Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Kalman Imre.
Cancer is one of the major deadly diseases globally. The alarming rise in the mortality rate due to this disease attracks attention towards discovering potent anticancer agents to overcome its mortality rate. Based on their particular activity, a number of other plant-derived bioactive compounds are in the clinical development phase against cancer, such as gimatecan, elomotecan, etc. Additionally, the conjugation of natural compounds with anti-cancerous drugs, or some polymeric carriers particularly targeted to epitopes on the site of interest to tumors, can generate effective targeted treatment therapies.
- natural products
- anticancer drugs
- medicinal plants
1. Introduction
Cancer is the anomalous growth of cells in the body; it is the leading cause of death and is also known as the biggest public health burden [1]. Cancer cells can also attack and damage the body’s normal cells [2]. Millions of people have died due to four common types of cancers every year, including breast, lung, prostate, and rectum/colon cancer with an unknown etiology. The present tenet indicates a conspicuous difference between cancer chemotherapy and chemoprevention. Cancer chemotherapy is the control of the developed disease, while cancer chemoprevention is the phenomenon of a carcinogenesis intervention by blocking the agents of the induction of the neoplastic process or averting the processing of transformed cells to the malignant phenotype using suppressing agents. Cancer chemoprevention may also implicate the reversal of the progression of cancer cells [3].
The investigation of anticancer agents through natural sources dates back to about 1550 BC. However, the scientific exploration of this research is very recent and originated in the 1950s with the generation of majorly found plant-derived anticancer agents, including vinca alkaloid analogs, camptothecin derivatives, podophyllotoxin derivatives, and taxol semi-synthetic analogs which are clinically helpful anticancer therapeutic drugs (Figure 1) [4,5][4][5]. Over 180,000 microbial-derived anticancer agents, 16,000 marine-derived organisms, and 114,000 plant-derived compounds were screened by the US National Cancer Institute (NCI) for their anti-cancerous activity from the 1960s to the 1980s [6]. Plant-based drug development also provided a platform for synthesizing efficient and safe anti-tumor drugs through the complete cognizance of a synergistic relation between numerous components of anti-tumor herbs [7,8][7][8]. According to the WHOs estimation, approximately 80% of African and Asian countries rely on traditional medicines for fundamental health care. A neoteric study shows that approximately more than 60% of patients use herbs or vitamins as cancer therapy [9,10][9][10]. Herbal remedies are among the most favored form of traditional medicine and are tremendously profit-making at the international commercial level. By the 2050s, the worldwide herbal medicine market is expected to hit USD 5 trillion [11].
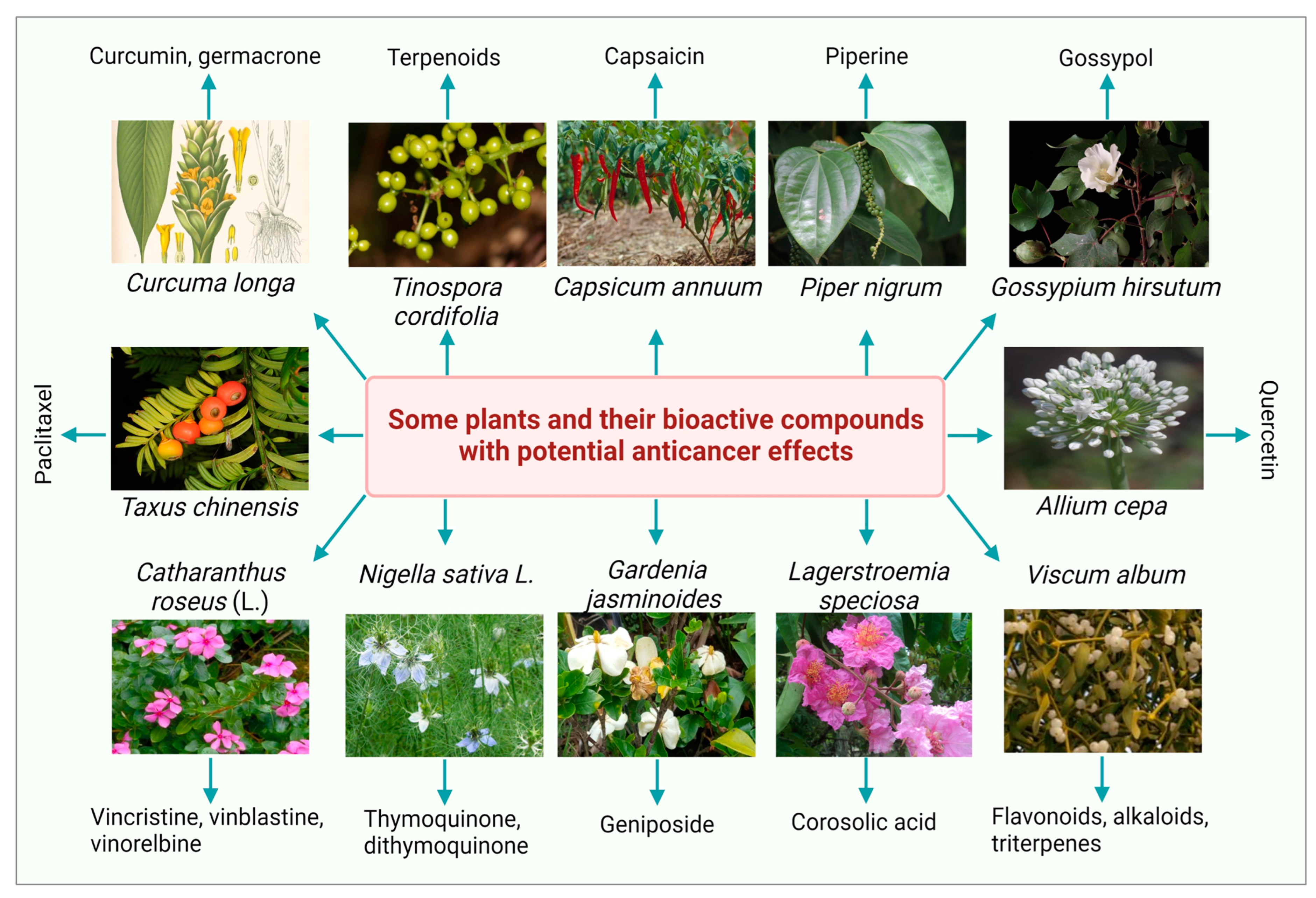
Figure 1.
Some medicinal plants and their bioactive compounds having potential anticancer properties.
Natural products provide a sustainable source with a considerable efficacy to treat and overcome several disorders and fatal diseases, including cancer. In the last time period, the role of the bioactive compound and natural products, as a source of anticancer drugs, has been marked within a collaborative, integrated, and multidisciplinary approach. Plants have long been known for having medicinal effects since aeon [12,13,14,15,16][12][13][14][15][16]. More than 50% of modern clinical drugs are of a natural source origin and have the capability to treat cancer cells [17]. A neoteric study shows that approximately more than 60% of patients use herbs or vitamins as cancer therapy. The ability of natural sources as anticancer agents were identified in the 1950s by the US National Cancer Institute and contributed to finding new naturally existing anti-tumor agents [18]. Plant-based drug development needs a specific production strategy with optimized environmental conditions and nutrient availability. In 1998, Sohn et al. estimated that an extraction from 10,000 kg of the bark of yew trees is required to produce 1 kg of taxol. The production of 25 kg of taxol required 38,000 yew trees for the treatment of 12,000 cancer patients [19]. The plant collection for finding anticancer agents ended in 1982, but in 1986, the generation of new screening strategies led to the amelioration of plants and the collection of other organisms mainly focused on the sub-tropical and tropical zones of the world. Hartwell listed more than 3000 plants in his review against cancer treatment [20]. Various anti-cancerous drugs are available to treat cancer, but they also exhibit toxic effects that limit their use [18,21][18][21]. Because of the severe side effects of radiotherapy and chemotherapy and the high mortality rate, recent research revolves around the need to design appropriate chemotherapy for cancer treatment without side effects [21,22][21][22]. Biodiversity has been determined to be a significant source of remarkable anticancer agents until now [23,24,25,26,27,28][23][24][25][26][27][28].
A significant investigation is devoted to finding more effective treatments with minimum undesirable toxic effects. However, many anti-tumor agents exhibit a restricted therapeutic window due to a lack of specificity of against cancer cells [29,30][29][30]. The ultimate objective of a cancer treatment is the generation of safe and effective drugs that can particularly kill malignant cancer cells or make them benign cancer cells without killing normal cells [31].
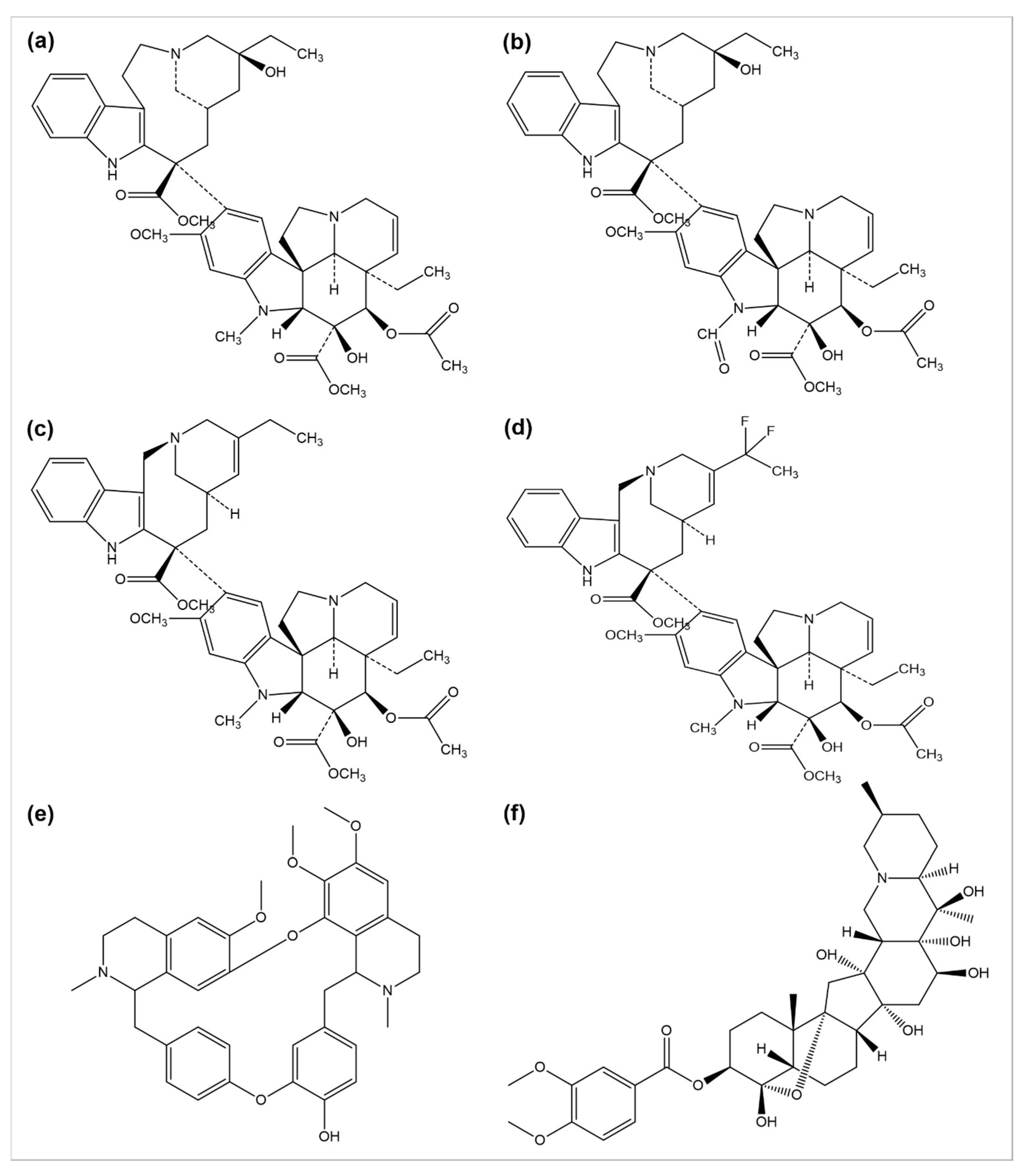 A number of semi-synthetic analogs of these two alkaloid drugs have been produced. Vindestine was produced by the replacement of the C acetyl group with an amino group in vinblastine [95][35], primarily applied for the treatment of acute lymphocytic leukemia (ALL) and rarely prescribed for chronic myelocytic leukemia (CML), breast cancer, non-small cell lungs cancer (NSCLC), colorectal cancer, and renal cancer treatment. Vinorelbine (also known as navelbine) is another semi-synthetic analog of vinblastine synthesized by shortening one carbon from the indole ring linking the bridge to piperidine nitrogen, resulting in a water elimination from the piperidine ring, and was approved in 1989 in France for the treatment of NSCLC under the brand name Navelbine. Vinflunine, a dihydrofluoro semi-synthetic analog of vinorelbine, is used as the second line of treatment in metastatic urothelial cancer. It was approved in 2009 by the European medical agency [96,97][36][37]. Alike other semi-synthetic analogs of vinca alkaloids, vinflunine also attaches to tubulin molecules resulting in the inhibition of microtubule polymerization and the formation of tubulins para crystals [98,99,100,101][38][39][40][41].
Cao et al. investigated the anticancer effects of 13 isoquinoline alkaloids extracted from Hylomecon japonica on MCF-7 breast cancer cells. Among these 13 alkaloids, 6,10-dimethoxydihydrochelerythrine, 6S/R-acroleinyl-dihydrochelerythrine, 9-methoxy-10-hydroxy-norchelerythrine, 10-methoxy boconoline, 6-methoxydihydrosanguinarine, dihydrosanguinaline, and 6-acetaldehyde-dihydrochelerythrine exhibited a significant inhibitory potential with an IC50 of ˂20 μM on MCF-7 cells [102][42]. Freeling et al. determined the tumor suppression potential of the plant-based alkaloid veratridine (VTD). VTD activates the expression of UBXN2A (an anti-tumor protein) by deactivating a dominant protein, mortalin, involved in the development of cancer [103][43]. Liu et al. evaluated the antiproliferative and anti-migratory effect of the alkaloid berbamine. Berbamine suppressed the growth of negative breast cancer cells by regulating the PI3K/Akt/mTOR and PI3K/Akt/MDM2/p53 pathways [104][44]. Esnaashari et al. investigated the synergistic effect of the alkaloid doxorubicin (DOX) with noscapine-loaded polymeric nanoparticles (NOS-NPs) for breast cancer treatment. The anticancer potential of NOS-NPs combined with DOX and alone was evaluated against 4T1 breast cancer cells (in vitro) and mice (in vivo). The NOS-NPs, in combination with DOX, significantly showed a 68.50% inhibition against the growth of breast cancer. The DOX and NOS-NPs alone exhibited a 32 to 55.10% inhibition, respectively [105][45].
A number of semi-synthetic analogs of these two alkaloid drugs have been produced. Vindestine was produced by the replacement of the C acetyl group with an amino group in vinblastine [95][35], primarily applied for the treatment of acute lymphocytic leukemia (ALL) and rarely prescribed for chronic myelocytic leukemia (CML), breast cancer, non-small cell lungs cancer (NSCLC), colorectal cancer, and renal cancer treatment. Vinorelbine (also known as navelbine) is another semi-synthetic analog of vinblastine synthesized by shortening one carbon from the indole ring linking the bridge to piperidine nitrogen, resulting in a water elimination from the piperidine ring, and was approved in 1989 in France for the treatment of NSCLC under the brand name Navelbine. Vinflunine, a dihydrofluoro semi-synthetic analog of vinorelbine, is used as the second line of treatment in metastatic urothelial cancer. It was approved in 2009 by the European medical agency [96,97][36][37]. Alike other semi-synthetic analogs of vinca alkaloids, vinflunine also attaches to tubulin molecules resulting in the inhibition of microtubule polymerization and the formation of tubulins para crystals [98,99,100,101][38][39][40][41].
Cao et al. investigated the anticancer effects of 13 isoquinoline alkaloids extracted from Hylomecon japonica on MCF-7 breast cancer cells. Among these 13 alkaloids, 6,10-dimethoxydihydrochelerythrine, 6S/R-acroleinyl-dihydrochelerythrine, 9-methoxy-10-hydroxy-norchelerythrine, 10-methoxy boconoline, 6-methoxydihydrosanguinarine, dihydrosanguinaline, and 6-acetaldehyde-dihydrochelerythrine exhibited a significant inhibitory potential with an IC50 of ˂20 μM on MCF-7 cells [102][42]. Freeling et al. determined the tumor suppression potential of the plant-based alkaloid veratridine (VTD). VTD activates the expression of UBXN2A (an anti-tumor protein) by deactivating a dominant protein, mortalin, involved in the development of cancer [103][43]. Liu et al. evaluated the antiproliferative and anti-migratory effect of the alkaloid berbamine. Berbamine suppressed the growth of negative breast cancer cells by regulating the PI3K/Akt/mTOR and PI3K/Akt/MDM2/p53 pathways [104][44]. Esnaashari et al. investigated the synergistic effect of the alkaloid doxorubicin (DOX) with noscapine-loaded polymeric nanoparticles (NOS-NPs) for breast cancer treatment. The anticancer potential of NOS-NPs combined with DOX and alone was evaluated against 4T1 breast cancer cells (in vitro) and mice (in vivo). The NOS-NPs, in combination with DOX, significantly showed a 68.50% inhibition against the growth of breast cancer. The DOX and NOS-NPs alone exhibited a 32 to 55.10% inhibition, respectively [105][45].
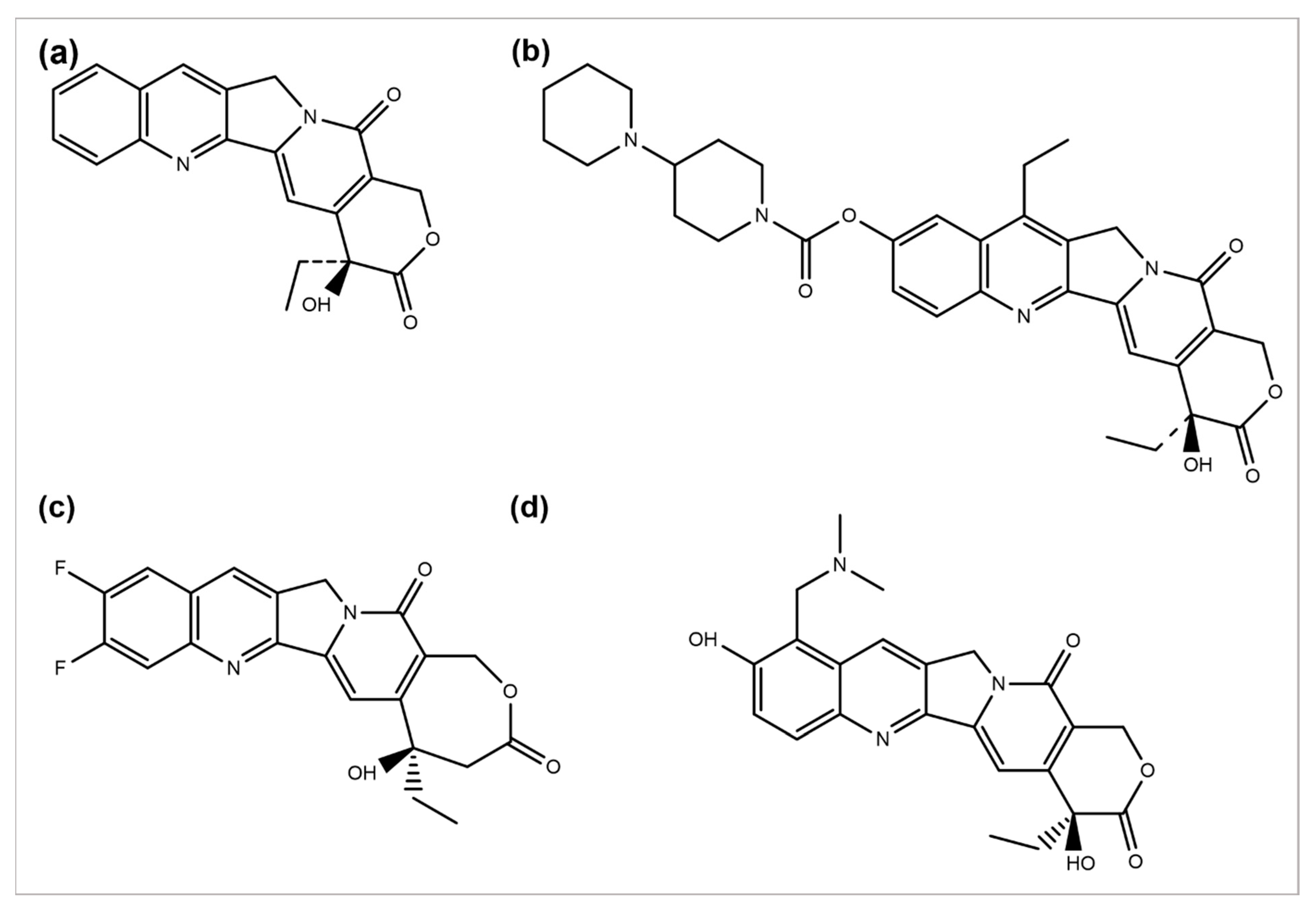 In 1993, 9-AC entered in phase-I trials and revealed the dose-dependent phenomenon of myelosuppression as a major toxic effect of the respective drug. Subsequently, in phase-II trials, the drug was found to be active against malignant and ovarian lymphoma and inactive against colon or lung cancer. Consequently, in 1999, it was terminated from any further development [108][48]. However, some phase-I or II trials have been reviewed to predict its efficacy, safety, and tolerability separately or in combination with some other analogs [109][49]. Several drugs such as diflomotecan (for advanced solid tumors treatment at phase I) (Figure 2c) [110][50], gimatecan (for advanced solid tumors treatment at phase I) [111][51], (for recurrent ovarian, peritoneal, or fallopian tumor treatment at phase II) [112][52], elomotecan (for advanced solid tumors treatment at phase I) [113][53], and EZN-2208 (for advanced malignancies treatment) [114][54] have been reported as clinical trial-based studies.
In 1993, 9-AC entered in phase-I trials and revealed the dose-dependent phenomenon of myelosuppression as a major toxic effect of the respective drug. Subsequently, in phase-II trials, the drug was found to be active against malignant and ovarian lymphoma and inactive against colon or lung cancer. Consequently, in 1999, it was terminated from any further development [108][48]. However, some phase-I or II trials have been reviewed to predict its efficacy, safety, and tolerability separately or in combination with some other analogs [109][49]. Several drugs such as diflomotecan (for advanced solid tumors treatment at phase I) (Figure 2c) [110][50], gimatecan (for advanced solid tumors treatment at phase I) [111][51], (for recurrent ovarian, peritoneal, or fallopian tumor treatment at phase II) [112][52], elomotecan (for advanced solid tumors treatment at phase I) [113][53], and EZN-2208 (for advanced malignancies treatment) [114][54] have been reported as clinical trial-based studies.
2. Plant-Derived Bioactive Compounds as Anti-Cancerous Agents
Over the last decade, several researchers have investigated the ethnopharmacological and ethnomedicinal properties of numerous plant-derived bioactive compounds and, recently, their antimicrobial and antibiofilm activities [35][32]. Several in vitro and in vivo experimental investigations revealed the therapeutic significance of numerous phytochemicals. Some photos of the most studied plants with a significant anticancer potential with their bioactive compounds are presented in Figure 1. The most common plant-derived anti-cancerous agents include vinca alkaloids and their derivatives, camptothecin and its derivatives, podophyllotoxin and its semi-synthetic analogs, and terpenes.2.1. Vinca Alkaloids and Their Derivatives
The use of plants as anticancer agents was established with two alkaloids’ isolation, vincristine, and vinblastine, using Catharanthus roseus and Madagascar periwinkle [93][33]. These drugs have been clinically used in oncology for about 50 years. They perform their function by blocking the polymerization phenomenon of tubulin molecules, averting the mitotic spindle formation, and resulting in apoptosis or metaphase arrest [94][34]. Several anticancer drugs, such as vincristine, vinblastine, vinorelbine, vinflunine, veratridine, and berbamine, are plant-derived natural alkaloids (Figure 2).
Figure 2. Chemical structures of (a) vinblastine, (b) vincristine, (c) vinorelbine, (d) vinflunine, (e) berbamine, and (f) veratridine.
2.2. Camptothecin and Its Derivatives
Camptotheca acuminata plant species are a source of the anticancer agent camptothecin (CPT), a quinoline alkaloid that acts by inhibiting the activity of topoisomerase-I, causing DNA damage and, ultimately, cell death [106][46]. Because of its severe toxicity and low aqueous solubility, it was terminated from clinical trials. Several CPT derivatives are developed and approved for clinical use to combat these limitations. Some of the CPT derivatives are irinotecan, belotecan, and topotecan, which actively inhibit DNA topoisomerase–I, an enzyme involved in DNA replication and transcription (Figure 3a,b,d) [107][47]. 9-aminocamptothecin (9-AC) is another CPT semi-synthetic derivative that exhibited a sound activity effect pre-clinical analysis but has not shown clinically effective anticancer activity hitherto.
Figure 3.
Chemical structures of (
a
) camptothecin, (
b
) irinotecan, (
c
) diflomotecan, and (
d
) topotecan.
2.3. Podophyllotoxin and Its Semi-Synthetic Analogs
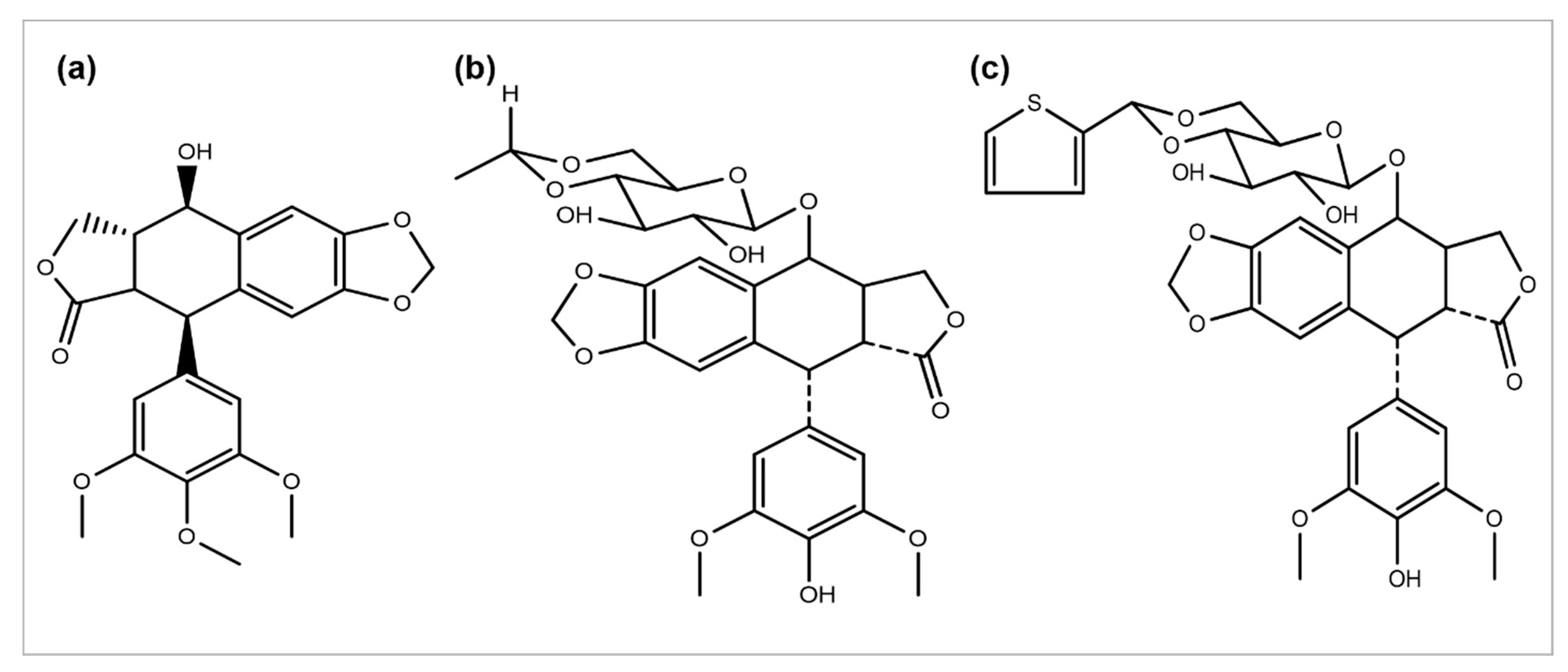
Podophyllum peltatum plant is an important source of the anticancer compound Podophyllotoxin and has two key analogs, Teniposide and Etoposide (Figure 4) [115][55], which are useful in the treatment of different types of cancer acts by inhibiting the function of the topoisomerase II enzyme [5]. The above two analogs combat some problems and issues, such as a metabolic inactivation, poor water solubility, and acquired drug resistance. The improved efficacy and potency led to the development of some semi-synthetic derivatives, including azatoxin, NK-611, Top-53, tafluposide, GL-331, and etoposide phosphate, either as clinical drugs or new trial candidates for cancer treatment [116][56].
Figure 4.
Chemical structures of (
a
) podophyllotoxin, (
b
) etoposide, and (
c
) teniposide.
2.4. Taxane Diterpenoids
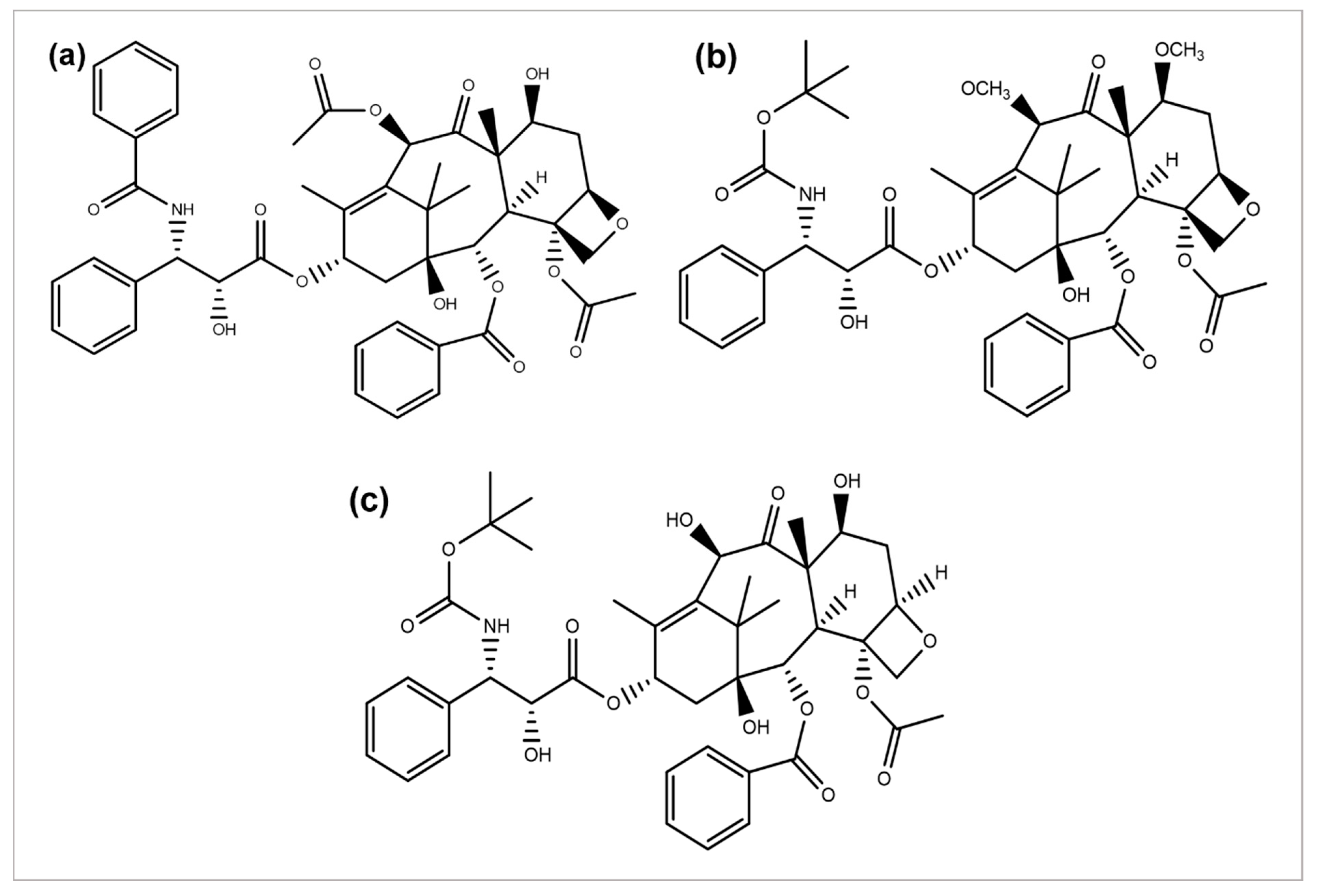
Paclitaxel discovery from the bark extract of the Yew tree further provided evidence for a successful drug discovery through natural products. Taxol was the first compound discovered for a microtubule synthesis promotion. It has been known to be used in treating several types of cancers, particularly breast, ovarian, and NSCLC [117][57]. A wide range of its derivatives has been produced (Figure 5). Docetaxel was the first to be clinically used with significant clinical activity against different tumors [118,119][58][59]. Both of the authorized taxane drugs, paclitaxel and docetaxel, still have limitations of use, and the researchers are trying to overcome their side effects by synthesizing the modified derivatives. Alterations in their structures has led to the discovery of new agents with a diminished toxicity, enhanced solubility, and refined cytotoxicity. The restricted ability of docetaxel and paclitaxel to cross the blood–brain barrier is concluded to be generated by the P-glycoprotein efflux pump tremendously expressed in the BBB [120,121,122][60][61][62]. In 2010, another FDA-approved taxane derivative, Cabazitaxel, was established in combination with prednisone for treating hormone-refractory prostate and prostate cancers. Cabazitaxel suppresses the proliferation of cancer cells by stabilizing tubulin and inhibiting the depolymerization of microtubules [123][63]. Nanoparticle formulations are also being applied to obtain better results. Abraxane is the albumin-bound nanoparticle-based formulation of paclitaxel free of any solvent, which acts as a mitotic inhibitor, and shows that it can have dramatically improved effects. New taxanes are also being developed to improve the therapeutic effect and pharmacology and replace docetaxel and paclitaxel which are currently used for the treatment of NSCLC [124][64].
Figure 5.
Chemical structures of (
a
) paclitaxel, (
b
) cabazitaxel, and (
c
) docetaxel.
References
- Tan, G.; Gyllenhaal, C.; Soejarto, D. Biodiversity as a source of anticancer drugs. Curr. Drug Targets 2006, 7, 265–277.
- De Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009, 123, 439–445.
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077.
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59.
- Srivastava, V.; Negi, A.S.; Kumar, J.; Gupta, M.; Khanuja, S.P. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–5908.
- Newman, D.J.; Cragg, G.M. The discovery of anticancer drugs from natural sources. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2005; pp. 129–168.
- Larkin, T. Herbs are often more toxic than magical. FDA Consumers 1983, 17, 4–10.
- Saxe, T. Toxicity of medicinal herbal preparations. Am. Fam. Physician 1987, 35, 135–142.
- Madhuri, S.; Pandey, G. Some dietary agricultural plants with anticancer properties. Plant Arch. 2008, 8, 13–16.
- Sivalokanathan, S.; Ilayaraja, M.; Balasubramanian, M. Efficacy of Terminalia arjuna (Roxb.) on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Indian J. Exp. Biol. 2005, 43, 264–267.
- Chaudhary, A.; Singh, N. Contribution of world health organization in the global acceptance of Ayurveda. J. Ayurveda Integr. Med. 2011, 2, 176–186.
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258.
- Semeniuc, C.A.; Socaciu, M.-I.; Socaci, S.A.; Mureșan, V.; Fogarasi, M.; Rotar, A.M. Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules 2018, 23, 2261.
- Pengelly, A.; Bone, K. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine; Routledge: Abingdon, UK, 2020.
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998, 62, 183–193.
- Singla, R.K.; De, R.; Efferth, T.; Mezzetti, B.; Sahab Uddin, M.d.; Sanusi; Ntie-Kang, F.; Wang, D.; Schultz, F.; Kharat, K.R.; et al. The International Natural Product Sciences Taskforce (INPST) and the power of Twitter networking exemplified through #INPST Hashtag Analysis. Phytomedicine 2023, 108, 154520.
- Rosangkima, G.; Prasad, S. Antitumour activity of some plants from Meghalaya and Mizoram against murine ascites Dalton's lymphoma. Indian J. Exp. Biol. 2004, 42, 981–988.
- Cragg, G.M.; Newman, D.J. Plants as a source of anticancer agents. J. Ethnopharmacol. 2005, 100, 72–79.
- Sohn, H.; Okos, M.R. Paclitaxel (taxol): From nutt to drug. J. Microbiol. Biotechnol. 1998, 8, 427–440.
- Kaur, R.; Kapoor, K.; Kaur, H. Plants as a source of anticancer agents. J. Nat. Prod. Plant Resour. 2011, 1, 119–124.
- Fouché, G.; Cragg, G.; Pillay, P.; Kolesnikova, N.; Maharaj, V.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461.
- Georgaki, S.; Skopeliti, M.; Tsiatas, M.; Nicolaou, K.A.; Ioannou, K.; Husband, A.; Bamias, A.; Dimopoulos, M.A.; Constantinou, A.I.; Tsitsilonis, O.E. Phenoxodiol, an anticancer isoflavene, induces immunomodulatory effects in vitro and in vivo. J. Cell Mol. Med. 2009, 13, 3929–3938.
- Kinghorn, A.; Farnsworth, N.; Beecher, C.; Soejarto, D.; Cordell, G.; Pezzuto, J.; Wall, M.; Wani, M.; Brown, D.; O'neill, M.M.; et al. Novel strategies for plant-derived anticancer agents. Int. J. Pharmacogn. 1995, 33, 48–58.
- Kinghorn, A.D.; Su, B.-N.; Jang, D.S.; Chang, L.C.; Lee, D.; Gu, J.-Q.; Carcache-Blanco, E.J.; Pawlus, A.D.; Lee, S.K.; Park, E.J. Natural inhibitors of carcinogenesis. Planta Med. 2004, 70, 691–705.
- Powis, G. Anticancer Drugs: Antimetabolite Metabolism and Natural Anticancer Agents, 1st ed.; Pergamon: Oxford, UK, 1994; Section 140.
- Yun, T.K. Update from Asia: Asian studies on cancer chemoprevention. Ann. N. Y. Acad. Sci. 1999, 889, 157–192.
- Kamath, K.; Park, K. Mucosal adhesive preparations. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1988; Volume 10, p. 133.
- Sagini, K.; Urbanelli, L.; Buratta, S.; Leonardi, L.; Emiliani, C. Nanovesicles from plants as edible carriers of bioactive compounds. AgroLife Sci. J. 2017, 6, 167–171.
- Shengquan, L.; Ngong, H.S. Design of low-molecular-weight prodrugs for targeted delivery of anticancer agents. In Proceedings of the 3rd International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, Chicago, IL, USA, 8–10 April 2013.
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. ChemMedChem 2008, 3, 20–53.
- Sporn, M.B.; Liby, K.T. Cancer chemoprevention: Scientific promise, clinical uncertainty. Nat. Clin. Pract. Oncol. 2005, 2, 518–525.
- Ertas Onmaz, N.; Demirezen Yilmaz, D.; Imre, K.; Morar, A.; Gungor, C.; Yilmaz, S.; Gundog, D.A.; Dishan, A.; Herman, V.; Gungor, G. Green synthesis of gold nanoflowers using Rosmarinus officinalis and Helichrysum italicum extracts: Comparative studies of their antimicrobial and antibiofilm activities. Antibiotics 2022, 11, 1466.
- Verma, A.; Singh, R. Induced dwarf mutant in Catharanthus roseus with enhanced antibacterial activity. Indian J. Pharm. Sci. 2010, 72, 655–657.
- Jordan, M.A.; Thrower, D.; Wilson, L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991, 51, 2212–2222.
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265.
- Mamtani, R.; Vaughn, D.J. Vinflunine in the treatment of advanced bladder cancer. Expert Rev. Anticancer Ther. 2011, 11, 13–20.
- Bachner, M.; De Santis, M. Vinflunine in the treatment of bladder cancer. Ther. Clin. Risk Manag. 2008, 4, 1243–1253.
- Bennouna, J.; Delord, J.-P.; Campone, M.; Nguyen, L. Vinflunine: A new microtubule inhibitor agent. Clin. Cancer Res. 2008, 14, 1625–1632.
- Kruczynski, A.; Barret, J.-M.; Etiévant, C.; Colpaert, F.; Fahy, J.; Hill, B.T. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem. Pharmacol. 1998, 55, 635–648.
- Pourroy, B.; Carré, M.; Honoré, S.; Bourgarel-Rey, V.; Kruczynski, A.; Briand, C.; Braguer, D. Low concentrations of vinflunine induce apoptosis in human SK-N-SH neuroblastoma cells through a postmitotic G1 arrest and a mitochondrial pathway. Mol. Pharmacol. 2004, 66, 580–591.
- Simoens, C.; Vermorken, J.B.; Korst, A.E.; Pauwels, B.; De Pooter, C.M.; Pattyn, G.G.; Lambrechts, H.A.; Breillout, F.; Lardon, F. Cell cycle effects of vinflunine, the most recent promising Vinca alkaloid, and its interaction with radiation, in vitro. Cancer Chemother. Pharmacol. 2006, 58, 210–218.
- Cao, Z.; Zhu, S.; Xue, Z.; Zhang, F.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhan, G.; Zhang, X.; Guo, Z. Isoquinoline alkaloids from Hylomecon japonica and their potential anti-breast cancer activities. Phytochemistry 2022, 202, 113321.
- Freeling, J.L.; Scholl, J.L.; Eikanger, M.; Knoblich, C.; Potts, R.A.; Anderson, D.J.; Rower, J.E.; Farjoo, M.H.; Zhao, H.; Pillatzki, A. Pre-clinical safety and therapeutic efficacy of a plant-based alkaloid in a human colon cancer xenograft model. Cell Death Discov. 2022, 8, 135.
- Liu, L.; Yan, J.; Cao, Y.; Yan, Y.; Shen, X.; Yu, B.; Tao, L.; Wang, S. Proliferation, migration and invasion of triple negative breast cancer cells are suppressed by berbamine via the PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. Oncol. Lett. 2021, 21, 70.
- Esnaashari, S.S.; Muhammadnejad, S.; Amanpour, S.; Amani, A. A combinational approach towards treatment of breast cancer: An analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech 2020, 21, 166.
- Hsiang, Y.-H.; Hertzberg, R.; Hecht, S.; Liu, L. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878.
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135.
- Pazdur, R.; Diaz-Canton, E.; Ballard, W.P.; Bradof, J.E.; Graham, S.; Arbuck, S.G.; Abbruzzese, J.L.; Winn, R. Phase II trial of 9-aminocamptothecin administered as a 72-hour continuous infusion in metastatic colorectal carcinoma. J. Clin. Oncol. 1997, 15, 2905–2909.
- Farray, D.; Ahluwalia, M.S.; Snyder, J.; Barnett, G.H.; Cohen, B.H.; Suh, J.H.; Peereboom, D.M. Pre-irradiation 9-amino camptothecin (9-AC) in patients with newly diagnosed glioblastoma multiforme. Invest. New Drugs. 2006, 24, 177–180.
- Scott, L.; Soepenberg, O.; Verweij, J.; de Jonge, M.; Th Planting, A.; McGovern, D.; Principe, P.; Obach, R.; Twelves, C. A multicentre phase I and pharmacokinetic study of BN80915 (diflomotecan) administered daily as a 20-min intravenous infusion for 5 days every 3 weeks to patients with advanced solid tumours. Ann. Oncol. 2007, 18, 569–575.
- Zhu, A.X.; Ready, N.; Clark, J.W.; Safran, H.; Amato, A.; Salem, N.; Pace, S.; He, X.; Zvereva, N.; Lynch, T.J. Phase I and pharmacokinetic study of gimatecan given orally once a week for 3 of 4 weeks in patients with advanced solid tumors. Clin. Cancer Res. 2009, 15, 374–381.
- Pecorelli, S.; Ray-Coquard, I.; Tredan, O.; Colombo, N.; Parma, G.; Tisi, G.; Katsaros, D.; Lhomme, C.; Lissoni, A.; Vermorken, J. Phase II of oral gimatecan in patients with recurrent epithelial ovarian, fallopian tube or peritoneal cancer, previously treated with platinum and taxanes. Ann. Oncol. 2009, 21, 759–765.
- Trocóniz, I.F.; Cendrós, J.-M.; Soto, E.; Pruñonosa, J.; Perez-Mayoral, A.; Peraire, C.; Principe, P.; Delavault, P.; Cvitkovic, F.; Lesimple, T. Population pharmacokinetic/pharmacodynamic modeling of drug-induced adverse effects of a novel homocamptothecin analog, elomotecan (BN80927), in a Phase I dose finding study in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 239–250.
- Kurzrock, R.; Goel, S.; Wheler, J.; Hong, D.; Fu, S.; Rezai, K.; Morgan-Linnell, S.K.; Urien, S.; Mani, S.; Chaudhary, I. Safety, pharmacokinetics, and activity of EZN-2208, a novel conjugate of polyethylene glycol and SN38, in patients with advanced malignancies. Cancer 2012, 118, 6144–6151.
- Imbert, T. Discovery of podophyllotoxins. Biochimie 1998, 80, 207–222.
- Sargent, J.M.; Elgie, A.W.; Williamson, C.J.; Hill, B.T. Ex vivo effects of the dual topoisomerase inhibitor tafluposide (F 11782) on cells isolated from fresh tumor samples taken from patients with cancer. Anti Cancer Drugs 2003, 14, 467–473.
- Kinghorn, A.D.; Seo, E.-K. Plants as sources of drugs. In Agricultural Materials as Renewable Resources; Fuller, G., McKeon, T.A., Bills, D.D., Eds.; ACS Publications: Washington, DC, USA, 1996; Volume 647, pp. 179–193.
- Gelmon, K. The taxoids: Paclitaxel and docetaxel. The Lancet 1994, 344, 1267–1272.
- Bissery, M.-C.; Nohynek, G.; Sanderink, G.-J.; Lavelle, F. Docetaxel (Taxotere): A review of preclinical and clinical experience. Part I: Preclinical experience. Anticancer Drugs 1995, 6, 339–355, 363–368.
- Beaulieu, E.; Demeule, M.; Ghitescu, L.; Béliveau, R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 1997, 326, 539–544.
- Fellner, S.; Bauer, B.; Miller, D.S.; Schaffrik, M.; Fankhänel, M.; Spruß, T.; Bernhardt, G.; Graeff, C.; Färber, L.; Gschaidmeier, H. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Invest. 2002, 110, 1309–1318.
- Kemper, E.M.; van Zandbergen, A.E.; Cleypool, C.; Mos, H.A.; Boogerd, W.; Beijnen, J.H.; van Tellingen, O. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin. Cancer Res. 2003, 9, 2849–2855.
- Paller, C.J.; Antonarakis, E.S. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011, 5, 117–124.
- Bao, R.; Chan, P. Novel compounds in the treatment of lung cancer: Current and developing therapeutic agents. J. Exp. Pharmacol. 2011, 3, 21–34.
More
