials
2.1.1. Au NPs
Au NPs are the most well-studied nanoparticles, and their interesting chemical and photophysical properties make them an integral part of nanoscience and ideal for biological and other commercial laboratories, which contain non-toxic, good biocompatibility, and unique chemical and optoelectronic properties. For example, Au NPs are characterized by adjustable size and physical size, catalytic activity, high surface volume ratio, high stability, easy synthesis and surface modification, and strong light absorption and scattering properties [5,14][5][14]. Figure 1 shows transmission electron microscopy (TEM) images of Au NPs in different morphologies [15,16][15][16].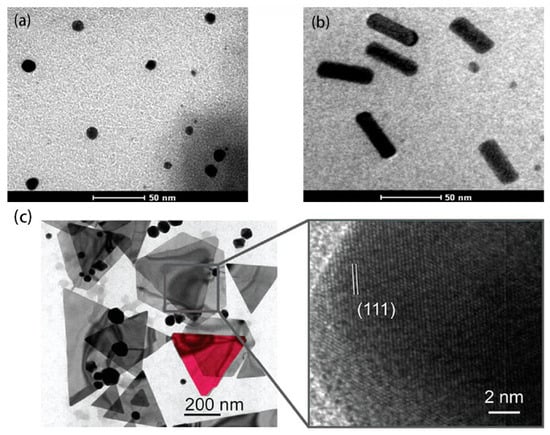
2.1.2. Ag NPs
At present, the use of metallic silver, silver nitrate, and silver sulfadiazine to treat burns, wounds, and bacterial infections has significantly declined because of the emergence of antibiotics. With the tremendous impetus of nanotechnology gains, nano-sized silver (Ag NPs) shows dramatically diverse chemical, physical, and optical properties and has high optical tunability, large absorption cross sections, and scattering properties [16]. Figure 2a,b are the TEM images of triangular NPs and spherical Ag NPs, respectively.
2.2. Carbon-Based Nanomaterials
2.2.1. CNTs
CNTs are greatly advantageous because of high electron transport without electronic scattering and electronic conductivity; thus, they provide high-performance sensing transistors. More specifically, CNTs possess a high aspect ratio (the ratio of lateral size to thickness), large specific surface area (SWCNT > 1600 m2/g, MWCNT > 430 m2/g), as well as good mechanical and electrical (~5000 s/cm) properties [26,27][26][27]. The SEM and TEM images of SWCNTs and MWCNTs are shown in Figure 3.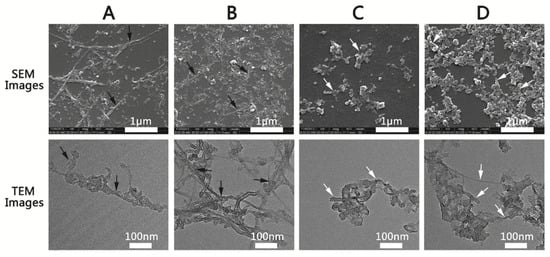
2.2.2. CNP
CNP has high specific surface area, strong adsorption, and high electrochemical capacity. As seen in Figure 4a, CNP has two broad peaks at 2θ of 25° and 43.8°, respectively. The diffraction peaks correspond to the planes (002) and (101) of graphite, indicating either a high degree of graphitization or a high degree of crystallinity, which can increase the thermal conductivity of CNP nanofluids due to the amorphous particles scatter phonon. This is probably the main reason why it enhances PCR. Figure 4b,c show the SEM photographs of the morphology of CNP. Clearly, the CNP is irregular, and the particles tend to aggregate with the diameter of CNP around 60 nm [32].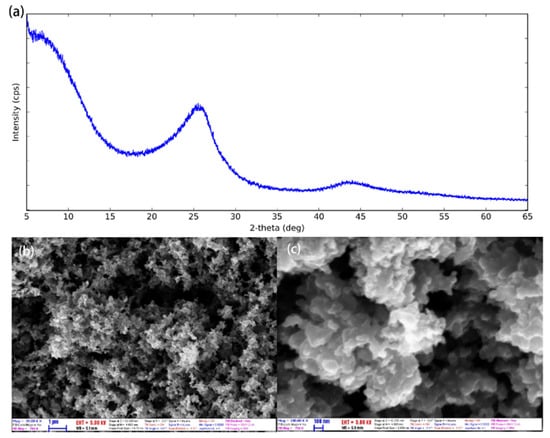
2.2.3. Graphene
Graphene, known as a 2D crystal of sp2-hybridized carbon atoms arranged in six-membered rings, has an extensive theoretical specific area, unparalleled thermal and electricity conductivity, and fascinating electronic properties such as an ambipolar electric field effect along with ballistic conduction of charge carriers [34]. However, during the preparation of GO, the oxygen-containing functional groups are usually introduced on the surface of graphene, and these heteroatoms will combine with adjacent carbon atoms through covalent bonds or weak van der Waals forces, resulting in a sharp decrease in thermal conductivity due to high-density defects caused to graphene [35]. Therefore, the thermal conductivity better enhances the properties of rGO than GO in PCR. In the study of graphene-enhanced PCR, Jia et al. found that the specificity of the PCR amplification could be improved by adding GO at concentrations from 12 mg∙mL−1 to 60 mg∙mL−1. However, GO did not affect the PCR when the GO concentration was lower than 12 mg∙mL−1, while it exhibited an inhibitory effect at concentrations higher than 70 mg∙mL−1. This study first demonstrated that rGO could significantly improve PCR specificity. It was then concluded that rGO was superior to GO in enhancing specificity [36]. Wang et al. further demonstrated that 1 μg∙mL−1 of GO effectively enhanced the specificity of the error-prone multi-round PCR [8]. In addition to conventional graphene, Abdul et al. explored the effect of graphene nanoflakes (GNFs) on PCR and found that 0.01% (w/w) GNFs provided an unambiguous 10-fold enhancement in the PCR yield. In addition, the thickness of the GNFs had a significant impact on the yield of PCR products. The 8 nm-thick GNFs increased the yield higher than other sizes [37]. Recently, Zhong et al. discussed the effects of GO through surface modification on PCR. The zwitterionic polymer-modified GO was found to be superior to other GO derivatives, with different charges enhancing the specificity of PCR [38].2.3. Oxide Nanomaterials
2.3.1. TiO2
TiO2 has been known as one of the cheapest and most widely-available types of NPs utilized for thermal conductivity enhancement [39]. Murshed et al. [40] demonstrated that TiO2 NPs have wonderful physical and chemical stability. It has been found that their particle size, shape, and volume fraction are the most critical factors that contribute to enhanced thermal conductivity. Both size and concentration of TiO2 NPs affects PCR. It was found that TiO2 NPs inhibited DNA synthesis in vitro more severely than the TiO2 particles in microscale at the equivalent concentration and the inhibition effect of TiO2 NPs was concentration-dependent in the dark [9]. About a decade ago, Rak et al. observed that TiO2 NPs with ∼25 nm diameter caused significant enhancement of PCR efficiency for various types of templates. The optimal concentration was determined to be 0.4 nM, resulting in up to a seven-fold increase in the amount of PCR product. As much as a 50% reduction in overall reaction time was also achieved by utilizing TiO2 NPs without compromising the PCR yield [41]. Upon the addition of TiO2 NPs with a particle size of 7 nm to the ordinary PCR, RT-qPCR, and RT-PCR (reverse transcription PCR), the effects of TiO2 NPs were investigated. The results indicated that 0.2 nM TiO2 NPs could achieve target amplification at a very low template concentration in an ordinary PCR system. Furthermore, relative to the larger TiO2 particles (25 nm) used in a previous study, the smaller TiO2 particles (7 nm) used in this study increased the yield of PCR by three-fold or more [42].2.3.2. ZnO
ZnO has been widely studied because it is non-toxic and easy to synthesize. Up to now, powdery ZnO in various morphologies, including nanowires, nanoflowers, and spherical and hierarchical structures have been successfully prepared and used to study their photocatalytic properties [43]. ZnO nanoflowers and their composites have been effectively used for PCR [44]. The XRD patterns and SEM images of ZnO nanoflowers are shown in Figure 5. The diffraction peaks are exactly the same as the standard card in the ZnO powder diffraction file (PDF) #36-1451. This clearly shows that the synthesized ZnO nanoflowers are of high purity. The SEM images show that the synthesized ZnO nanoflowers are self-assembling and clearly depict the nanopetal-like structure that emerges from the center of the flower. The synthesized ZnO nanoflowers are clear, uncrowded, and well dispersed, with an average diameter of about 1–2 μm [44].
2.3.3. Fe3O4
Magnetic NPs like Fe3O4 are characteristic of good magnetization and super-para-magnetism. Compared with other nanomaterials, the surface of magnetic NPs is more able to be functionalized. For instance, Fe3O4 nanomaterials have been found to be able to improve the sensitivity of PCR with a detection limit reaching 4.26 mol∙L−1. Kambli et al. compared the PCR efficiency enhanced by three transition metal NPs in the form of stable colloidal suspensions at varying concentrations. The AFM images of three nanoparticles are shown in Figure 6. Compared to the citrate stabilized Ag NPs (25 nm, 45%) and Au NPs (15.19 nm, 134%), the highest amplification efficiency of 190% was received using the ammonium salt of oleic acid-coated Fe3O4 NPs with an average size of 33 nm at a concentration of 7.2 × 10−3 nM in a conventional PCR system [45].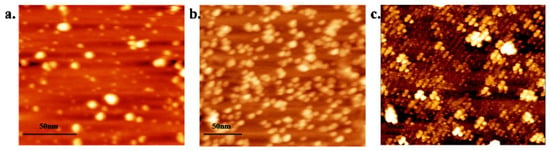
2.3.4. MgO
MgO nanomaterials have unique properties such as being highly stable with good dispensability and less toxic effects. For example, Narang et al. introduced MgO NPs to a PCR system and caused significant improvement in PCR efficiency [48].2.3.5. SiO2
SiO2 nanomaterials with well-defined morphology and porosity were first prepared and characterized by Stober. Carbonized polydopamine silica (C-PDA silica) were synthesized and employed to increase PCR efficiency (Figure 8). As compared with the effects of SiO2 NPs and PDA silica on PCR, C-PDA silica exhibited about 1.5 and 1.2 times higher efficiency. As a result, C-PDA silica can not only reduce the PCR cycle but also increase the final quantity of the PCR product [49].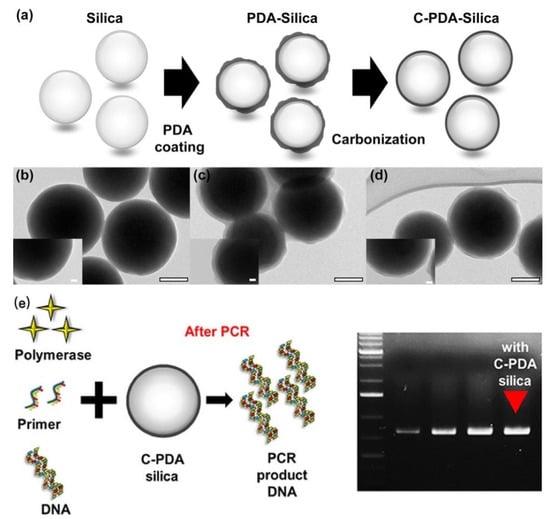
2.4. Fluorescent Nanomaterials
2.4.1. QDs
QDs, as a new kind of fluorescent material, possess many excellent characteristics such as size-tunable emission, wide absorbance bands, narrow symmetric emission bands, high photostability, etc. In 2009, Wang et al. [6] first found that CdTe QDs could increase the specificity of the PCR at different annealing temperatures with DNA templates of different lengths. Also, CdTe QDs were reported to accelerate PCR speed [50]. Then a Pfu polymerase based multi-round PCR technique was developed through being assisted by CdTe QDs, and the specificity could be retained even in ninth-round amplification [51]. The stacking of the primers on graphene QDs(GQDs) could improve the sensitivity and specificity of PCR by improving the efficiency of base-pairing between the primer and the template. By increasing polymerase activity, GQDs could improve the yield of PCR, where GQDs are tuned through chelating magnesium ions with their peripheral carboxylic groups [52].2.4.2. Up-Conversion Nanomaterials
Photon up-conversion is the phenomenon where high-energy photons are emitted upon the excitation of low-energy photons (Figure 9). Nucleic acid detection based on up-conversion NPs (UCNPs) displays a high signal-to-noise ratio and no photobleaching and has been widely applied. For example, Wang et al. [53] demonstrated that the addition of UCNPs to the reaction mixtures at appropriate concentrations could improve PCR specificity.
2.5. Others
2.5.1. Hybrid Nanocomposites
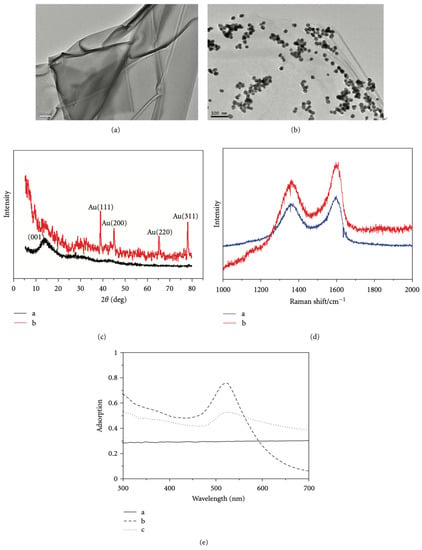
In the past decade, the application of composite nanomaterials in PCR has emerged to optimize the disadvantages of nanoparticles such as easy aggregation, poor adsorption capacity, poor thermal conductivity, etc., through surface modification or compounding of multiple NPs for the purpose of improving the characteristic properties of nanoparticles. Although Au NPs have been used maturely in PCR, it has been found that surface-modified Au NPs have also had a strong enhancement effect on PCR in recent years. In addition, some Au modified complexes have further specific effects on PCR. Chen et al. synthesized the dendrimer-entrapped Au NPs (Au DENPs) using amine-terminated G5 dendrimers as templates and different compositions as additives to investigate their effects on the specificity and efficiency of PCR amplification. It was found that the optimum concentration of Au DENPs could be reduced to as low as 0.37 nM, much lower than that of NH2-G5 dendrimers without Au NPs entrapped [54]. One year later, using poly (diallyl dimethylammonium) chloride (PDDA) as novel PCR enhancers, Yuan et al. verified the improvement of three kinds of Au NPs modified with different surface charge polarities in the efficiency and specificity of an error-prone two-round PCR system. The optimum concentrations of positively charged PDDA-Au NPs were different and as low as 1.54 pM, while the negatively charged Na3Ct-Au NPs were over three orders of magnitude higher than the positive ones [11]. Additionally, polyethylene glycol (PEG)-modified polyethylenimine (PEI)-entrapped Au NPs (PEG-Au PENPs) showed potential capacity to enhance the specificity and efficiency in both two-round PCR and GC-rich PCR. As the proportion of gold content increased, the optimum concentration of the modified Au NPs decreased (Figure 10) [55].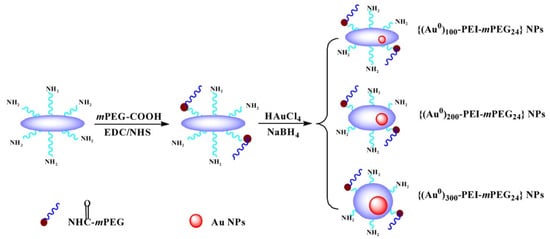
2.5.2. Other NPs
Metal-organic frameworks (MOFs) are special organic–inorganic hybrid porous solids with extraordinarily high surface areas, tunable pore sizes, adjustable internal surface properties, and an extraordinary degree of variable structures. These features endow MOFs with potential gas or liquid adsorption/storage applications, such as drug delivery, polymerization, catalysis, and biosensors. Recently, Sun et al. used UiO-66 and ZIF-8 to optimize the error-prone two-round PCR and found that both UiO-66 and ZIF-8 not only enhanced the sensitivity and efficiency of the first-round PCR but also increased the specificity and efficiency of the second-round PCR. Moreover, both MOFs could widen the annealing temperature range of the second-round PCR [59]. Also, Rasheed et al. developed a hexagonal boron nitride (hBN) NP-based PCR assay for the rapid detection of Acanthamoeba to amplify DNA from low amoeba cell density. As low as 1 × 10−4 (wt%) was determined as the optimum concentration of hBN NPs, which increased Acanthamoeba DNA yield up to ~16%. Further, it was able to reduce PCR temperature, which led to a ~2.0-fold increase in Acanthamoeba DNA yield at an improved PCR specificity at 46.2 °C low annealing temperature. hBN nanoparticles further reduced standard PCR step time by 10 min and cycles by 8 min, thus enhancing Acanthamoeba detection rapidly [60].References
- Kuypers, J.; Jerome, K.R. Applications of Digital PCR for Clinical Microbiology. J. Clin. Microbiol. 2017, 55, 1621–1628.
- Elizaquivel, P.; Aznar, R.; Sanchez, G. Recent developments in the use of viability dyes and quantitative PCR in the food microbiology field. J. Appl. Microbiol. 2014, 116, 1–13.
- Matheson, C.D.; Marion, T.E.; Hayter, S.; Esau, N.; Fratpietro, R.; Vernon, K.K. Technical note: Removal of metal ion inhibition encountered during DNA extraction and amplification of copper-preserved archaeological bone using size exclusion chromatography. Am. J. Phys. Anthropol. 2009, 140, 384–391.
- Huang, Y.-H.; Hu, X.-X.; Xu, W.-Z.; Gao, Y.; Feng, J.-D.; Sun, H.; Li, N. The factors affecting the efficiency of mutiplex PCR. Yi Chuan = Hered. 2003, 25, 65–68.
- Yang, W.; Li, X.; Sun, J.; Shao, Z. Enhanced PCR amplification of GC-rich DNA templates by Au NPs. ACS Appl. Mater. Interfaces 2013, 5, 11520–11524.
- Wang, L.; Zhu, Y.; Jiang, Y.; Qiao, R.; Zhu, S.; Chen, W.; Xu, C. Effects of QDs in Polymerase Chain Reaction. J. Phys. Chem. B 2009, 113, 7637–7641.
- Cui, D.; Tian, F.; Kong, Y.; Titushikin, I.; Gao, H. Effects of SWCNTs on the polymerase chain reaction. Nanotechnology 2004, 15, 154–157.
- Wang, Y.; Wang, F.; Wang, H.; Song, M. GO enhances the specificity of the polymerase chain reaction by modifying primer-template matching. Sci. Rep. 2017, 7, 16510.
- Li, S.; Zhu, H.; Zhu, R.; Sun, X.; Yao, S.; Wang, S. Impact and mechanism of TiO2 NPs on DNA synthesis in vitro. Sci. China Ser. B Chem. 2008, 51, 367–372.
- Nie, L.; Gao, L.; Yan, X.; Wang, T. Functionalized tetrapod-like ZnO nanostructures for plasmid DNA purification, polymerase chain reaction and delivery. Nanotechnology 2007, 18, 015101.
- Yuan, L.; He, Y. Effect of surface charge of PDDA-protected Au NPs on the specificity and efficiency of DNA polymerase chain reaction. Analyst 2013, 138, 539–545.
- Bai, Y.; Cui, Y.; Paoli, G.C.; Shi, C.; Wang, D.; Shi, X. Nanoparticles Affect PCR Primarily via Surface Interactions with PCR Components: Using Amino-Modified Silica-Coated Magnetic Nanoparticles as a Main Model. ACS Appl. Mater. Interfaces 2015, 7, 13142–13153.
- Wang, Z.D.; Zhang, L.Y.; Wang, X. Molecular toxicity and defense mechanisms induced by silver nanoparticles in Drosophila melanogaster. J. Environ. Sci. 2023, 125, 616–629.
- Wang, W.; Wang, X.; Liu, J.; Lin, C.; Liu, J.; Wang, J. The Integration of Gold Nanoparticles with Polymerase Chain Reaction for Constructing Colorimetric Sensing Platforms for Detection of Health-Related DNA and Proteins. Biosensors 2022, 12, 421.
- Nunes, A.M.; da Silva Filho, R.C.; da Silva, K.R.M.; Bezerra, S.M.; de Figueiredo, R.C.B.Q.; Saraiva, K.L.A.; Leite, A.C.R.; Meneghetti, M.R. Gold nanoparticles with different shapes can cause distinct effect on mitochondria bioenergetics. J. Nanoparticle Res. 2022, 24, 31.
- Kadu, P.; Pandey, S.; Neekhra, S.; Kumar, R.; Gadhe, L.; Srivastava, R.; Sastry, M.; Maji, S.K. Machine-Free Polymerase Chain Reaction with Triangular Au and Ag NPs. J. Phys. Chem. Lett. 2020, 11, 10489–10496.
- Li, M.; Lin, Y.C.; Wu, C.C.; Liu, H.S. Enhancing the efficiency of a PCR using Au NPs. Nucleic Acids Res. 2005, 33, e184.
- Pan, J.; Li, H.; Cao, X.; Huang, J.; Zhang, X.; Fan, C.; Hu, J. Nanogold-assisted multi-round polymerase chain reaction (PCR). J. Nanosci. Nanotechnol. 2007, 7, 4428–4433.
- Binh, V. Vu, D.L., Richard C. Willson. Gold Nanoparticle Effects in Polymerase Chain Reaction: Favoring of Smaller Products by Polymerase Adsorption. Anal. Chem. 2008, 80, 5462–5467.
- Mi, L.; Zhu, H.; Zhang, X.; Hu, J.; Fan, C. Mechanism of the interaction between Au nanoparticles and polymerase in nanoparticle PCR. Chin. Sci. Bull. 2007, 52, 2345–2349.
- Lin, Y.; Li, J.; Yao, J.; Liang, Y.; Zhang, J.; Zhou, Q.; Jiang, G. Mechanism of gold nanoparticle induced simultaneously increased PCR efficiency and specificity. Chin. Sci. Bull. 2013, 58, 4593–4601.
- Lou, X.; Zhang, Y. Mechanism studies on nanoPCR and applications of Au NPs in genetic analysis. ACS Appl. Mater. Interfaces 2013, 5, 6276–6284.
- Mandal, S.; Hossain, M.; Muruganandan, T.; Kumar, G.S.; Chaudhuri, K. Au NPs alter Taq DNA polymerase activity during polymerase chain reaction. RSC Adv. 2013, 3, 20793–20799.
- Wang, Q.; Li, J.; Cao, X.; Zhang, C. Ag NPs Enhance the Specificity of Repeated Long PCR Amplification. J. Tianjin Univ. Sci. Technol. 2007, 22, 1–5.
- Liu, P.; Guan, R.; Liu, M.; Huang, G.; Dai, X. Effect of PCR Amplification with Nano-silver on DNA Synthesis and Its Mechanism. J. Agric. Biotechnol. 2010, 18, 876–881.
- Lee, K.-Y.; Pham, X.-H.; Rho, W.-Y.; Chang, H.; Lee, S.H.; Kim, J.; Hahm, E.; Lee, J.H.; Lee, Y.-S.; Jun, B.-H. Nanotechnology for Bioapplications. Adv. Exp. Med. Biol. 2021, 1309, 235–255.
- Suo, L.; Li, Z.; Luo, F.; Chen, J.; Jia, L.; Wang, T.; Pei, X.; Wan, Q. Effect of dentin surface modification using carbon nanotubes on dental bonding and antibacterial ability. Dent. Mater. J. 2018, 37, 229–236.
- Zhang, Z.; Shen, C.; Wang, M.; Han, H.; Cao, X. Aqueous suspension of CNTs enhances the specificity of long PCR. Biotechniques 2008, 44, 537–538, 540, 542.
- Cao, X.; Chen, J.; Wen, S.; Peng, C.; Shen, M.; Shi, X. Effect of surface charge of polyethyleneimine-modified MWCNTs on the improvement of polymerase chain reaction. Nanoscale 2011, 3, 1741–1747.
- Yuce, M.; Budak, H. Dispersion quality of amine functionalized MWCNTs plays critical roles in polymerase chain reaction enhancement. J. Nanoparticle Res. 2014, 16, 2768.
- Yüce, M.; Uysal, E.; Budak, H. Amplification yield enhancement of short DNA templates using bulk and surface-attached amine-functionalized SWCNTs. Appl. Surf. Sci. 2015, 349, 147–155.
- Thong Le, B.; Bohus, M.; Lukacs, I.E.; Wongwises, S.; Grof, G.; Hernadi, K.; Szilagyi, I.M. Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids. Nanomaterials 2021, 11, 608.
- Zhang, Z.; Wang, M.; An, H. An aqueous suspension of CNP enhances the efficiency of a polymerase chain reaction. Nanotechnology 2007, 18, 355706.
- Wei, W.; Qu, X. Extraordinary physical properties of functionalized graphene. Small 2012, 8, 2138–2151.
- Li, Y.; Li, J.-l.; Zhu, Q.-s.; Liang, J.-f.; Guo, J.-q.; Wang, X.-d. Research progress in graphene based thermal conductivity materials. J. Mater. Eng. 2021, 49, 1–13.
- Jia, J.; Sun, L.; Hu, N.; Huang, G.; Weng, J. Graphene enhances the specificity of the polymerase chain reaction. Small 2012, 8, 2011–2015.
- Abdul Khaliq, R.; Kafafy, R.; Salleh, H.M.; Faris, W.F. Enhancing the efficiency of polymerase chain reaction using GNFs. Nanotechnology 2012, 23, 455106.
- Zhong, Y.; Huang, L.; Zhang, Z.; Xiong, Y.; Sun, L.; Weng, J. Enhancing the specificity of polymerase chain reaction by graphene oxide through surface modification: Zwitterionic polymer is superior to other polymers with different charges. Int. J. Nanomed. 2016, 11, 5989–6002.
- Amadeh, A.; Ghazimirsaeed, E.; Shamloo, A.; Dizani, M. Improving the performance of a photonic PCR system using TiO2 nanoparticles. J. Ind. Eng. Chem. 2021, 94, 195–204.
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2-water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373.
- Abdul Khaliq, R.; Sonawane, P.J.; Sasi, B.K.; Sahu, B.S.; Pradeep, T.; Das, S.K.; Mahapatra, N.R. Enhancement in the efficiency of polymerase chain reaction by TiO2 NPs: Crucial role of enhanced thermal conductivity. Nanotechnology 2010, 21, 255704.
- Lenka, G.; Weng, W.-H. Nanosized Paticles of TIitanium Dioxide Specifically Increase the Efficiency of Conventional Polymerase Chain Reaction. Dig. J. Nanomater. Biostructures 2013, 8, 1435–1445.
- Zhu, Y.F.; Yan, J.Y.; Zhou, L.; Feng, L.D. ZnO Nanorods Grown on Rhombic ZnO Microrods for Enhanced Photocatalytic Activity. Nanomaterials 2022, 12, 3085.
- Upadhyay, A.; Yang, H.; Zaman, B.; Zhang, L.; Wu, Y.; Wang, J.; Zhao, J.; Liao, C.; Han, Q. ZnO Nanoflower-Based NanoPCR as an Efficient Diagnostic Tool for Quick Diagnosis of Canine Vector-Borne Pathogens. Pathogens 2020, 9, 122.
- Kambli, P.; Kelkar-Mane, V. Nanosized Fe3O4 an efficient PCR yield enhancer Comparative study with Au, Ag nanoparticles. Colloids Surf. B-Biointerfaces 2016, 141, 546–552.
- Ozalp, V.C.; Bayramoglu, G.; Arica, M.Y. Magnetic silica nanoparticle-Taq polymerase hybrids for multiple uses in polymerase chain reaction. Rsc Adv. 2015, 5, 87672–87678.
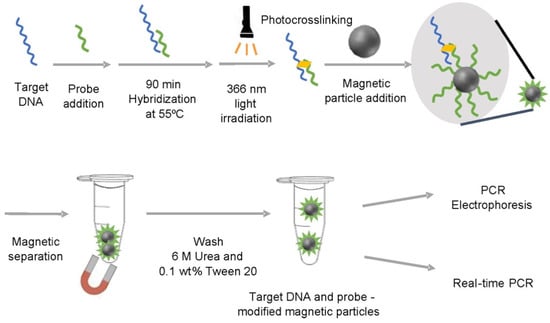
- Yajima, S.; Koto, A.; Koda, M.; Sakamoto, H.; Takamura, E.; Suye, S.-i. Photo-Cross-Linked Probe-Modified Magnetic Particles for the Selective and Reliable Recovery of Nucleic Acids. Acs Omega 2022, 7, 12701–12706.
- Narang, J.; Malhotra, N.; Narang, S.; Singhal, C.; Kansal, R.; Chandel, V.; Vastan, A.V.; Pundir, C.S. Replacement of magnesium chloride with magnesium NPs in polymerase chain reaction. Protoc. Exch. 2016.
- Park, J.Y.; Back, S.H.; Chang, S.J.; Lee, S.J.; Lee, K.G.; Park, T.J. Dopamine-assisted synthesis of carbon-coated silica for PCR enhancement. ACS Appl. Mater. Interfaces 2015, 7, 15633–15640.
- Fuming, S.; Yang, Y.; Hexiang, Z.; Meirong, M.; Zhizhou, Z. CdTe QDs accelerate the speed of Pfu-based polymerase chain reaction. J. Exp. Nanosci. 2013, 10, 476–482.
- Sang, F.; Zhang, Z.; Yuan, L.; Liu, D. QDs for a high-throughput Pfu polymerase based multi-round polymerase chain reaction (PCR). Analyst 2018, 143, 1259–1267.
- Zhu, M.; Luo, C.; Zhang, F.; Liu, F.; Zhang, J.; Guo, S. Interactions of the Primers and Mg2+ with GQDs Enhance PCR Performance. RSC Adv. 2015, 5, 74515–74522.
- Hwang, S.H.; Im, S.G.; Hah, S.S.; Cong, V.T.; Lee, E.J.; Lee, Y.S.; Lee, G.K.; Lee, D.H.; Son, S.J. Effects of upconversion NPs on polymerase chain reaction. PLoS ONE 2013, 8, e73408.
- Chen, J.; Cao, X.; Guo, R.; Shen, M.; Peng, C.; Xiao, T.; Shi, X. A highly effective polymerase chain reaction enhancer based on Au DENPs. Analyst 2012, 137, 223–228.
- Li, A.; Zhou, B.; Alves, C.S.; Xu, B.; Guo, R.; Shi, X.; Cao, X. Mechanistic Studies of Enhanced PCR Using PEG-Au PENPs. ACS Appl. Mater. Interfaces 2016, 8, 25808–25817.
- Jeong, H.Y.; Baek, S.H.; Chang, S.-J.; Yang, M.; Lee, S.J.; Lee, K.G.; Park, T.J. A hybrid composite of gold and GO as a PCR enhancer. RSC Adv. 2015, 5, 93117–93121.
- Song, M.; Yu, L.; Wu, Y. Simple Synthesis and Enhanced Performance of Graphene Oxide-Gold Composites. J. Nanomater. 2012, 2012, 135138.
- Dao Van, Q.; Nguyen Minh, H.; Pham Thi, T.; Nguyen Hoang, N.; Nguyen Hoang, H.; Nguyen Thai, S.; Phan Tuan, N.; Nguyen Thi Van, A.; Tran Thi, H.; Nguyen Hoang, L. Synthesis of Silica-Coated Magnetic Nanoparticles and Application in the Detection of Pathogenic Viruses. J. Nanomater. 2013, 2013, 603940.
- Sun, C.; Cheng, Y.; Pan, Y.; Yang, J.; Wang, X.; Xia, F. Efficient polymerase chain reaction assisted by MOFs. Chem. Sci. 2019, 11, 797–802.
- Rasheed, A.K.; Siddiqui, R.; Ahmed, S.M.K.; Gabriel, S.; Jalal, M.Z.; John, A.; Khan, N.A. hBN Nanoparticle-Assisted Rapid Thermal Cycling for the Detection of Acanthamoeba. Pathogens 2020, 9, 824.
