You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Meysam Najaflou.
Tumor cell-derived extracellular vesicles (TEVs) are an important means of tumor communication with, and manipulation of, the patient’s physiology. TEVs influence the local tumor environment as well as the systemic conditions of the patient.
- exosomes
- immunoediting
- cancer immunity
- immune escape
- immunosurveillance
- tumor microenvironment
1. Introduction
The tumor microenvironment (TME) within and around a tumor is a complex interacting mixture of tumor cells with various stromal cells, including endothelial cells, fibroblasts, and immune cells. In the early steps of tumor formation, the local microenvironment tends to oppose carcinogenesis, while with cancer progression, the microenvironment skews into a protumoral TME and the tumor influences stromal cells to provide tumor-supporting functions. The creation and development of cancer are dependent on escape from immune recognition predominantly by influencing stromal cells, particularly immune cells, to suppress antitumor immunity. This overall process is generally called immunoediting and has been categorized into three phases; elimination, equilibrium, and escape. Interaction of tumor cells with stromal cells in the TME is mediated generally by cell-to-cell contact, cytokines, growth factors, and extracellular vesicles (EVs). The least well studied are EVs (especially exosomes), which are nanoparticle-sized bilayer membrane vesicles released by many cell types that participate in cell/cell communication. EVs carry various proteins, nucleic acids, lipids, and small molecules that influence cells that ingest the EVs. Tumor-derived extracellular vesicles (TEVs) play a significant role in every stage of immunoediting, and their cargoes change from immune-activating in the early stages of immunoediting into immunosuppressing in the escape phase.
TEV early in tumor development can stimulate antitumor immunity. The interaction between immune cells and cancer in TME is categorized into seven potential steps (Figure 1) [4][1] which is called the “cancer-immunity cycle”. In the right conditions, TEV from tumor cells can also support antitumor immunity. TEVs contain and transfer TAs and damage-associated molecular patterns (DAMPs) to innate immune cells, especially dendritic cells (Step 1) [85,86][2][3]. Tumor-derived EVs are a source of shared TAs for CTL cross-priming.
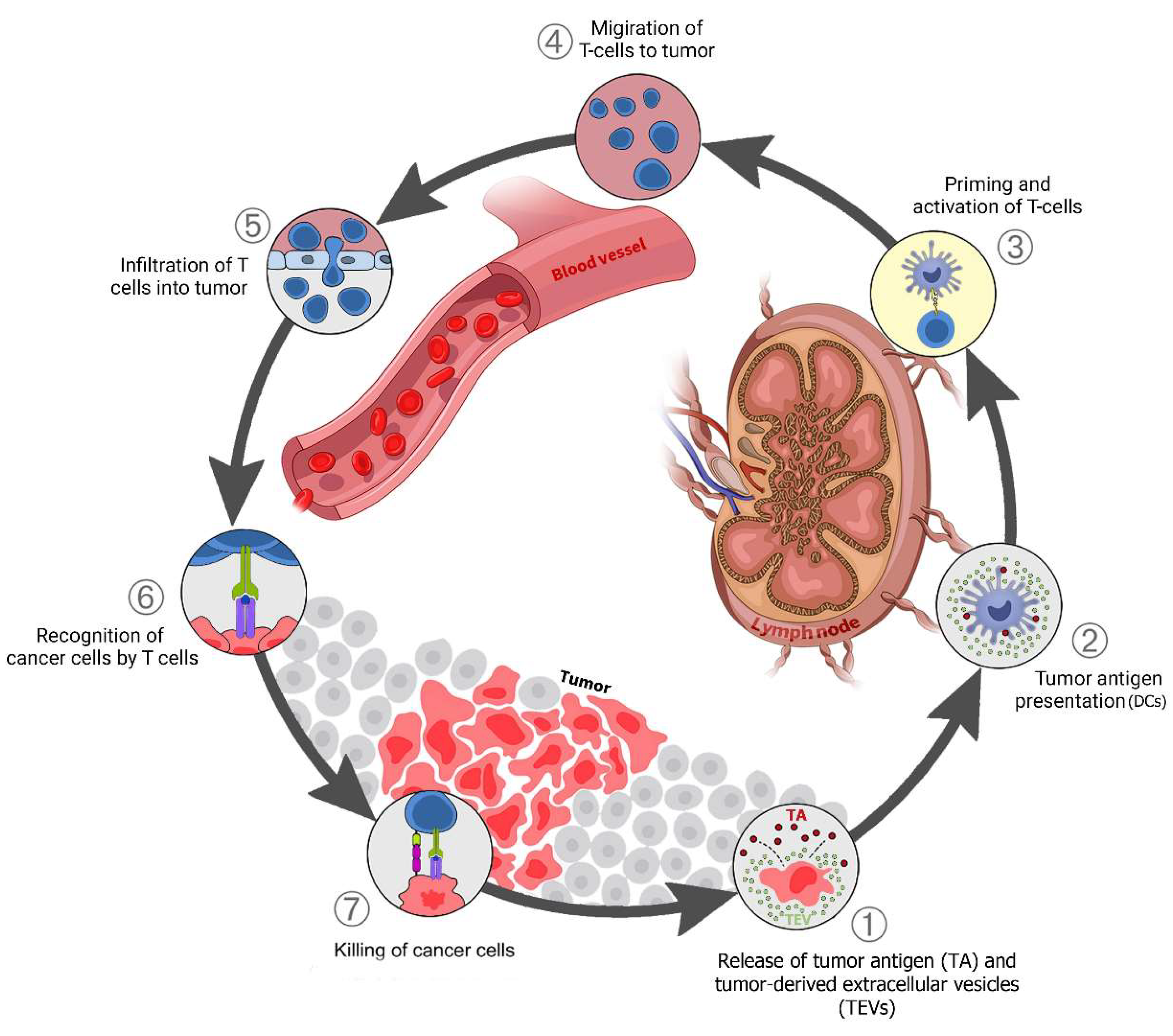
Figure 1. TEV in the cancer-immunity cycle: (1) Release of tumor antigens (TAs) along with tumor-derived extracellular vesicles (TEVs) that carry TA + DAMPs from dying cancer cells; (2) Presentation of TAs on the major histocompatibility complex (MHC) by dendritic cells; (3) T-cell receptor recognition of TAs on the MHC, leading to T-cell activation; (4) Migration of activated T cells to the tumors; (5) T-cell infiltration into the tumor; (6) Recognition of cancer antigens within the tumor; (7) Attack and killing of tumor cells.
Dendritic cells (DCs) respond to TEVs carrying DAMPs and TAs, mature, and migrate to lymph nodes (Steps 1–2). The tumor antigen is cross-presented on MHC class I (MHC-I) in the lymph nodes where it activates naive CD8 T cells (Step 3). The activated effector T cells go to the tumor site (Step 4), penetrate the tumor tissue (Step 5), identify cancer cells by tumor antigens presented on MHC-I (Step 6), then attack and kill them (Step 7). One or more of these stages may be disrupted in many cancer patients, resulting in ineffective immune responses to cancer. Disruption at any stage of this cycle is caused by cancer cells and their secreted factors, including via TEVs [86,87][3][4]. This disruption and immune system suppression block antitumor immunity and support cancer progression.
2. Elimination Phase TEV Involvement
This phase has not been directly detected in vivo in humans since it occurs with very small tumors. The innate and adaptive immune systems collaborate to identify and eliminate tumors that have evaded intrinsic tumor suppressor mechanisms in developing tumors [14][5]. Cancer immunosurveillance is proposed to remove newly generated neoplastic cells that have the potential to develop tumors. The processes of how the immune system is alerted of the presence of primary tumor cells remain unknown. Among the possibilities, the generation of neoantigens by abnormal cells within the created inflammatory environment (via immune cell subsets, recognition molecules, and effector cytokines) results in the detection of nascent cancers, and the traditional warning signals such as IFNs are likely involved [88,89,90][6][7][8]. T cells are the primary immune cells that identify and eliminate tumor cells [88,91][6][9]. However, B cells and their antibodies also seem to play a role in recognizing and removing these cells [92][10]. IFN-γ has a direct anti-proliferative impact on tumors through the STAT1 pathway and causes the release of cytokines such as CXCL9, 10, and 11 that increase immune activation by recruiting effector T cells [93,94][11][12]. IFN alpha and beta (type I IFNs) also play an important role in activating CD103+ DCs to cross-present tumor antigens [15,95][13][14].
Physical characteristics of the tumor environment such as hypoxia can cause tumor cell death, potentially resulting in the release of DAMPs such as Heat shock proteins (HSPs) and high mobility group box 1 (HMGB1), which act as ligands for Toll-like receptors on innate immune cells [85][2]. EVs can carry TAs, interferon, and DAMPs that stimulate immunological responses against tumors [96,97][15][16]. EVs carried CEA and HER2 TAAs that triggered immune responses and improved anti-tumor responses in vivo [98][17]. The release of tumor antigens and EVs can be altered under various TME situations. For example, an acidic microenvironment, quite common for tumors, increased the number of secreted EVs [99][18].
TEVs can play a key role in NK cell activation, DC maturation, and CD8+ effector T-cell development [100,101][19][20]. TEVs may also carry surface proteins derived from cancer cells which promote the uptake of TEVs by DCs. There are reports supporting LFA-1/CD54 and mannose-rich C-type lectin receptor interactions as enabling TEV uptake by DCs [102,103][21][22]. Uptake of TEVs by DCs enhanced DC expression of co-stimulatory receptors such as CD80, CD86, and also MHC II expression and boosted interferon and cytokine production along with DC maturation [104,105,106][23][24][25]. Breast cancer cells generated EVs that convey dsDNA to DCs, causing IFN alpha and beta expression in a STING-dependent manner and elevation of costimulatory molecules in DCs [107][26].
Furthermore, TEVs carry molecules that promoted CD8+ T-cell activation and enhanced tumor cytotoxic T lymphocyte (CTL) responses in vivo in mice [105,106,108][24][25][27]. EVs generated from brain tumors were delivered to mice on days 7 and 14 post-tumor inoculation, stimulating antibody production and T-cell activation. Antitumor antibodies and T cells present at the time of tumor inoculation appear to have caused enough tumor cell death to generate further T-cell antitumor response [109][28].
Tumor-Derived Immunostimulatory Vesicular DAMPs
During an immunogenic cell death (ICD), cancer cells release danger signals (DAMPs) raising the immunogenicity of dying cancer cells [85,110,111,112][2][29][30][31]. ICD is more immune stimulatory than necrosis which can suppress immunological responses [113][32] and necrosis generally does not strongly stimulate CD8+ T-cell-dependent immune responses [114][33]. DAMPs are secreted as a result of endoplasmic reticulum (ER) stress induced by mitochondrial ROS, membrane-lipid peroxidation, and ER-directed ROS generation [115,116,117][34][35][36]. DAMPs can also be released during necroptosis, pyroptosis, and ferroptosis [118,119,120][37][38][39]. EVs from cancer cells can carry DAMPs including HSPs, HMGB1, histones, ATP, vesicular RNAs, and cell-free DNA inside or on the surface [121,122,123,124,125,126][40][41][42][43][44][45]. Interestingly, EVs with surface-bound HSP70 stimulate more helper T cells (Th1) and CTL than TEVs with cytoplasmic HSP70 inside EVs [126][45]. Hsp70-enriched TEVs elicited significant CD4+ Th1 immune responses and promoted the production of MHC class II molecules on antigen-presenting cells, leading to the elimination of cancer cells [127][46]. CD94+ NK cells in the presence of TEVs possessing membrane HSP70 released granzyme B [126,128][45][47] and expressed stimulating receptors such as the NKG2D, CD69, and NKp44 while also down-regulating inhibitory receptor CD94 [129][48].
3. Equilibrium Phase TEV Involvement
Molecular processes that initiate immune-mediated cancer dormancy/control, i.e., the equilibrium phase (EqP), are not well understood in part because this phase is hard to model and has been minimally characterized in humans [130][49]. Not surprisingly, when overall mechanisms are poorly understood, there is not much known about the involvement of EVs in the equilibrium phase. In the equilibrium phase, the adaptive effector functions and the resistance of the tumor are in a dynamic balance. There are clear indications that tumors in the escape phase having metastasized, can return to equilibrium following chemotherapy and be dormant for many years before relapse. This occurs in particular with metastatic breast tumors where metastatic cells stop proliferating but survive in a quiescent state [131][50]. The role, if any that the immune system plays in maintaining this dormancy is not clear.
In the EqP, TEVs may suppress different adaptive immune cell types through various mechanisms such as inhibiting effector cells such as CD8+ T cells and NK cells, suppressing DC maturation and activation, increasing M2 and TAM immune suppressive polarization, and stimulating CAF differentiation [64,132,133][51][52][53]. However, as noted previously, TEV can also mediate tumor-suppressing signals. TEVs containing miR-23b derived from mesenchymal bone marrow cancer stem cells (CSC) can induce cancer dormancy via downregulation of the MARCKS gene that mediates breast cancer cells’ differentiation into CSCs through the Wnt-β-catenin pathway [134,135][54][55].
Considering PD-L1 and IFN-γ in the EqP of tumors is of interest for understanding the involvement of TEV and highlighting the complexity of molecular interactions. While IFN-γ supports CD8 T-cell effector function, IFN-γ stimulation also increases the quantity of PD-L1 on melanoma-released EVs that in turn suppressed the effector function of CD8+ T cells [136][56]. IFN-γ induced tumor dormancy when the interferon-gamma receptor 1 (IFNGR1) expression level was low but resulted in tumor elimination when it was high [137][57]. GW4869 treatment or Rab27a knockdown can inhibit vesicular-PD-L1 secretion, and significantly augment anti-PD-L1 therapeutic efficacy in 4T1 tumor growth [138][58]. Animal studies have shown that TEVs can also impair the production of interferons as well as decrease innate immune activity via EGFR- and MEKK2- dependent pathways [139][59].
4. Escape Phase TEV Involvement
Clinically recognized tumors have generally moved from equilibrium to escape. In the equilibrium phase, genome instability and accumulation of mutations in cancer cells over time leads to selection for low immunogenicity, expression of immune suppressive ligands, and escape from the immune system [140][60]. Tumors can eventually overcome antitumor immunity through mechanisms already mentioned, including tumor antigen editing, loss of MHC I expression, and expression of immune inhibitors such as PD-L1 [141,142,143][61][62][63] or suppressive mediators such as IL-10 [144][64], TGF-β [145][65], and TRAIL decoy receptors [146,147][66][67]. Recruitment and activation of immune-suppressing cells such as Tregs also contribute to escape [148][68].
4.1. Effect of TEVs on Dendritic Cells
Maturation of DCs requires inflammation-related stimuli which stimulate the expression of co-stimulatory molecules such as CD86, CD80, and CD40. TEVs can modify or block the differentiation of immature myeloid cells (IMC) to DC or divert the DCs maturation from IMC to MDSC or M2 macrophage by interacting with bone marrow IMC and inducing the production of IL-6, and decreasing expression of CD83 and CD86, as reported for breast cancer, murine mammary adenocarcinoma, and melanoma [149,150][69][70]. TEVs also can disrupt DC maturation and T-cell immune response with HLA-G-associated mechanisms in renal cancer [133][53] (Table 1). Some vesicular proteins such as MALAT1 directly interact with DCs and induce DC autophagy, which decreases DC-mediated T-cell activation [151][71]. Furthermore, TEV-treated DCs were ineffective at inducing CD4+ T-cell proliferation and activation but promoted differentiation into Treg [152][72]. TEVs fatty acids can create immunologically dysfunctional DCs by increasing intracellular lipid content by activating the peroxisome proliferator-activated receptor (PPAR) resulting in extra fatty acid oxidation (FAO) which shifts the DCs’ metabolism toward oxidative phosphorylation of mitochondria and the disruption of the function of DCs [153,154,155][73][74][75]. It was reported that human prostate cancer-derived extracellular vesicles purified from cultured cells contained PGE2 and triggered the expression of CD73 and CD39 on DCs in vitro, resulting in the generation of adenosine from ATP and inhibition of TNF-α and IL-12-production which reduced T-cell activation [156][76].
Table 1.
Effect of the tumor-derived extracellular vesicles on Dendritic cells.
| Cancer Type. | Cancer Type | Cellular Source | Vesicular Cargo | The Main Result | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Prostate cancer | DU145 | Ascites of ovarian patients OVCAR3 SKOV3 PGE2 |
Triggered the expression of CD73 and then CD39 on DCs, resulting in inhibition of TNFα- and IL-12-production via an ATP-dependent manner | AD10[156][76] | |||||
| TGF-β1, | IL-10 | Increased IL-10, FasL, TGF-β1, CTLA-4, which promoted Treg proliferation, suppressor activity, and Treg cell survival. | [190][113] | NSCLC | Blood samples from NSCLC patients | Galectin-9 and Tim-3 | |||
| Blood samples from ovarian patients Ascites of ovarian patients OVCAR-3 | Interacted with TIM-3 on DCs | AD10 A2780 Skov3 CaOv-3 MDAH2774 OvCa-14 OVP-10 |
Arginase-1 | Inhibited antigen-specific T-cell proliferation[ | [191][114]157][77] | ||||
| Renal cancer | |||||||||
| Prostate cancer | CD105+ CSCs CD105− TCs |
HLA-G | Disrupted maturation of DCs and T-cell immune responses | Pleural fluid samples of malignant pleural mesothelioma patients DU145 PC3[133][53] |
|||||
| PGE2 | T-cell inhibition was mediated through the adenosine A2A receptor | [ | 192 | ][115] | Glioblastoma | CSF samples from glioma patients GL261 U87MG U118 MG |
Galectin-9 | ||
| DU145 | Inhibited antigen recognition, processing, and presentation by interacting with TIM-3 on DCs | PC3 | TGF-β1 | Skewed IL-2 responses in T cells and suppressed cytotoxicity | [184][106][142][62] | ||||
| Ascites of glioma patients | PD-L1 | Impaired DCs maturation via formation of immunosuppressive monocytes | |||||||
| Melanoma | Blood samples from melanoma patients Blood samples from melanoma tumor-bearing mice WM1552C WM35 WM793 WM902B UACC-903 1205Lu WM9 WM164 | [ | 77][78] | ||||||
| PD-L1 | Suppressed the function of CD8 T cells | [ | 136 | ][56] | Blood samples from glioma patients GSC20 GSC267 GSC17 MEC-1 |
Vesicular cargo | Skewed monocytes toward an immune suppressive phenotype and induced programmed PD-L1 expression on monocytes through STAT3 phosphorylation and TLR7-dependent manner | [33,158][79][80] | |
| Colorectal cancer | Blood samples from colorectal patients SW403 CRC28462 1869col |
FasL, TRAIL | Induced T-cell apoptosis | [168][90] | Melanoma | SKMEL28 A375 C32TG |
S100, A8/A9 | Inhibited DCs maturation and reduced expression of CD83, CD86, Th1 polarizing chemokines (such as Flt3L, IL-15), and migration chemokines (MIP-1α and MIP-1β) | [150][70] |
| lymphatic fluid sample of melanoma patients ATCC |
S100A9 | Inhibited DCs maturation and prepared metastatic niche in lymph nodes | [159][81] | ||||||
| B16-F0 | TGF-β1 | Increased mRNA levels of IL-4 and TGF-β1 which inhibited DCs’ maturation | [160][82] | ||||||
| Blood samples from melanoma patients B16-F0 |
HSP72 and HSP105 | Induced secretion of IL-6 from DCs via TLR4- and TLR2-dependent manner activating STAT3-dependent MMP 9 activity | [161][83] | ||||||
| lymphocytic leukemia | Blood samples from CLL patients | S100A8/A9 | CD83, CD86, IL-12, and IL-15 expressions were all downregulated via activating the NFκB pathway | [162,[84163]][85] | |||||
| lung carcinoma | LLC | PD-L1 | Myeloid precursor cells were unable to differentiate into CD11c+ DCs in the presence of vesicular PD-L1 and resulted in DCs death | [152][72] | |||||
| LLC A549 |
MALAT1 | Inhibited DC function and T-cell proliferation and increased DC autophagy via AKT/mTOR Pathway | [151][71] | ||||||
| Breast cancer | MDA-MB-231 TS/A |
Vesicular cargo | Inhibited the development of myeloid precursor cells into DCs by increasing IL-6 production and reducing CD83 and CD86 expression | [149][69] | |||||
| 4T1 | PD-L1 | Myeloid precursor cells were unable to differentiate into CD11c+ DCs in the presence of vesicular PD-L1 and resulted in DC death | [152][72] | ||||||
| Blood samples from melanoma patients 4T1 |
HSP72 and HSP105 | Promoted DCs to IL-6 secretion in a TLR2- and TLR4-dependent manner which activated STAT3-dependent MMP 9 activity | [161][83] |
HSP72 and HSP105 on the membrane of TEVs interact with TLR2 and TLR4 on DCs which induced IL-6 secretion by DCs that increased STAT3-dependent MMP-9 transcription activity in cancer cells resulting in tumor invasion [161][83]. Galectin-9 on glioblastoma-derived EVs binds to the TIM3 DCs receptor and inhibits antigen presentation by DCs, leading to disrupted antitumor immune responses of cytotoxic T cells [142][62]. Important DC receptors such as Tim-3 and galectin-9 [157][77] and SIRPα as the ligand for CD47 were up-regulated on the tumor cells’ membranes and derived TEV [143,164][63][86]. TLR4 on the DCs decreased after treatment with pancreatic cancer-derived vesicular miR-203 resulting in reduced expression of cytokines such as TNF-α and IL-12, subsequently reducing DC maturation and Th1 differentiation [125][44]. Besides the vesicular proteins, vesicular miRs also affect DC’s function. For example, miR-212-3p transferred to DCs by pancreatic cancer-derived extracellular vesicles suppressed regulatory factor X-associated protein (RFXAP), decreased MHC II expression, and reduced antigen presentation by DCs [165][87]. Table 1 summarizes reports of TEV impacts on DC.
4.2. Effect of TEVs on T Cells
TEVs have a broad array of mechanisms by which they impact T cells. TEVs modify antitumor response by reducing T-cell viability, proliferation, and effector activities [166,167,168][88][89][90]. TEVs can disrupt T-cell effector function indirectly by blocking APC maturation [142,151,152][62][71][72] or directly by inhibiting activated CD8+ T-cell function, inducing CD8+ T-cell death through pro-apoptotic molecules (galectin-group proteins and FasL), promoting Treg expansion, and inducing T-cell exhaustion [169,170][91][92]. PD-L1 enriched glioblastoma-derived EVs perhaps surprisingly suppress monocytes rather than T-cells [77][78]. Nasopharyngeal carcinoma-derived vesicular galectin-9 induced apoptosis in CD4+ T cells via interaction with Tim-3 [171][93], as well as impairing T-cell function by interaction with TIM3 receptor on DCs in glioblastoma [142][62]. TEVs can carry pro-apoptotic Bax that induces apoptosis in CD8+T cells [172][94] and downregulates JAK3 expression which blocks CD8+ T-cell activation [167,173][89][95]. In Treg cell activation, both CD45 negative and positive EVs derived from plasma in head and neck cancer induced Treg differentiation of CD4 cells, but CD45(-) EVs also reduced CD8+ T-cell activation due to their higher adenosine concentrations [174][96]. EVs generated from multiple myeloma reduced the viability of CD4+ T cells and boosted the proliferation of Treg cells [175][97].
Vesicular PD-L1 promotes CD8+ T-cell apoptosis via PD-1/PD-L1 and PD-L1/CD80 signaling pathways [176][98], blocks T-cell activation in the draining lymph node in TRAMP- C2 prostate cancer mouse model [177[99][100],178], and reduces the proliferation of CD8+ T cells by decreasing IL- 2 and IFN-γ in the TME [136][56]. FasL on the TEVs decreased T-cell receptor (TCR) and CD3ζ expression in T cells leading to T-cell apoptosis [179][101], and melanoma-derived vesicular TNF downregulates TCR via redox signaling in T cells [180][102].
Pancreatic cancer cell EVs can stimulate p38 MAP kinase signaling in T lymphocytes that causes ER stress, which triggers the PERK–eIF2–ATF4–CHOP signaling cascade resulting in T-cell death [181][103]. Vesicular microRNAs in the serum of patients with nasopharyngeal carcinoma influenced T-cell differentiation and activation through suppression of the MAPK1 signaling pathway [182][104], while EVs with a high amount of miR-24–3 reduced CD4+ and CD8+ T-cell proliferation by targeting FGF11 [183][105]. In addition, mesothelioma cells’ EVs carrying TGF-β decreased proliferative response to IL-2 in T effector cells, but not in T-reg cells [184][106].
Vesicular galectin-1 plays a role in the induction of T-cell suppression [185][107]. TEVs also can induce T-cell exhaustion, by carrying inhibitory molecules, including PD-L1, CTLA- 4, TIM3, LAG3, and TIGIT [186,187][108][109]. miR-146a-5p and 14-3-3ζ in HCC-derived EVs induced T-cell exhaustion via activating M2-macrophages by inhibiting transcription factor SALL4 [30,188][110][111]. EVs carrying circRNA-002178 from patients’ serum with lung adenocarcinoma could boost PD-L1 production by sponging miR-34 in cancer cells, leading to CD8+T-cell exhaustion in vitro [132][52].
In addition, cancer patients’ plasma TEVs can prevent the activation of Th1 and Th17 lymphocytes and change them to immunosuppressive Treg phenotype cells [167,182][89][104]. The mutant KRAS gene is involved in the NSCLC-generated EVs-mediated transition of naive CD4+ T cells towards a FoxP3+ T-reg phenotype in a cytokine-independent manner in an NSCLC xenograft mouse model [189][112]. Table 2 summarizes reports on TEV suppressive effects on T cells.
Table 2.
Effect of the tumor-derived extracellular vesicles on T cells.
| Cancer Type | Cellular Source | Vesicular Cargo | Mechanism of Action | Refs. |
|---|---|---|---|---|
| Ovarian cancer | ||||
| DLD-1 | ||||
| WiDr | ||||
| TGF-β1 | Induced differentiation of T cells to Treg-like cells via the TGF-β pathway while inactivating the SAPK signaling pathway | [ | 193][116] | |
| Caco-2 | Galectin- 1 | Induced suppressor phenotype in human CD8+ T cells | [185][107] | |
| Head and neck cancer |
Tu167 SCC0209 HN60 |
Galectin- 1 | Induced suppressor phenotype in human CD8+ T cells | [185][107] |
| Blood samples from HNSCC patients | Vesicular cargo | Induced apoptosis in CD8+ T cells by converting CD4+ T cells to Treg | [174][96] | |
| Glioblastoma | Blood samples from glioma patients UPN933 E3-2 E6-5 |
Vesicular cargo | Deactivated T cells by FasL-dependent mechanisms and inhibit secretion of IL-2 | [194][117] |
| Nasopharyngeal cancer (NPC) | Blood samples from NPC patients Blood samples from NPC tumor-bearing mice C15 C17 |
Galectin- 9 | Induced huge apoptosis in T cells via membrane receptor Tim-3 | [171][93] |
| Blood samples from NPC patients C15 C17 |
CCL20 | Facilitated Treg recruitment and expansion that increased secretion of immunosuppressive cytokines (IL10, TGFB1) | [195][118] | |
| Blood samples from NPC patients TW03 C666 CNE2 |
miR- 24–3p | Blocked T-cell proliferation and Th1 and Th17 differentiation and promoted Treg induction via dephosphorylating ERK, STAT1, and STAT3 by reducing IL-2, IFNγ, and IL-17 secretion and phosphorylating STAT5 with increasing IL-6, IL-1β, and IL-10 secretion | [182,183][104][105] | |
| Oral squamous cell carcinoma(OSCC) | SCC-9 SCC-4 CAL-27 |
HSP70 | Altered development and cytotoxicity of T cells in an HSP70-dependent way via miR-21/PTEN/PD-L1 regulatory pathway | [170][92] |
| Blood samples from OSCC patients PCI-13 |
FasL | Induced apoptotic pathways in T cells through triggering caspase-3 cleavage, the release of cytochrome c that led to disrupting mitochondrial membrane, and decreased TCR-ζ chain production | [172][94] | |
| Breast cancer | MCF7 | CD73, CD39 | Inhibited T cells via the adenosine A2A receptor | [192][115] |
| BT-474 MDA-MB-231 |
TGF-β1 | Suppressed T-cell proliferation | [196][119] | |
| Lung cancer | Blood samples from lung cancer patients A549 PC9 95D |
circRNA- 002178 |
Enhanced PDL1 expression led to induced T-cell exhaustion | [132][52] |
| Hepatocellular Carcinoma (HCC) |
Blood samples from HCC patients MHCC97H |
14- 3- 3ζ | Inhibited the functions of T cells against cancer in the HCC microenvironment | [188][111] |
| Hepa1-6 H22 |
SALL4/miR-146a- 5p | T cells were exhausted by reducing IFN-γ and TNF-α expression while increasing the expression of inhibitory receptors such as PD-1 and CTLA-4 | [34][120] | |
| Pancreatic cancer | BxPC-3 tdTomato-BxPC-3 |
Vesicular cargo | Induced ER stress-mediated apoptosis via activating the p38 MAP kinase signaling | [181][103] |
4.3. Effect of TEVs on NK Cells
NK cells play an important role in cancer immunosurveillance by expressing death-inducing ligands such as FasL, TRAIL and JAK/STAT pathway [197,198][121][122]. However, like most immune cells, the activation of NK cells is controlled by a complex balance of activating and inhibiting signals. Tumor cells trigger several activating receptors, such as NKG2D, natural cytotoxicity receptors (NCRs), and DNAX accessory molecule-1 (DNAM-1/CD226) [199][123]. Vesicular NKG2D, TGF-β, and MICA*008 suppress or downregulate the expression of NKG2D in both NK and CD8+ T cells resulting in decreasing cytotoxicity of these cells by reducing the expression of cytotoxic molecules [200,201,202,203,204,205,206][124][125][126][127][128][129][130].
References
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875.
- Guo, Y.; Wang, S.-Z.; Zhang, X.; Jia, H.-R.; Zhu, Y.-X.; Zhang, X.; Gao, G.; Jiang, Y.-W.; Li, C.; Chen, X. In situ generation of micrometer-sized tumor cell-derived vesicles as autologous cancer vaccines for boosting systemic immune responses. Nat. Commun. 2022, 13, 1–20.
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570.
- Łuksza, M.; Sethna, Z.M.; Rojas, L.A.; Lihm, J.; Bravi, B.; Elhanati, Y.; Soares, K.; Amisaki, M.; Dobrin, A.; Hoyos, D. Neoantigen quality predicts immunoediting in survivors of pancreatic cancer. Nature 2022, 606, 389–395.
- DuPage, M.; Mazumdar, C.; Schmidt, L.M.; Cheung, A.F.; Jacks, T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 2012, 482, 405–409.
- Lakatos, E.; Williams, M.J.; Schenck, R.O.; Cross, W.C.; Househam, J.; Zapata, L.; Werner, B.; Gatenbee, C.; Robertson-Tessi, M.; Barnes, C.P. Evolutionary dynamics of neoantigens in growing tumors. Nat. Genet. 2020, 52, 1057–1066.
- Rao, S.; Gharib, K.; Han, A. Cancer immunosurveillance by T cells. Int. Rev. Cell Mol. Biol. 2019, 342, 149–173.
- Hu, Q.; Hong, Y.; Qi, P.; Lu, G.; Mai, X.; Xu, S.; He, X.; Guo, Y.; Gao, L.; Jing, Z. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun. 2021, 12, 1–13.
- Cole, K.E.; Strick, C.A.; Paradis, T.J.; Ogborne, K.T.; Loetscher, M.; Gladue, R.P.; Lin, W.; Boyd, J.G.; Moser, B.; Wood, D.E. Interferon–inducible T cell alpha chemoattractant (I-TAC): A novel Non-ELR CXC Chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 1998, 187, 2009–2021.
- Yan, Y.; Zheng, L.; Du, Q.; Yazdani, H.; Dong, K.; Guo, Y.; Geller, D.A. Interferon regulatory factor 1 (IRF-1) activates anti-tumor immunity via CXCL10/CXCR3 axis in hepatocellular carcinoma (HCC). Cancer Lett. 2021, 506, 95–106.
- von Locquenghien, M.; Rozalén, C.; Celià-Terrassa, T. Interferons in cancer immunoediting: Sculpting metastasis and immunotherapy response. J. Clin. Investig. 2021, 131.
- Dunn, G.P.; Bruce, A.T.; Sheehan, K.C.; Shankaran, V.; Uppaluri, R.; Bui, J.D.; Diamond, M.S.; Koebel, C.M.; Arthur, C.; White, J.M. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 2005, 6, 722–729.
- Jan, A.T.; Rahman, S.; Khan, S.; Tasduq, S.A.; Choi, I. Biology, pathophysiological role, and clinical implications of exosomes: A critical appraisal. Cells 2019, 8, 99.
- Yang, M.-q.; Du, Q.; Varley, P.R.; Goswami, J.; Liang, Z.; Wang, R.; Li, H.; Stolz, D.B.; Geller, D.A. Interferon regulatory factor 1 priming of tumour-derived exosomes enhances the antitumour immune response. Br. J. Cancer 2018, 118, 62–71.
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.-Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.-B. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367.
- Logozzi, M.; Capasso, C.; Di Raimo, R.; Del Prete, S.; Mizzoni, D.; Falchi, M.; Supuran, C.T.; Fais, S. Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 272–278.
- Shi, S.; Rao, Q.; Zhang, C.; Zhang, X.; Qin, Y.; Niu, Z. Dendritic cells pulsed with exosomes in combination with PD-1 antibody increase the efficacy of sorafenib in hepatocellular carcinoma model. Transl. Oncol. 2018, 11, 250–258.
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Thery, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303.
- Hao, Q.; Wu, Y.; Wu, Y.; Wang, P.; Vadgama, J.V. Tumor-Derived Exosomes in Tumor-Induced Immune Suppression. Int. J. Mol. Sci. 2022, 23, 1461.
- Segura, E.; Guérin, C.; Hogg, N.; Amigorena, S.; Théry, C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J. Immunol. 2007, 179, 1489–1496.
- Liu, H.; Chen, L.; Peng, Y.; Yu, S.; Liu, J.; Wu, L.; Zhang, L.; Wu, Q.; Chang, X.; Yu, X. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget 2018, 9, 2887.
- Ren, G.; Wang, Y.; Yuan, S.; Wang, B. Dendritic cells loaded with HeLa-derived exosomes simulate an antitumor immune response. Oncol. Lett. 2018, 15, 6636–6640.
- Dai, S.; Wan, T.; Wang, B.; Zhou, X.; Xiu, F.; Chen, T.; Wu, Y.; Cao, X. More efficient induction of HLA-A* 0201-restricted and carcinoembryonic antigen (CEA)–specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin. Cancer Res. 2005, 11, 7554–7563.
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.-P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920.
- Hao, S.; Bai, O.; Yuan, J.; Qureshi, M.; Xiang, J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol. Immunol. 2006, 3, 205–211.
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009, 23, 1541–1557.
- Garg, A.; Martin, S.; Golab, J.; Agostinis, P. Danger signalling during cancer cell death: Origins, plasticity and regulation. Cell Death Differ. 2014, 21, 26–38.
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 1–16.
- Wang, Z.; Yang, C.; Li, L.; Jin, X.; Zhang, Z.; Zheng, H.; Pan, J.; Shi, L.; Jiang, Z.; Su, K. Tumor-derived HMGB1 induces CD62L dim neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis 2020, 9, 1–17.
- Sachet, M.; Liang, Y.Y.; Oehler, R. The immune response to secondary necrotic cells. Apoptosis 2017, 22, 1189–1204.
- Gamrekelashvili, J.; Ormandy, L.A.; Heimesaat, M.M.; Kirschning, C.J.; Manns, M.P.; Korangy, F.; Greten, T.F. Primary sterile necrotic cells fail to cross-prime CD8+ T cells. Oncoimmunology 2012, 1, 1017–1026.
- Panaretakis, T.; Kepp, O.; Brockmeier, U.; Tesniere, A.; Bjorklund, A.C.; Chapman, D.C.; Durchschlag, M.; Joza, N.; Pierron, G.; Van Endert, P. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009, 28, 578–590.
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 1–13.
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. The emergence of phox-ER stress induced immunogenic apoptosis. Oncoimmunology 2012, 1, 786–788.
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4, e128834.
- Hou, L.; Yang, Z.; Wang, Z.; Zhang, X.; Zhao, Y.; Yang, H.; Zheng, B.; Tian, W.; Wang, S.; He, Z. NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab. Investig. 2018, 98, 1052–1064.
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 2015, 43, 923–932.
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front. Immunol. 2020, 11, 229.
- Nair, R.R.; Mazza, D.; Brambilla, F.; Gorzanelli, A.; Agresti, A.; Bianchi, M.E. LPS-challenged macrophages release microvesicles coated with histones. Front. Immunol. 2018, 9, 1463.
- Vulpis, E.; Cecere, F.; Molfetta, R.; Soriani, A.; Fionda, C.; Peruzzi, G.; Caracciolo, G.; Palchetti, S.; Masuelli, L.; Simonelli, L. Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: Role of HSP70/TLR2/NF-kB axis. Oncoimmunology 2017, 6, e1279372.
- Jella, K.; Nasti, T.; Li, Z.; Lawson, D.; Ahmed, R.; Dynan, W.; Khan, M. Post-irradiated tumor-derived exosomes lead to melanoma tumor growth delay, potentially mediated by death associated molecular pattern (damps) proteins. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, S155.
- Zhou, M.; Chen, J.; Zhou, L.; Chen, W.; Ding, G.; Cao, L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014, 292, 65–69.
- Xie, Y.; Bai, O.; Zhang, H.; Yuan, J.; Zong, S.; Chibbar, R.; Slattery, K.; Qureshi, M.; Wei, Y.; Deng, Y. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8+ CTL-and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J. Cell Mol. Med. 2010, 14, 2655–2666.
- Cho, J.-a.; Lee, Y.-S.; Kim, S.-H.; Ko, J.-K.; Kim, C.-W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009, 275, 256–265.
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247.
- Lv, L.-H.; Wan, Y.-L.; Lin, Y.; Zhang, W.; Yang, M.; Li, G.-L.; Lin, H.-M.; Shang, C.-Z.; Chen, Y.-J.; Min, J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012, 287, 15874–15885.
- Koebel, C.M.; Vermi, W.; Swann, J.B.; Zerafa, N.; Rodig, S.J.; Old, L.J.; Smyth, M.J.; Schreiber, R.D. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007, 450, 903–907.
- Neophytou, C.M.; Kyriakou, T.-C.; Papageorgis, P. Mechanisms of metastatic tumor dormancy and implications for cancer therapy. Int. J. Mol. Sci. 2019, 20, 6158.
- Sung, J.S.; Kang, C.W.; Kang, S.; Jang, Y.; Chae, Y.C.; Kim, B.G.; Cho, N.H. ITGB4-mediated metabolic reprogramming of cancer-associated fibroblasts. Oncogene 2020, 39, 664–676.
- Wang, J.; Zhao, X.; Wang, Y.; Ren, F.; Sun, D.; Yan, Y.; Kong, X.; Bu, J.; Liu, M.; Xu, S. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020, 11, 1–11.
- Grange, C.; Tapparo, M.; Tritta, S.; Deregibus, M.C.; Battaglia, A.; Gontero, P.; Frea, B.; Camussi, G. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer 2015, 15, 1–11.
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.-u.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, ra63.
- Sandiford, O.A.; Donnelly, R.J.; Markos, H.; Burgmeyer, L.M.; Sinha, G.; Pamarthi, S.H.; Sherman, L.S.; Ferrer, A.I.; DeVore, D.E.; Patel, S.A. Mesenchymal Stem Cell–Secreted Extracellular Vesicles Instruct Stepwise Dedifferentiation of Breast Cancer Cells into Dormancy at the Bone Marrow Perivascular Region. Cancer Res. 2021, 81, 1567–1582.
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386.
- Liu, Y.; Liang, X.; Yin, X.; Lv, J.; Tang, K.; Ma, J.; Ji, T.; Zhang, H.; Dong, W.; Jin, X. Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells. Nat. Commun. 2017, 8, 1–15.
- Yang, Y.; Li, C.-W.; Chan, L.-C.; Wei, Y.; Hsu, J.-M.; Xia, W.; Cha, J.-H.; Hou, J.; Hsu, J.L.; Sun, L. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018, 28, 862–864.
- Gao, L.; Wang, L.; Dai, T.; Jin, K.; Zhang, Z.; Wang, S.; Xie, F.; Fang, P.; Yang, B.; Huang, H. Tumor-derived exosomes antagonize innate antiviral immunity. Nat. Immunol. 2018, 19, 233–245.
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.-A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.; Taunk, N.K. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472.
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 469.
- Wang, M.; Cai, Y.; Peng, Y.; Xu, B.; Hui, W.; Jiang, Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020, 11, 1–16.
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667.
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107.
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limón, P. The polarization of immune cells in the tumour environment by TGFβ. Nat. Rev. Immunol. 2010, 10, 554–567.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Dhani, S.; Zhao, Y.; Zhivotovsky, B. A long way to go: Caspase inhibitors in clinical use. Cell Death Dis. 2021, 12, 1–13.
- Malchow, S.; Leventhal, D.S.; Nishi, S.; Fischer, B.I.; Shen, L.; Paner, G.P.; Amit, A.S.; Kang, C.; Geddes, J.E.; Allison, J.P. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013, 339, 1219–1224.
- Yu, S.; Liu, C.; Su, K.; Wang, J.; Liu, Y.; Zhang, L.; Li, C.; Cong, Y.; Kimberly, R.; Grizzle, W.E. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J. Immunol. 2007, 178, 6867–6875.
- Maus, R.L.; Jakub, J.W.; Nevala, W.K.; Christensen, T.A.; Noble-Orcutt, K.; Sachs, Z.; Hieken, T.J.; Markovic, S.N. Human melanoma-derived extracellular vesicles regulate dendritic cell maturation. Front. Immunol. 2017, 8, 358.
- Liu, Y.; Yin, Z.; Lu, P.; Ma, Y.; Luo, B.; Xiang, L.; Zhang, W.; He, Y.; Liang, X. Lung carcinoma cells secrete exosomal MALAT1 to inhibit dendritic cell phagocytosis, inflammatory response, costimulatory molecule expression and promote dendritic cell autophagy via AKT/mTOR pathway. OncoTargets Ther. 2020, 13, 10693.
- Ning, Y.; Shen, K.; Wu, Q.; Sun, X.; Bai, Y.; Xie, Y.; Pan, J.; Qi, C. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol. Lett. 2018, 199, 36–43.
- Yin, X.; Zeng, W.; Wu, B.; Wang, L.; Wang, Z.; Tian, H.; Wang, L.; Jiang, Y.; Clay, R.; Wei, X. PPARα inhibition overcomes tumor-derived exosomal lipid-induced dendritic cell dysfunction. Cell Rep. 2020, 33, 108278.
- Zhao, F.; Xiao, C.; Evans, K.S.; Theivanthiran, T.; DeVito, N.; Holtzhausen, A.; Liu, J.; Liu, X.; Boczkowski, D.; Nair, S. Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity 2018, 48, 147–160.e147.
- Wculek, S.K.; Khouili, S.C.; Priego, E.; Heras-Murillo, I.; Sancho, D. Metabolic control of dendritic cell functions: Digesting information. Front. Immunol. 2019, 10, 775.
- Salimu, J.; Webber, J.; Gurney, M.; Al-Taei, S.; Clayton, A.; Tabi, Z. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracell. Vesicles 2017, 6, 1368823.
- Gao, J.; Qiu, X.; Li, X.; Fan, H.; Zhang, F.; Lv, T.; Song, Y. Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 498, 409–415.
- Himes, B.T.; Peterson, T.E.; de Mooij, T.; Garcia, L.M.C.; Jung, M.-Y.; Uhm, S.; Yan, D.; Tyson, J.; Jin-Lee, H.J.; Parney, D. The role of extracellular vesicles and PD-L1 in glioblastoma-mediated immunosuppressive monocyte induction. Neuro-Oncology 2020, 22, 967–978.
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909.
- Haderk, F.; Schulz, R.; Iskar, M.; Cid, L.L.; Worst, T.; Willmund, K.V.; Schulz, A.; Warnken, U.; Seiler, J.; Benner, A. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci. Immunol. 2017, 2, eaah5509.
- Maus, R.L.; Jakub, J.W.; Hieken, T.J.; Nevala, W.K.; Christensen, T.A.; Sutor, S.L.; Flotte, T.J.; Markovic, S.N. Identification of novel, immune-mediating extracellular vesicles in human lymphatic effluent draining primary cutaneous melanoma. Oncoimmunology 2019, 8, e1667742.
- Yang, C.; Kim, S.-H.; Bianco, N.R.; Robbins, P.D. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS ONE 2011, 6, e22517.
- Shen, Y.; Guo, D.; Weng, L.; Wang, S.; Ma, Z.; Yang, Y.; Wang, P.; Wang, J.; Cai, Z. Tumor-derived exosomes educate dendritic cells to promote tumor metastasis via HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology 2017, 6, e1362527.
- Prieto, D.; Sotelo, N.; Seija, N.; Sernbo, S.; Abreu, C.; Durán, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, C. S100-A9 protein in exosomes from chronic lymphocytic leukemia cells promotes NF-κB activity during disease progression. Blood J. Am. Soc. Hematol. 2017, 130, 777–788.
- Cheng, P.; Corzo, C.A.; Luetteke, N.; Yu, B.; Nagaraj, S.; Bui, M.M.; Ortiz, M.; Nacken, W.; Sorg, C.; Vogl, T. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008, 205, 2235–2249.
- Kaur, S.; Elkahloun, A.G.; Arakelyan, A.; Young, L.; Myers, T.G.; Otaizo-Carrasquero, F.; Wu, W.; Margolis, L.; Roberts, D.D. CD63, MHC class 1, and CD47 identify subsets of extracellular vesicles containing distinct populations of noncoding RNAs. Sci. Rep. 2018, 8, 1–17.
- Ding, G.; Zhou, L.; Qian, Y.; Fu, M.; Chen, J.; Chen, J.; Xiang, J.; Wu, Z.; Jiang, G.; Cao, L. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget 2015, 6, 29877.
- Thommen, D.S.; Schumacher, T.N. T cell dysfunction in cancer. Cancer Cell 2018, 33, 547–562.
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730.
- Huber, V.; Fais, S.; Iero, M.; Lugini, L.; Canese, P.; Squarcina, P.; Zaccheddu, A.; Colone, M.; Arancia, G.; Gentile, M. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology 2005, 128, 1796–1804.
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Chin, J.L.; Min, W.-P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173.
- Li, L.; Cao, B.; Liang, X.; Lu, S.; Luo, H.; Wang, Z.; Wang, S.; Jiang, J.; Lang, J.; Zhu, G. Microenvironmental oxygen pressure orchestrates an anti-and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene 2019, 38, 2830–2843.
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Le Moulec, S.; Guigay, J.; Hirashima, M.; Guemira, F. Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells. Blood J. Am. Soc. Hematol. 2009, 113, 1957–1966.
- Kim, J.W.; Wieckowski, E.; Taylor, D.D.; Reichert, T.E.; Watkins, S.; Whiteside, T.L. Fas ligand–positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. 2005, 11, 1010–1020.
- Taylor, D.; Gercel-Taylor, C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br. J. Cancer 2005, 92, 305–311.
- Beccard, I.J.; Hofmann, L.; Schroeder, J.C.; Ludwig, S.; Laban, S.; Brunner, C.; Lotfi, R.; Hoffmann, T.K.; Jackson, E.K.; Schuler, P.J. Immune suppressive effects of plasma-derived exosome populations in head and neck cancer. Cancers 2020, 12, 1997.
- Shao, Q.; Deng, L.; Liu, H.; Liu, Z.; Chen, J.; Jiang, F.; Yan, S.; Fu, R. Involvement of MM cell-derived exosomes in T lymphocytes immune responses. Oncol. Lett. 2020, 20, 1.
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.-E.; Sung, K.J.; Sung, Y.H.; Pack, C.-G.; Jung, M.-k.; Han, B. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13.
- Poggio, M.; Hu, T.; Pai, C.-C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019, 177, 414–427.e413.
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New insights into tumor immune escape mechanisms and therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 569219.
- Taylor, D.D.; Gerçel-Taylor, Ç.; Lyons, K.S.; Stanson, J.; Whiteside, T.L. T-cell apoptosis and suppression of T-cell receptor/CD3-ζ by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin. Cancer Res. 2003, 9, 5113–5119.
- Söderberg, A.; Barral, A.M.; Söderström, M.; Sander, B.; Rosén, A. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic. Biol. Med. 2007, 43, 90–99.
- Shen, T.; Huang, Z.; Shi, C.; Pu, X.; Xu, X.; Wu, Z.; Ding, G.; Cao, L. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB J. 2020, 34, 8442–8458.
- Ye, S.-b.; Li, Z.-L.; Luo, D.-h.; Huang, B.-j.; Chen, Y.-S.; Zhang, X.-s.; Cui, J.; Zeng, Y.-x.; Li, J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 2014, 5, 5439.
- Ye, S.B.; Zhang, H.; Cai, T.T.; Liu, Y.N.; Ni, J.J.; He, J.; Peng, J.Y.; Chen, Q.Y.; Mo, H.Y.; Zhang, X.S. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016, 240, 329–340.
- Clayton, A.; Mitchell, J.P.; Mason, M.D.; Tabi, Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007, 67, 7458–7466.
- Maybruck, B.T.; Pfannenstiel, L.W.; Diaz-Montero, M.; Gastman, B.R. Tumor-derived exosomes induce CD8+ T cell suppressors. J. Immunother. Cancer 2017, 5, 1–15.
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792.
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019, 37, 457–495.
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156.
- Wang, X.; Shen, H.; Zhangyuan, G.; Huang, R.; Zhang, W.; He, Q.; Jin, K.; Zhuo, H.; Zhang, Z.; Wang, J. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018, 9, 1–14.
- Kalvala, A.; Wallet, P.; Yang, L.; Wang, C.; Li, H.; Nam, A.; Nathan, A.; Mambetsariev, I.; Poroyko, V.; Gao, H. Phenotypic switching of naive T cells to immune-suppressive Treg-like cells by mutant KRAS. J. Clin. Med. 2019, 8, 1726.
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS ONE 2010, 5, e11469.
- Czystowska-Kuzmicz, M.; Sosnowska, A.; Nowis, D.; Ramji, K.; Szajnik, M.; Chlebowska-Tuz, J.; Wolinska, E.; Gaj, P.; Grazul, M.; Pilch, Z. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 2019, 10, 1–16.
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011, 187, 676–683.
- Yamada, N.; Kuranaga, Y.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Akao, Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-β1-mediated suppression. Oncotarget 2016, 7, 27033.
- Hellwinkel, J.E.; Redzic, J.S.; Harland, T.A.; Gunaydin, D.; Anchordoquy, T.J.; Graner, M.W. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro-Oncology 2015, 18, 497–506.
- Mrizak, D.; Martin, N.; Barjon, C.; Jimenez-Pailhes, A.-S.; Mustapha, R.; Niki, T.; Guigay, J.; Pancré, V.; de Launoit, Y.; Busson, P. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J. Natl. Cancer Inst. 2015, 107, dju363.
- Rong, L.; Li, R.; Li, S.; Luo, R. Immunosuppression of breast cancer cells mediated by transforming growth factor-β in exosomes from cancer cells. Oncol. Lett. 2016, 11, 500–504.
- Yin, C.; Han, Q.; Xu, D.; Zheng, B.; Zhao, X.; Zhang, J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology 2019, 8, e1601479.
- Dianat-Moghadam, H.; Mahari, A.; Heidarifard, M.; Parnianfard, N.; Pourmousavi-Kh, L.; Rahbarghazi, R.; Amoozgar, Z. NK cells-directed therapies target circulating tumor cells and metastasis. Cancer Lett. 2021, 497, 41–53.
- Bottos, A.; Gotthardt, D.; Gill, J.W.; Gattelli, A.; Frei, A.; Tzankov, A.; Sexl, V.; Wodnar-Filipowicz, A.; Hynes, N.E. Decreased NK-cell tumour immunosurveillance consequent to JAK inhibition enhances metastasis in breast cancer models. Nat. Commun. 2016, 7, 1–12.
- Kruse, P.H.; Matta, J.; Ugolini, S.; Vivier, E. Natural cytotoxicity receptors and their ligands. Immunol. Cell Biol. 2014, 92, 221–229.
- Hedlund, M.; Stenqvist, A.-C.; Nagaeva, O.; Kjellberg, L.; Wulff, M.; Baranov, V.; Mincheva-Nilsson, L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: Evidence for immunosuppressive function. J. Immunol. 2009, 183, 340–351.
- Ashiru, O.; Boutet, P.; Fernández-Messina, L.; Agüera-González, S.; Skepper, J.N.; Valés-Gómez, M.; Reyburn, H.T. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA* 008 that is shed by tumor cells in exosomes. Cancer Res. 2010, 70, 481–489.
- Labani-Motlagh, A.; Israelsson, P.; Ottander, U.; Lundin, E.; Nagaev, I.; Nagaeva, O.; Dehlin, E.; Baranov, V.; Mincheva-Nilsson, L. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumor Biol. 2016, 37, 5455–5466.
- Lundholm, M.; Schröder, M.; Nagaeva, O.; Baranov, V.; Widmark, A.; Mincheva-Nilsson, L.; Wikström, P. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: Mechanism of immune evasion. PLoS ONE 2014, 9, e108925.
- Clayton, A.; Mitchell, J.P.; Linnane, S.; Mason, M.D.; Tabi, Z. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 2008, 180, 7249–7258.
- López-Cobo, S.; Campos-Silva, C.; Moyano, A.; Oliveira-Rodríguez, M.; Paschen, A.; Yáñez-Mó, M.; Blanco-López, M.C.; Valés-Gómez, M. Immunoassays for scarce tumour-antigens in exosomes: Detection of the human NKG2D-Ligand, MICA, in tetraspanin-containing nanovesicles from melanoma. J. Nanobiotechnol. 2018, 16, 1–12.
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978.
More
