Leishmaniasis is a vector-born disease caused by a group of protozoan parasites belonging to the genus Leishmania.
- Leishmania
- zoonotic diseases
- Parasites
- anthroponoses
Leishmaniasis is a vector-born disease caused by a group of protozoan parasites belonging to the genus Leishmania [1]. (Draft for definition)
1. Introduction
Systemic/localized lymphadenomegaly associated with fever and other nonspecific signs or symptoms are common causes of access to the ED, but the differential diagnosis is usually hard. The combination of anamnesis, physical examination, laboratory tests, and instrumental diagnosis are extremely important to orientate toward a rapid and appropriate therapy, but currently, a prompt discrimination of the lymphadenomegaly etiology is not often possible. The management of a differential diagnosis between hematological and infective diseases such as Leishmaniasis usually represents a challenge for the emergency physician; hence, we suggest a quick diagnostic test that might be useful for the early identification.
Most Leishmania infections are zoonotic diseases except for those that have Leishmania Tropica and Leishmania Donovani as causative agents and are considered anthroponoses[1] [2].
The infection begins with the bite of the vector, a female specimen of sandflies. In Africa, Europe, and Asia, the sand-fly Phlebotomus is widespread, while in the New Continent, the sand-fly Lutzomyia is responsible for the spread of Leishmania; however, they all are very similar morphologically.
After the parasite enters human cells, cutaneous macrophages phagocytize promastigote, which is the primary stage of the parasite. An immunocompetent system is commonly able to kill promastigotes, blocking the spread of parasites in other organs through cellular lysis. This phenomenon occurs in a small percentage of cases, where promastigotes resist the destruction and evolve into amastigotes, which replicate and provoke cellular lysis. The next progression step is the spreading of amastigotes into other reticular-endothelial system cells showing different clinical conditions as for gravity, clinical signs, and outcome[2] [3].
Cutaneous Leishmaniasis is a severe but not deadly disease which usually manifests with self-limited ulcerative lesions that spontaneously heal in 6–18 months. Only 10% of cases evolve into systemic disease, which is potentially lethal (mucosal and mucocutaneous forms)[1][3] [2,4].
The severity and chronicity of the skin lesion of leishmaniasis depend on two fundamental factors: the infecting species and the host's immune response.
The lesion generally starts from the vector injection site and develops within about 2 weeks as papules or nodules; with the involvement of the lymph nodes draining the site of infection, eventually, a granuloma can develop from this lesion, and it will hesitate in healing, or it can ulcerate causing skin lesions that tend to become chronic[1] [2].
Visceral Leishmaniasis is a severe form, typically occurring in rural areas, that requires prompt treatment to avoid fatal outcomes. The rate mortality is 10–20%, but this is just a poor estimation due to the lack of appropriate epidemiological methods and the numerous misdiagnosed cases[4][5] [5,6].
The patient who approaches the emergency department with visceral leishmaniasis typically reports rapid weight loss in the preceding weeks. The presenting symptoms include fever, asthenia, weakness, anorexia, and night sweats. On physical examination, we may find hepatomegaly, splenomegaly, and lympho-adenomegaly.
A special class of patients with visceral leishmaniasis is represented by those with HIV: in these patients, visceral leishmaniasis infection is much more severe and leads to a progression of the acquired immunodeficiency, worsening the prognosis of HIV patients.
It has also been shown that HIV can lead to the re-activation of leishmania infection that was latent[6][7][8] [7–9].
Leishmaniasis is characterized by an endemic diffusion in East Africa, Latin America, and South-East Asia, which are areas where malnutrition is associated with a high concentration of parasite; indeed, it is not well elucidated if the parasite is the etiology or a consequence of the poor nutritional status[9] [10]. Despite the numerous efforts to contain the infection, developing countries failed to achieve their goal to eliminate Leishmaniasis as a public health issue by 2015[10] [11].
Nowadays, thanks to the increased migration of people, Leishmaniasis has recently spread worldwide, especially to several Mediterranean countries[11] [1], forcing the consideration of Leishmaniasis as a potential differential diagnosis in patients presenting no specific symptoms associated to fever and lymphadenomegaly.
There is no effective pre-exposure prophylaxis or effective vaccine, and the only protective measures include individual devices (mask, gloves, distance) and public health measures.
In the case of leishmaniasis, people need to know the risk of infection and epidemiology:
(1) The infection is transmitted through sand-fly bites between dusk and dawn;
(2) Covering every part of body with clothing is protective, because sand-fly mouthparts do not penetrate clothing (in contrast, mosquito mouthparts do penetrate clothing). Moreover, clothes impregnated with permethrin make a stronger protection for exposed skin areas (face, neck, hands, forearms, feet, ankle, joints) and DEET (NN-diethyl-3-methylbenzamide) should be used an insect repellent.
Regarding public health prevention, the only measures are vector control (sand flies) and reservoir control (domestic and sylvatic animals)[12] [12].
In areas where sylvatic rodents live and grow up, the reservoir control is not applicable due to the high concentration of rats to treat. Indeed, rats may harbor some of the Leishmania species, but it is not sure if they infect the sandflies. In contrast, the control of infection with domestic reservoir measures is more simple. In fact, the use of deltametrin-impregnated collars in dogs has been associated with decreased seroconversion rates of visceral leishmaniasis in humans and dogs, but its efficacy for the prevention of CL has not been evaluated[13] [13].
2. Clinical and Diagnostic Tips for the Emergency Physicians
Leishmaniasis presents several clinical forms depending on the involved species, which are Leishmania mexicana, Leishmania (Viannia) braziliensis, Leishmania panamensis, L. major, and L. tropica. All of these cause the cutaneous Leishmaniasis, which is the consequence of an inefficient cellular-mediated response, and although it cannot be considered a life-threatening condition, people who are affected usually suffer from social stigmatization[2][14][15] [3,14,15]. The incubation period lasts around 1–2 months, after which one or more reddish papulo-nodular lesions will appear on the inoculation point (face, neck, legs, or arms). These lesions can be ulcerated or not, and they can have different sizes.
Lympho-adenomegaly is frequently found near the skin lesion[15] [15]: it is harmless, indolent, and self-limited within a few months; it is extremely rare that a disfiguring scar remains, although the skin is heavily infiltrated with parasites.
Approximately 10% of cutaneous forms evolve into mucocutaneous leishmaniasis (MCL), which is a disfiguring disease characterized by the progression into mucosal inflammation because of a combination of host cell‐mediated immunity[15] [15], parasite virulence, and inadequate treatment. For all of this, mucocutaneous leishmaniasis needs to be promptly treated[16] [16]. Mucocutaneous leishmaniasis causes destructive lesions mainly on the lips, nasal septum, and palate. Lesions can easily be confused with other infectious diseases such as fungal infections. In most cases, the first symptom is nasal congestion, but with the disease’s progression, the symptoms worsen[1][15] [2,15], and erythema, dysphagia, dysphonia, tooth loss, severe respiratory obstruction, and dyspnea may arise. When promptly recognized, MCL can be treated and solved before any consequence occurs[3] [4].
Visceral leishmaniasis aka kala-azar occurs when a parasite spreads from the reticuloendothelial system to many organs. If left untreated, this is a harmful and potentially fatal condition that typically leads to death within 2 years. Early symptoms and signs include prolonged, persistent, and irregular fever, hepatomegaly, splenomegaly, pancytopenia, progressive anemia, and weight loss, despite not all of these features always being present at the same time[16] [16].
As happened in our case report, nonspecific clinical presentation might often be misleading for the physician, who is primarily tempted to address the diagnosis toward a hematologic disease[15] [15]. In fact, the most common signs—such as hepatomegaly, splenomegaly, and fever—are also present in infective, liver, autoimmune, and infiltrative diseases. Considering these signs intertwining, other pathologies must be considered in the diagnostic process.
3. Differential Diagnosis
The differential diagnosis of VL includes[17] [17] the following:
Malaria—Both malaria and VL may present with fever, malaise, and splenomegaly; the main difference regards the symptoms onset: malaria generally occurs acutely, while VL tends to be chronic. The diagnosis of malaria is established by blood smear or rapid diagnostic testing.
Histoplasmosis—Patients with acute histoplasmosis present with fever, fatigue, hepatosplenomegaly, and pancytopenia; this is a disease that occurs in the setting of immunosuppression. The diagnosis is made by antigen testing, culture, or histopathology.
Amebic Liver Abscess—This pathology is characterized by one to two weeks of right upper quadrant pain and fever and sweating, malaise, weight loss, and anorexia. A rapid and cheap diagnostic toll is radiographic imaging.
Schistosomiasis—The principal manifestation is hepatosplenomegaly due to granulomatous inflammation and subsequent fibrosis of the periportal spaces of the liver, with subsequent portal hypertension. The diagnosis is established by the visualization of eggs on microscopy and/or serology.
Lymphoma—Lymphoma shares the principal symptoms of leishmaniasis as lymphadenopathy, hepatomegaly, splenomegaly, cytopenia, fever, night sweats, and weight loss. The only valid diagnosis is established by histopathology.
Tuberculosis—Only symptoms of extrapulmonary tuberculosis may present with seeding of nearly any organ of the body, including hepatic and/or splenic disease. The diagnosis is established by culture of acid-fast bacilli from the sputum or other fluid/tissue.
In lympho-hematologic diseases, there is a high hepatomegaly/splenomegaly prevalence that drives the physicians to consider more such a disease than an infectious one.
The onset of visceral Leishmaniasis can be acute and hard to make; anyway, no delay is allowed for treatment to prevent a potentially fatal evolution[18] [18] (Table 2).
Table 2. Summary of the most common differential diagnosis that could mimic leishmaniasis.
|
|
Splenomegaly |
Hepatomegaly |
Fever |
Weight Loss |
Cytopenia |
|
Liver diseases (e.g., fibrosis, cirrhosis) |
Common |
Common |
Rare |
Common |
Common |
|
Hematologic Malignancies |
|
|
|
|
|
|
Chronic leukemia |
Common |
Common |
Rare |
Common |
Common |
|
Lymphoma |
Common |
Common |
Common |
Common |
Common |
|
Myeloproliferative diseases |
Common |
Common |
Rare |
Common |
Common |
|
Multiple myeloma |
Common |
Possible |
Common |
Common |
Common |
|
Autoimmune cytopenia (e.g., ITP1, AIHA2) |
Common |
Rare |
Possible |
Rare |
Common |
|
Infectious diseases |
|
|
|
|
|
|
Viral (e.g., hepatitis, EBV, HIV/AIDS) |
Common |
Common |
Common |
Common |
Common |
|
Bacterial (e.g., mycobacteria, leptospirosis, brucellosis) |
Possible |
Possible |
Common |
Common |
Common |
|
Parasitic (e.g., malaria, schistosomiasis) |
Common |
Possible |
Common |
Rare |
Rare |
|
Fungal (e.g., histoplasmosis) |
Common |
Possible |
Common |
Common |
Common |
|
Infiltrative diseases (i.e., amyloidosis, sarcoidosis, Felty sindrome, SLE3, HLH4) |
Common |
Possible |
Possible |
Possible |
Common |
4. How Physicians Diagnose Visceral Leishmaniasis in ED
First, nonspecific symptoms are more common than specific ones, and the suspicion of visceral Leishmaniasis must be confirmed through accurate diagnostic tests.
The traditional diagnostic method is the direct amastigote microscope visualization of biopsied samples of spleen, lymph nodes, bone marrow, or liver. However, sensitivity strictly depends on the analyzed tissue, ranging from 50% to 90%; even blood samples have a low sensitivity, except in HIV-positive patients who have a higher parasitemia level[19][20] [19,20]. Polymerase Chain Reaction (PCR) on bone marrow, peripheral blood, or buffy coat samples has a sensitivity >95% both for L. donovani in Asia and east Africa and L. Infantum in the Mediterraneum, but the low specificity, the high costs, and the complexity of the technique make it hard to be introduced in the diagnostic algorithm[21] [21]. A latex antigenic test that aims to detect a heat-stable low molecular weight carbohydrate antigen in urine is now available, but it is rarely used in clinical practice due to a low sensibility (64%), despite an excellent specificity (93%)[22] [22].
Several serologic tests are available, including the enzyme-linked immunosorbent assay (ELISA), the indirect fluorescent antibody test (IFAT), the indirect hemagglutination assay (IHA), and Western blot (WB). All of these methods involve antibody detection tests, hence sharing the same issues. Indeed, both sensitivity and specificity range from 80% to 100%, techniques are expensive and complicated to perform (limiting their use in endemic countries), asymptomatic infected patients often result positive, and sensitivity is much lower in immunocompromised patients[23] [23]. An RK39-based rapid diagnostic test (RDT) is currently widely used in North America. RK39 is a 39-amino-acid protein produced by a Brazilian L. infantum/chagasi strain. The test has shown a sensitivity of 97% in the Indian subcontinent, although it resulted only 85% sensitivity in eastern Africa. More recently, an RK28-RDT has been introduced, and it has maintained a high sensitivity in India while improving its sensitivity to 95% in eastern Africa[24][25][26] [24–26]. However, RTDs have the same limitations of other serologic tests, except for them being cheaper and easier to use[26] [26].
Antigen-based immune-chromatic tests consist of analyzing a peripheral blood drop sample with a nitrocellulose membrane pre-coated with the RK39 antigen. The test is quick and easy to perform, and it has been largely used worldwide, especially in rural areas with a lack of health facilities. However, sensitivity strongly varies based on the tested area, with values ranging from 92.8% to 100% in India to 36.8% to 92% in Brazil and East Africa; therefore, a negative result cannot rule out the diagnosis of visceral leishmaniasis, especially in patients with HIV. In addition, specificity is limited by the cross-reaction with a large number of different diseases, such as infective endocarditis, hepatic insufficiency, malaria, enteric fever, disseminated tuberculosis, lymphoma, sepsis, and toxoplasmosis[27] [27].
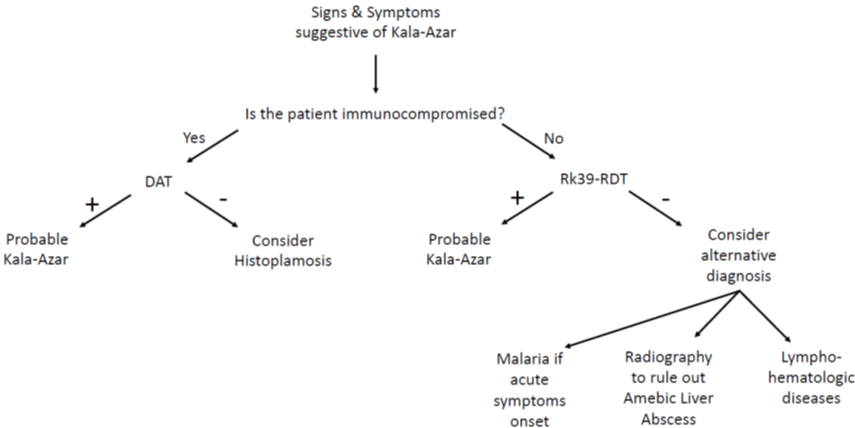
The diagnosis of visceral leishmaniasis is particularly challenging in immunocompromised patients for whom serologic tests are usually unable to detect the disease due to the low antibodies levels[23][24][25][26][28] [23–26,28]. The best results have been obtained with WB, which showed a sensitivity between 75% and 91%. However, due to the lack of data comparing WB and direct agglutination test (DAT), it is not possible to recommend the use of WB for immunocompromised patients: in these individuals, it would be preferred to perform two serologic tests and PCR[29] [29].
The direct agglutination test (DAT) is widely used in South America, Iran, and in some European countries[30][31][32][33] [30–33]. This is a semi-quantitative test that aims to detect if agglutination occurs when serial dilutions of patient’s serum are mixed with stained killed Leishmania sp promastigote. Therefore, DAT is not influenced by the involved species (L. donovani or L. infantum)[34] [34]. Sensitivity is 70.5–99% and specificity is 82.2–100%, with high values even for HIV-positive individuals (89.1–91.3% and 89.3–89.7%, respectively), thus making it a very useful test for the diagnosis of visceral leishmaniasis in a large population with or without HIV[30][31][32][33][34][35][36][37][38] [35–38]. However, false positive can result in asymptomatic patients or individuals affected by malaria or other parasites [23][31][33][39][23,31,33,39]. In 2006, Chappuis and coworkers conducted a meta-analysis to compare the diagnostic performances of the DAT test and the rK39 dipstick. They only analyzed studies conducted on patients with a certain diagnose of VL by splenic aspirate and finally included 30 studies evaluating the DAT test and 13 evaluating the RK39 test. The results showed that both tests perform good or excellent for the diagnosis of VL, with a sensitivity of 94.8% and a specificity of 85.9% for DAT, and a sensitivity of 93.9% and a specificity of 90.6% for rk39 dipstick (Figure 1)[40] [40].
Figure 1. Clinical flowchart in emergency department.