Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Yong Huang.
The development of local percutaneous drug delivery systems can reduce the side effects of sinomenine (SIN) and enhance patient compliance. Many percutaneous drug delivery systems have thus been developed, including liposomes, hydrogels, and microneedles. In addition, some equipment-assisted percutaneous drug delivery methods were also used, such as electroporation and dual-frequency ultrasound.
- rheumatoid arthritis
- sinomenine
- half-life
- side effect
1. Liposomes
Liposomes have shown excellent biocompatibility and are often used for transdermal drug delivery. The local delivery of liposome-encapsulated SIN may enrich SIN in the target site and thus reduce the side effects caused by systemic absorption. Conventional liposomes prepared with soybean phospholipid, cholesterol, and vitamin E can be used to encapsulate SIN, while they still suffer limited permeability [49,50][1][2]. Ethosomes are a new type of liposomes containing a high concentration of alcohol inside [51][3], which would effectively deliver SIN through the stratum corneum to the deeper layers of skin, and even into the blood circulation [52][4]. The optimized SIN-loaded ethosomes (SE) with negative charges and diameter of 157.08 nm were prepared. The skin penetration and deposition of SIN in ethosomes were 663.8 and 18.5 μg/cm2 within 24 h, while those of SIN in ethanol-water solution were only 329.2 and 5.2 μg/cm2, respectively. These results indicated that SE could significantly improve the transdermal property of SIN. Skin irritation tests showed that ethosomes caused no skin rash and edema in rabbits within 72 h, suggesting excellent biocompatibility. In a xylene-induced mouse ear swelling model, SIN encapsulated ethosome had an inhibition rate of 30.01% in ear swelling, significantly higher than that treated by SIN-HCl ethanol-water solution (20.83%). Transfersomes (TFSs) are a class of elastic liposomes composed of phospholipids and edge activators; the addition of edge activators could interfere and deform the phospholipid bilayers of vesicles and lead to their deformability [53,54][5][6]. The resulting flexible membranes enable TFSs to be transported through the skin and bypass the cuticle barrier, thereby increasing drug deposition in the skin and prolonging the duration of effective drug concentration [55][7].
SIN-HCl TFSs were prepared using sodium deoxycholate as the edge activator, and SIN-HCl liposomes were used as a control [56][8]. The in vitro permeation experiment showed that the cumulative transdermal permeated amount of SIN-HCl from SIN-HCl TFSs was 1.7 times higher than that from SIN-HCl liposomes at 36 h. SIN-HCl TFSs showed about 8.8 and 8.0 times of in vivo steady-state blood concentration (Css) and the area under the drug concentration–time curve from time zero to t (AUC0–t) compared to those of SIN-HCl liposomes in a skin pharmacokinetic investigation. In blood pharmacokinetic tests, SIN-HCl TFSs exhibited 3.7 and 2.9 times of Css and AUC0–t compared to that of the control group. These data suggest that TFSs could effectively improve the transdermal absorption of SIN-HCl. A mixture of monoterpene edge-activated PEGylated transfersomes (MMPTs) was also used to improve the in vivo transdermal delivery efficiency of SIN [41][9] (Figure 31A). In the in vitro skin penetration test, the cumulative skin penetration of SIN in the optimized formulation TFSs3 was 1.5 and 3 times of those of sodium deoxycholate edge activated transfersomes (DTFS) and ordinary liposomes, respectively. Pharmacokinetic evaluation in rats revealed that the equilibrium concentration and area under the curve of SIN encapsulated in TFSs3 were 8.7 and 8.2 times of those in ordinary liposome, respectively, indicating more effective skin penetration of SIN. Confocal laser scanning microscopy and double-sited microdialysis coupled with LC-MS/MS were used to reveal the biodistribution of MMPTs in different cortex and the pharmacokinetic properties of SIN in blood and joint cavity, as well as intrinsic mechanisms of the local transdermal delivery [57][10]. The control liposomes only reached to the cuticle, while MMPTs reached to the deep cortex. The equilibrium concentration and area under the curve of SIN delivered by MMPTs in articular cavity were 2.1 and 2.5 times higher than that of control liposomes, respectively, while only about one third compared to that of control liposomes in blood. Taken together, MMPTs could efficiently deliver SIN into the deep cortex and subsequently be enriched in the joint cavity. Further delivery of SIN using the combination of the TFSs and ethanosomes resulted in a transsethosome (TE) for transdermal administration of SIN, featuring even more effectively delivery of SIN through the skin [42][11]. The surface of TE was also modified with the antioxidant ascorbic acid (AS-TE) to rebalance reactive oxygen species in the inflammatory microenvironment and achieve targeted drug delivery [58][12]. The TE and AS-TE had similar transdermal effects, which were about 7.6 times as large as that of SIN-HCl aqueous solution. Subsequent micro-dialysis on synovial fluid showed that the AS-TE group exhibited higher drug concentration in the synovial fluid of rabbits with RA than the TE group, indicating its excellent targeting ability to inflammatory microenvironment. In a rat RA model, AS-TE encapsulated SIN showed better therapeutic effect, which significantly reduced the symptoms of joint swelling after three weeks of treatment.
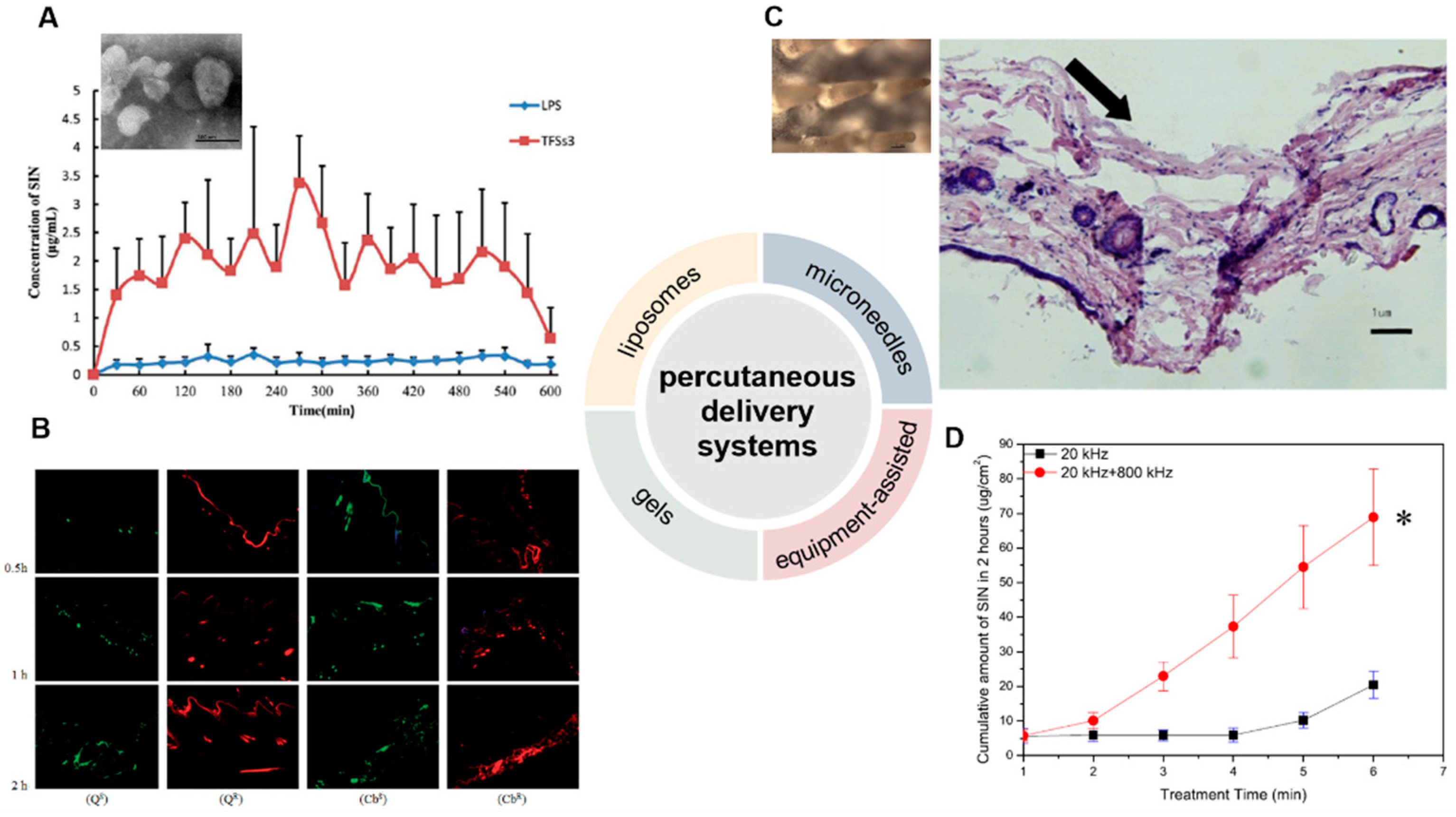
Figure 31. Development of percutaneous delivery systems of SIN. (A) The transmission electron microscope of monoterpene edge activated PEGylated transfersomes (×20,000) and the release of SIN after its application to the abdominal skin of rats (n = 5) [41][9]. (B) laser scanning confocal microscopy images of skin samples for sodium fluorescein-loaded cubic LC gel (QS), rhodamine B-loaded cubic LC gel (QR), sodium fluorescein-loaded carbomer gel (CbS), and rhodamine B-loaded carbomer gel (CbR) in Franz cells during different periods (×100) [44][13]. (C) The close-up views of microneedles and the methylene blue-stained frozen section of rat’s abdominal skins treated by microneedles [45][14]. The arrow means the pierced position. (D) Cumulative release of SIN under ultrasound with single- or dual-frequency treatment [48][15]. (Reprinted with permission from Refs. [41,44,45,48][9][13][14][15]).
2. Gels
Gels belong to semi-solid materials with three-dimensional network structures and good biocompatibility, and are widely used in drugs, e.g., piroxicam gel, terbinafine hydrochloride gel, and ofloxacin gel, and various cosmetics [59][16]. An optimized pluronic lecithin organogel (PLO)-based SIN formulation was prepared, which showed a SIN infiltration rate of 146.55 ± 2.93 μg/cm2/h into the skin, higher than that of SIN-loaded carbomer gel (120.39 μg/cm2/h). In addition, more SIN was deposited into skin from PLO (10.08 ± 0.86 μg/cm2) than that from carbomer gel (6.01 ± 0.04 μg/cm2). Subsequent in vivo skin microdialysis studies revealed that PLO showed much higher SIN maximum concentration in permeation and drug-deposition studies (150.27 ± 20.85 and 67.95 ± 5.21 μg/mL) than that of carbomer gel (29.66 ± 1.50 and 6.73 ± 0.88 μg/mL). Cubic liquid crystal gels are useful for the controlled release of small molecules, proteins, peptides, and even nucleic acids, due to their stable thermodynamic properties and highly ordered internal structures [60,61][17][18]. These gels were used for the transdermal delivery of SIN, and showed increased cumulative release of SIN with the increase of SIN loading when evaluated in Franz diffusion cells using the rat ventral skin dermis oriented to the receiving chamber [62][19]. A double-loaded cubic liquid crystal gels containing cinnamaldehyde and SIN-HCl with pseudoplastic fluid behavior was further developed, in which cinnamaldehyde further enhances the transdermal delivery of SIN [44][13] (Figure 31B).
3. Microneedles
Microneedles combine the features of conventional injections and patches, and can be divided into solid, drug-coated, and drug-loaded dissolving microneedles for transdermal drug delivery [63,64,65][20][21][22]. The solid microneedle only punctures the skin to form a drug delivery channel, followed by the application of drugs for transdermal delivery. Drugs can be either coated on the surface of microneedles or embedded into soluble microneedles; the former are difficult in continuous drug administration, while the later ones and the embedded drugs are completely dissolved or degraded in the skin [66][23]. A SIN-loaded dissolving microneedles (SH-DM) composed of maltose and poly (lactic acid-glycolic acid) copolymer was prepared using casting method and shown good mechanical strength [67][24]. The SH-DM showed a higher cumulative permeability and faster penetration rate compared to the SIN gel (SH-G) group. Pharmacokinetic study revealed that SH-DM had a later peak time and larger maximum concentration (Cmax) of SIN than that of SH-G, which holds 1.99 times the area under the curve of SIN-HCl of that from SH-G. Similar SH-DM prepared with maltose and polyvinyl alcohol also exhibited ideal mechanical strength and better transdermal drug delivery behavior and bioavailability than the control hydrogel in both in vitro infiltration and pharmacokinetic experiments [45][14] (Figure 31C). New composite microneedles were casted with chondroitin sulfate and PVP, which integrated with phytriol/water system containing SIN-HCl [68][25]. The composite microneedles can achieve continuous SIN release in transdermal drug delivery, since it had longer peak time and a lower peak SIN concentration [69][26].
4. Physically Assisted Delivery Systems
Electroporation has been used for the transdermal delivery of small-molecule drugs by forming water channels in the stratum corneum through short high-voltage pulses and thus resulting in instantaneous penetration [70][27]. The electroporated transdermal delivery of SIN-HCl showed the highest CSF/Cplasma (SIN concentration in synovial fluid vs. that in plasma) among oral, intravenous, and electroporation transdermal delivery systems in a rabbit animal model [46][28]. In addition, the electroporation administration parameters, including frequency, waveform of exponential curve, and intensity of pulses could be optimized to improve delivery efficiency by 1.9- to 10.1-fold or 1.6- to 47.1-fold than that of the passive diffusion in mouse skin and miniature pig skin, respectively [47][29]. The concentration of SIN in synovial fluid reached to 20.84 ng/mL with the highest CSF/Cplasma after electroporation in observatory clinical trials, suggesting SIN-HCl could be effectively delivered to the site of lesions in patients with RA. Dual-frequency ultrasound (20 kHz + 800 kHz) was used for transdermal delivery of SIN-HCl, showing a significant higher cumulative penetration than that of each single frequency ultrasound (20 kHz or 800 kHz) and the sum of them [48][15] (Figure 31D).
References
- Zhou, L.I.; Wang, Y.; Liu, Q.F.; Ling, J.J. Study on determination of entrapment efficiency of sinomenine liposomes. Zhongguo Zhong Yao Za Zhi 2006, 31, 731–734. (In Chinese)
- Wang, Y.; Cong, Z.; Liu, Q.; Ling, J.; Zhou, L. Study on optimization of formulation and preparation process of sinomenine liposomes. Zhongguo Zhong Yao Za Zhi 2009, 34, 275–278. (In Chinese)
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 63–102.
- Yan, Y.; Zhang, H.F.; Sun, J.Y.; Wang, P.C.; Dong, K.; Dong, Y.L.; Xing, J.F. Enhanced transdermal delivery of sinomenine hydrochloride by ethosomes for anti-inflammatory treatment. J. Drug Deliv. Sci. Technol. 2016, 36, 201–207.
- Cevc, G.; Schäitzlein, A.; Blume, G. Transdermal drug carriers: Basic properties, optimization and transfer efficiency in the case of epicutaneously applied peptides. J. Control. Release 1995, 36, 3–16.
- Cevc, G.; Schatzlein, A.; Richardsen, H. Ultradeformable lipid vesicles can penetrate the skin and other semi-permeable barriers unfragmented. Evidence from double label clsm experiments and direct size measurements. Biochim. Biophys. Acta-Biomembr. 2002, 1564, 21–30.
- Chaudhary, H.; Kohli, K.; Kumar, V. Nano-transfersomes as a novel carrier for transdermal delivery. Int. J. Pharm. 2013, 454, 367–380.
- Fan, Y.; Lu, Y.; Cheng, B.; Wei, Y.; Wei, Y.; Piao, J.; Li, F.; Zheng, H. Correlation between in vivo microdialysis pharmacokinetics and ex vivo permeation for sinomenine hydrochloride transfersomes with enhanced skin absorption. Int. J. Pharm. 2022, 621, 121789.
- Wang, J.; Wei, Y.; Fei, Y.R.; Fang, L.; Zheng, H.S.; Mu, C.F.; Li, F.Z.; Zhang, Y.S. Preparation of mixed monoterpenes edge activated pegylated transfersomes to improve the in vivo transdermal delivery efficiency of sinomenine hydrochloride. Int. J. Pharm. 2017, 533, 266–274.
- Zheng, H.S.; Xu, C.; Fei, Y.R.; Wang, J.; Yang, M.S.; Fang, L.; Wei, Y.H.; Mu, C.F.; Sheng, Y.J.; Li, F.Z.; et al. Monoterpenes-containing pegylated transfersomes for enhancing joint cavity drug delivery evidenced by clsm and double-sited microdialysis. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 113, 110929.
- Song, H.; Wen, J.; Li, H.; Meng, Y.; Zhang, Y.J.; Zhang, N.; Zheng, W.S. Enhanced transdermal permeability and drug deposition of rheumatoid arthritis via sinomenine hydrochloride-loaded antioxidant surface transethosome. Int. J. Nanomed. 2019, 14, 3177–3188.
- Jukanti, R.; Devaraj, G.; Devaraj, R.; Apte, S. Drug targeting to inflammation: Studies on antioxidant surface loaded diclofenac liposomes. Int. J. Pharm. 2011, 414, 179–185.
- Chu, X.Q.; Wang, X.Q.; Tian, C.L.; Liu, L.; Xia, M.Q.; Jiang, J.Q.; Gui, S.Y. Dual drug-loaded cubic liquid crystal gels for transdermal delivery: Inner structure and percutaneous mechanism evaluations. Drug Dev. Ind. Pharm. 2019, 45, 1879–1888.
- Cao, Y.J.; Tao, Y.T.; Zhou, Y.J.; Gui, S.Y. Development of sinomenine hydrochloride-loaded polyvinylalcohol/maltose microneedle for transdermal delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 1–7.
- Yin, L.; Qin, F.H.; Zhou, Y.; Qi, X. Enhancing percutaneous permeability of sinomenine hydrochloride using dual-frequency sonophoresis. J. Drug Deliv. Sci. Technol. 2016, 36, 62–67.
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440.
- Phan, S.; Fong, W.K.; Kirby, N.; Hanley, T.; Boyd, B.J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharm. 2011, 421, 176–182.
- Guo, C.Y.; Wang, J.; Cao, F.L.; Lee, R.J.; Zhai, G.X. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040.
- Chu, X.Q.; Li, Q.; Gui, S.Y.; Li, Z.G.; Cao, J.J.; Jiang, J.Q. Characterization and in vitro permeation study of cubic liquid crystal containing sinomenine hydrochloride. AAPS PharmSciTech 2018, 19, 2237–2246.
- Matsuo, K.; Yokota, Y.; Zhai, Y.; Quan, Y.S.; Kamiyama, F.; Mukai, Y.; Okada, N.; Nakagawa, S. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J. Control. Release 2012, 161, 10–17.
- Martin, C.J.; Allender, C.J.; Brain, K.R.; Morrissey, A.; Birchall, J.C. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J. Control. Release 2012, 158, 93–101.
- Vrdoljak, A.; McGrath, M.G.; Carey, J.B.; Draper, S.J.; Hill, A.V.S.; O’Mahony, C.; Crean, A.M.; Moore, A.C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release 2012, 159, 34–42.
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568.
- Wu, X.X.; Chen, Y.L.; Gui, S.Y.; Wu, X.Q.; Chen, L.; Cao, Y.J.; Yin, D.K.; Ma, P. Sinomenine hydrochloride-loaded dissolving microneedles enhanced its absorption in rabbits. Pharm. Dev. Technol. 2016, 21, 787–793.
- Gui, Z.P.; Wu, X.X.; Wang, S.Y.; Cao, Y.J.; Wan, J.; Shan, Q.Q.; Yang, Z.Z.; Zhang, J.W.; Gui, S.Y. Dissolving microneedles integrated with liquid crystals facilitate transdermal delivery of sinomenine hydrochloride. J. Pharm. Sci. 2017, 106, 3548–3555.
- Shu, Z.X.; Cao, Y.J.; Tao, Y.T.; Liang, X.; Wang, F.Y.; Li, Z.; Li, Z.B.; Gui, S.Y. Polyvinylpyrrolidone microneedles for localized delivery of sinomenine hydrochloride: Preparation, release behavior of in vitro & in vivo, and penetration mechanism. Drug Deliv. 2020, 27, 642–651.
- Denet, A.R.; Vanbever, R.; Preat, V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004, 56, 659–674.
- Yan, H.; Yan, M.; Li, H.D.; Jiang, P.; Deng, Y.; Cai, H.L. Pharmacokinetics and penetration into synovial fluid of systemical and electroporation administered sinomenine to rabbits. Biomed. Chromatogr. 2015, 29, 883–889.
- Feng, S.; Zhu, L.J.; Huang, Z.S.; Wang, H.J.; Li, H.; Zhou, H.; Lu, L.L.; Wang, Y.; Liu, Z.Q.; Liu, L. Controlled release of optimized electroporation enhances the transdermal efficiency of sinomenine hydrochloride for treating arthritis in vitro and in clinic. Drug Des. Devel. Ther. 2017, 11, 1737–1752.
More
