With the development of nanotechnology, various types of polymer-based drug delivery systems have been designed for biomedical applications. Polymer-based drug delivery systems with desirable biocompatibility can be efficiently delivered to tumor sites with passive or targeted effects and combined with other therapeutic and imaging agents for cancer theranostics. As an effective vehicle for drug and gene delivery, polyethyleneimine (PEI) has been extensively studied due to its rich surface amines and excellent water solubility.
- polyethylenimine
- drug delivery
- cancer treatment
- cancer imaging
- cancer theranostics
1. Introduction
2. Overview of PEI
PEI is a commercially widely used cationic polymer containing primary, secondary, and tertiary amino groups in a ratio of 1:2:1 with strong positive charges [22]. PEI can be synthesized as linear PEI (Figure 1a) or branched PEI (Figure 1b) with a molecular weight ranging from 700 Da to 1000 kDa according to the degree of polymerization [23]. PEI can be easily prepared using an AB-type monomer via a simple one-step reaction [24]. In addition, PEI can be considered a low-cost option compared to dendrimers with the same molecular weight [25]. PEI has been widely used in different fields because of its unique structure and abundant amino groups. For example, in industry, PEI can be used as a flocculant to remove oil present in synthetically produced water, or as a wet strength agent in paper-making and the manufacture of shampoo [26,27][26][27]. In biomedicine, PEI is widely used in enzyme immobilization [28], virus immobilization on cellulose [29], cell adhesion [30], gene transfection [31], and the synthesis of NPs to enhance their stability and anticancer efficacy [32].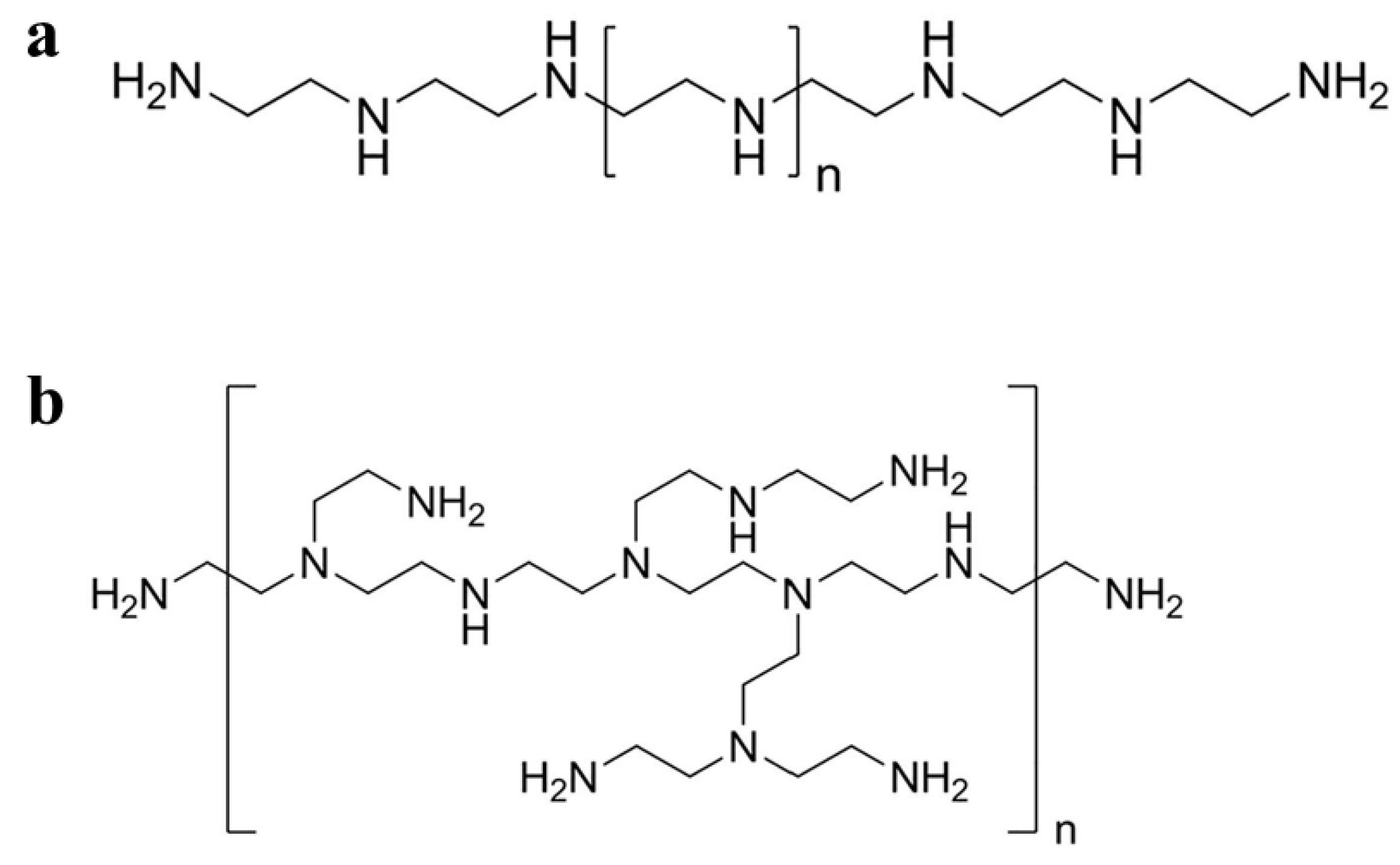
3. PEI Modifications
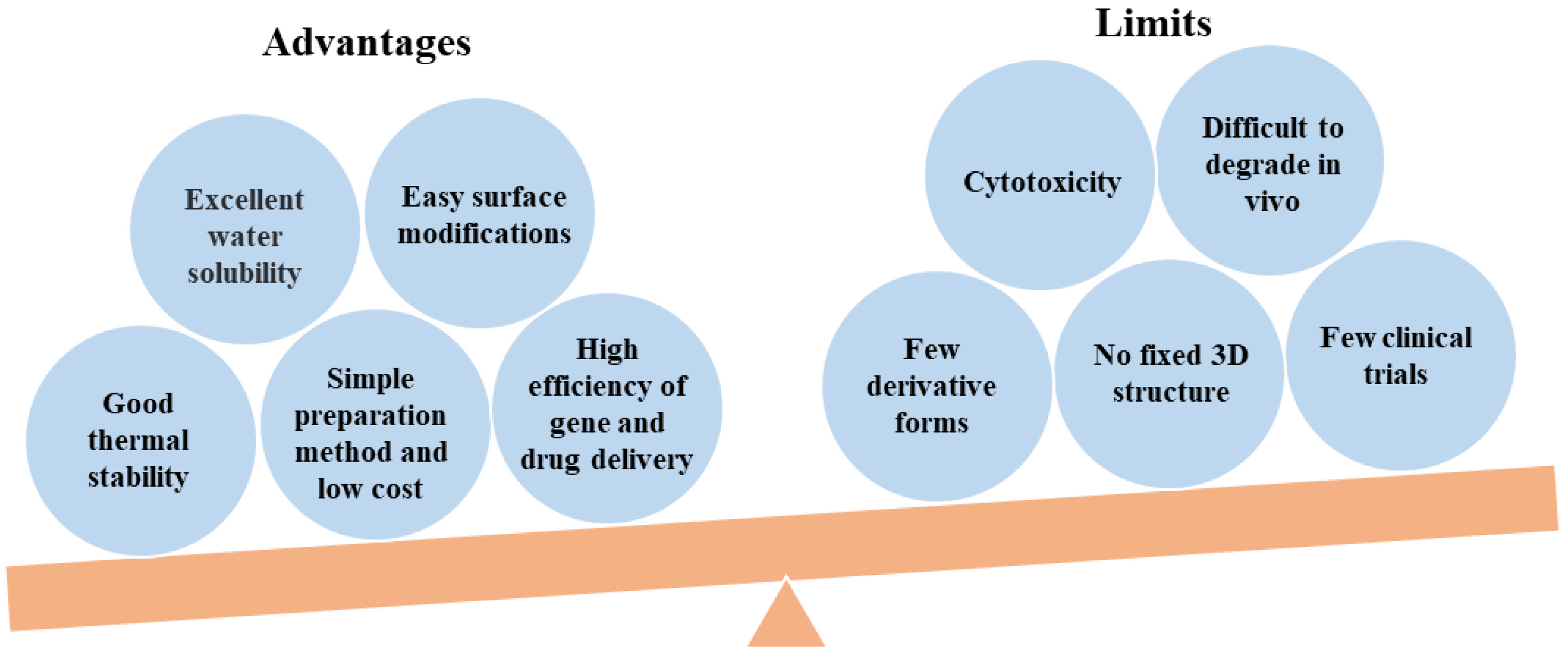
As a cationic polymer, PEI contains abundant amino groups and as a result has a certain degree of cytotoxicity. Cationic PEI enters cells by adhering to negatively charged transmembrane heparanproteoglycans, which can cause cell damage through membrane destabilization [44]. Additionally, the internalized PEI causes apoptotic cell death by forming pores in the mitochondrial membrane [45,46][45][46]. PEI is not well-degraded in organisms, and its cytotoxicity is closely related to its molecular weight and branching degree [47]. Branched PEI with a higher molecular weight has a higher cytotoxicity. The surface amines of PEI can be shielded with simple modifications, thus significantly improving the biocompatibility of PEI [21]. At present, the surface amines of PEI are mainly shielded with covalent bonds such as carboxylation, acetylation, and hydroxylation, or with electrostatic modification of negatively charged proteins. However, currently, there is a lack of systematic research to contrast the benefits and challenges of these approaches for the surface modifications of PEI. For example, Wen et al. improved the biocompatibility of PEI through carboxylation, acetylation, hydroxylation, and PEGylation [21]. These methods effectively reduced or shielded the positive charge of the PEI, thus reducing cytotoxicity. Various functional groups including polyethylene glycol (PEG), folic acid (FA), hyaluronic acid (HA), fluorescent tags, and protein can be modified with PEI for biomedical applications [24,25,48,49,50,51,52,53,54,55,56,57,58][24][25][48][49][50][51][52][53][54][55][56][57][58]. PEI modifications for biomedical applications in recent years are summarized in Table 1.|
Modification Types |
Aims |
Ref. |
|---|---|---|
|
Carboxylation modification |
Gene delivery, absorption of heavy metals in sewage. |
|
SMMC-7721 | ||
SMMC-7721 | ||
[ | ||
] | ||
[ | ||
] | ||
5.2. PEI-Based Drug Delivery System for Cancer Imaging
Contrast agents are widely used in molecular imaging to enhance imaging resolution. Owing to its unique physicochemical structure, PEI can effectively stabilize or encapsulate various agents for cancer imaging applications. A variety of imaging contrast agents can be constructed based on PEI including computed tomography (CT), magnetic resonance (MR), and single-photon emission CT (SPECT) imaging [59,165][137][138]. This section summarizes the progress of research concerning the use of PEI to construct multifunctional nanosystems as contrast agents for single-modal and multimodal molecular imaging. Table 3 summarizes PEI-based imaging or imaging-guided cancer therapies. Table 3.|
Imaging Types |
Imaging Agents |
Cell Line Models |
In Vivo Models |
Ref. |
|||
|---|---|---|---|---|---|---|---|
[ | |||||||
|
CT |
AuNPs | ||||||
A549 |
A549 |
[53] |
Acetylation modification |
Gene delivery efficiency improvement, cytotoxicity reduction. |
[63 | ||
|
AuNPs |
MCF-7 | ||||||
|
Hydroxylation modification |
Biocompatibility enhancement, gene delivery, transformation improvement of NPs. |
||||||
|
PEG modification |
Stability and transfection efficiency improvement. |
||||||
|
FA modification |
Tumor-targeted delivery. |
||||||
|
HA modification |
Tumor-targeted gene delivery, stability improvement. |
||||||
|
Protein modification |
Gene delivery, protein transduction. |
||||||
|
FI modification |
Fluorescence imaging. |
[57] |
4. Synthesis of PEI-Based NPs
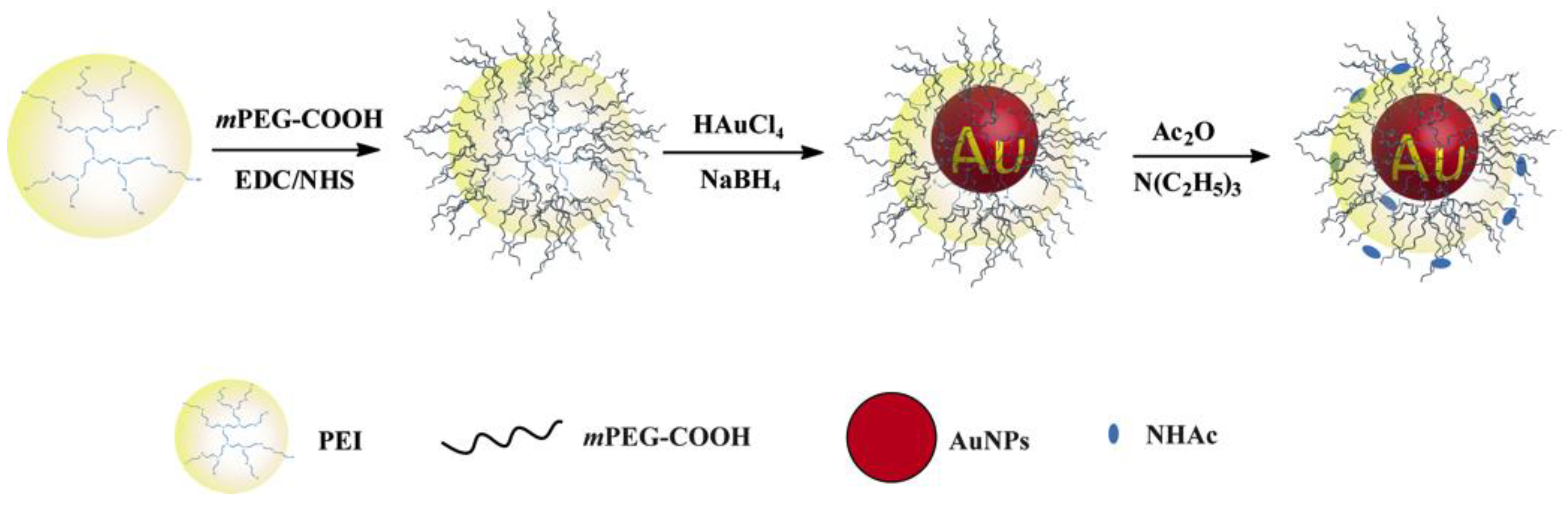
5. PEI-Based Drug Delivery Systems
5.1. PEI-Based Drug Delivery Systems for Cancer Treatment
|
Therapeutic Modalities |
Therapeutic Agents |
Cell Line Models |
In Vivo Models |
Ref. |
|||
|---|---|---|---|---|---|---|---|
|
Chemotherapy |
DOX |
HeLa |
HeLa |
[57] |
|||
|
MTX |
HCT 116 |
/ |
MCF-7 |
||||
[ | ][139] |
PTX |
HepG2 |
||||
|
AuNPs | / |
HeLa |
HeLa |
||||
[ |
DOX |
C6 |
|||||
|
Bi2Se3 NPs | / |
A549, U14 [140 |
U14 ][121] |
||||
[ | ][ |
DOX |
HeLa |
HeLa |
|||
] | |||||||
|
MR |
Gd ions |
KB |
KB |
DOX |
4T1, HepG2 |
/ |
|
|
Superparamagnetic iron oxide nanocrystals |
MCF-7/Adr | [149 |
/ ][123] |
||||
[ | ][144] |
DOX |
A549 |
/ |
|||
|
DOX, siRNA |
|||||||
|
Superparamagnetic iron oxide NPs |
Chondrolyte cells |
/ |
MDA-MB-231, HeLa, EAT |
EAT |
|||
|
Ultrasmall iron oxide NPs |
4T1 | ||||||
4T1 |
DOX |
||||||
|
Gd(OH)(3)-doped Fe3 | SKBR3 |
O4 NPs |
KB SKBR3 |
/ |
|||
[ | ][146] |
Gene therapy |
pDNA |
||||
|
Fe3O4 NPs | HeLa, 16HBE14o−, HepG2 |
HepG2 |
HepG2 / |
||||
|
pDNA |
Huh7 |
||||||
|
Fe3O4 NPs |
U87MG, HeLa Huh7 |
U87MG, HeLa |
|||||
[ | ][148] |
DNA |
|||||
|
SPECT |
NIH/3T3 |
131I / |
4T1 [45] |
||||
4T1 |
pDNA |
HeLa |
|||||
|
99mTc | / |
C6 [51 |
C6 ] |
||||
[ | ][150] |
DNA |
HeLa, CT26 |
||||
|
MR/CT |
CT26 |
AuNPs, Gd2O3 [148][ |
HeLa129] |
||||
HeLa |
mRNA |
||||||
|
Fe3O4@Au nanostars |
B16-OVA |
HeLa B16-OVA |
HeLa |
||||
[ | ][152] |
Other therapies |
|||||
|
Fe3O4@Au nanocomposites |
RNase A |
KB MDA-MB-231 |
/ / |
[49] |
|||
|
Oxidized mesoporous carbon nanospheres, pDNA |
MCF-7 |
MCF-7 |
|||||
|
Au-Gd NPs |
HeLa |
HeLa |
CAT-Ce6 |
T24 |
|||
|
MR/PA | T24 | ||||||
Gd/CuS |
KB |
KB |
GO, DTX, anti-miRNA21 |
||||
|
SPECT/CT |
MDA-MB-231 |
99m / |
Tc, AuNPs [ |
HCC-LM3 |
|||
HCC-LM3 | [58] |
CuS, DTX, CpG |
4T1 |
||||
|
99mTc, AuNPs | 4T1 |
SKOV-3 |
/] |
||||
[ |
pDNA, 9B9 mAb | ||||||
|
AuNPs, 131I |
C6 |
C6 |
|||||
|
MR/CT/PA |
Fe3O4 NPs, Au nanostars |
HeLa |
HeLa |
||||
|
MR/SPECT/PA |
19F,99mTc, ICG |
HepG2 |
HepG2 |
||||
|
CT/MR/upconversion luminescence |
Yb3+- and Gd3+-doped UCNPs |
A2780 |
A2780 |
5.3. PEI-Based Drug Delivery Systems for Cancer Theranostics
The development of nanotechnology provides new strategies with regard to the combination of therapeutic drugs and imaging agents for imaging-guided cancer therapy, namely cancer theranostics [18,213,214][18][158][159]. As a highly cationic polymer, PEI has the advantages of low cost, easy surface functionalization, stable chemical properties, and high loading of small molecules and NPs, enabling it to be used to construct PEI-based drug delivery systems for cancer theranostics. As an example, Shi et al. used an inverse mini-emulsion method to prepare PEI-based hybrid nanogels for incorporation with ultrasmall iron oxide NPs and the anticancer drug DOX for T1 MR imaging-guided chemotherapy of tumors [104][85]. The nanogels displayed excellent water solubility and colloidal stability, high DOX loading efficiency (51.4%), and a pH-dependent release of the DOX with an accelerated release rate under acidic pH. Compared to free ultrasmall iron oxide NPs, the nanogels showed a much higher r1 relaxivity at 2.29 mM−1 s−1. Additionally, under the guidance of T1-weighted MR imaging, the nanogels effectively inhibited tumor growth. HA-modified PEI-stable Fe3O4@Au core–shell nanostars (NSs) were used for trimodal CT-, MR-, and photothermal- imaging-guided PTT of tumors [92][152]. Here, HA-modified PEI provided the NSs with desirable colloidal stability, biocompatibility, and targeted specificity to cancer cells overexpressing CD44 receptors. With the Fe3O4 core NPs and Au star shell, the NSs could be used as a contrast agent for efficient MR and CT imaging of tumors in vivo. Furthermore, because of the NIR absorption property, the NSs could also be used as a nanoprobe for thermal imaging and PTT of tumors. Laponite (LAP) is a synthetic biodegradable nanoclay with a large specific surface area and cation exchange capacity [215][160]. Combining LAP with PEI not only can improve the drug loading rate of the complex, but also produce good stability. Zhuang and colleagues created PEI-modified LAP using a polylactic acid-PEG-COOH spacer. The PEI-LAP was used as a nanoplatform to embed AuNPs and load DOX for targeted CT imaging and chemotherapy of tumors [201][140]. The formed nanocomplexes displayed excellent colloidal stability and a high drug loading efficiency of up to 91.0 ± 1.8%, which significantly inhibited the growth of tumors and reduced the side effects of DOX. Alkoxyphenyl acylsulfonamide (APAS) as a zwitterionic polymer can enhance the cellular uptake of NPs at the pH of tumor microenvironment [216][161]. Zhu et al. used APAS-linked PEI as a vehicle to entrap AuNPs and labeled it with radioactive 131I to enhance dual-modal SPECT/CT imaging-guided radiotherapy of tumors [121][102]. Because of the charge conversion property of APAS, the AuNPs can change from neutral to positively charged in a weak acid environment, thus promoting cellular uptake. In addition, after 131I labeling, the therapeutic agents can enhance SPECT/CT dual mode imaging and radiotherapy of tumors in vivo.References
- Yu, Z.; Gao, L.; Chen, K.; Zhang, W.; Zhang, Q.; Li, Q.; Hu, K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16, 88.
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972.
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651.
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782.
- Rojas-Quijano, F.A.; Benyo, E.T.; Tircso, G.; Kalman, F.K.; Baranyai, Z.; Aime, S.; Sherry, A.D.; Kovacs, Z. Lanthanide(III) complexes of tris(amide) PCTA derivatives as potential bimodal magnetic resonance and optical imaging agents. Chem. Eur. J. 2009, 15, 13188–13200.
- Tseng, Y.C.; Xu, Z.; Guley, K.; Yuan, H.; Huang, L. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials 2014, 35, 4688–4698.
- Guo, R.; Shi, X. Dendrimers in Cancer Therapeutics and Diagnosis. Curr. Drug Metab. 2012, 13, 1097–1109.
- Gotov, O.; Battogtokh, G.; Ko, Y.T. Docetaxel-Loaded Hyaluronic Acid-Cathepsin B-Cleavable-Peptide-Gold Nanoparticles for the Treatment of Cancer. Mol. Pharm. 2018, 15, 4668–4676.
- Yin, W.; Zhao, Y.; Kang, X.; Zhao, P.; Fu, X.; Mo, X.; Wang, Y.; Huang, Y. BBB-penetrating codelivery liposomes treat brain metastasis of non-small cell lung cancer with EGFR(T790M) mutation. Theranostics 2020, 10, 6122–6135.
- Yu, Y.; Wang, Z.H.; Zhang, L.; Yao, H.J.; Zhang, Y.; Li, R.J.; Ju, R.J.; Wang, X.X.; Zhou, J.; Li, N.; et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials 2012, 33, 1808–1820.
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147.
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191.
- Ma, X.; Yang, S.; Zhang, T.; Wang, S.; Yang, Q.; Xiao, Y.; Shi, X.; Xue, P.; Kang, Y.; Liu, G.; et al. Bioresponsive immune-booster-based prodrug nanogel for cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 451–466.
- Su, W.; Chen, C.; Wang, T.; Li, X.; Liu, Y.; Wang, H.; Zhao, S.; Zuo, C.; Sun, G.; Bu, W. Radionuclide-labeled gold nanoparticles for nuclei-targeting internal radio-immunity therapy. Mater. Horiz. 2020, 7, 1115–1125.
- Rhim, W.-K.; Kim, M.; Hartman, K.L.; Kang, K.W.; Nam, J.-M. Radionuclide-labeled nanostructures for in vivo imaging of cancer. Nano Converg. 2015, 2, 10.
- He, H.; Du, L.; Guo, H.; An, Y.; Lu, L.; Chen, Y.; Wang, Y.; Zhong, H.; Shen, J.; Wu, J.; et al. Redox Responsive Metal Organic Framework Nanoparticles Induces Ferroptosis for Cancer Therapy. Small 2020, 16, 2001251.
- Zhou, B.; Liu, J.; Wang, L.; Wang, M.; Zhao, C.; Lin, H.; Liang, Y.; Towner, R.A.; Chen, W.R. Iron oxide nanoparticles as a drug carrier reduce host immunosuppression for enhanced chemotherapy. Nanoscale 2022, 14, 4588–4594.
- Zhou, B.; Wu, Q.; Wang, M.; Hoover, A.; Wang, X.; Zhou, F.; Towner, R.A.; Smith, N.; Saunders, D.; Song, J.; et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem. Eng. J. 2020, 396, 125239.
- Zhou, B.; Song, J.; Wang, M.; Wang, X.; Wang, J.; Howard, E.W.; Zhou, F.; Qu, J.; Chen, W.R. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale 2018, 10, 21640–21647.
- Lungu, C.N.; Diudea, M.V.; Putz, M.V.; Grudzinski, I.P. Linear and Branched PEIs (Polyethylenimines) and Their Property Space. Int. J. Mol. Sci. 2016, 17, 555.
- Wen, S.; Zheng, F.; Shen, M.; Shi, X. Surface modification and PEGylation of branched polyethyleneimine for improved biocompatibility. J. Appl. Polym. Sci. 2013, 128, 3807–3813.
- Hernandez-Montelongo, J.; Lucchesi, E.G.; Nascimento, V.F.; Franca, C.G.; Gonzalez, I.; Macedo, W.A.A.; Machado, D.; Lancellotti, M.; Moraes, A.M.; Beppu, M.M.; et al. Antibacterial and non-cytotoxic ultra-thin polyethylenimine film. Mater. Sci. Eng. C 2017, 71, 718–724.
- Vicennati, P.; Giuliano, A.; Ortaggi, G.; Masotti, A. Polyethylenimine In Medicinal Chemistry. Curr. Med. Chem. 2008, 15, 2826–2839.
- Zhou, B.; Zheng, L.; Peng, C.; Lo, D.; Li, J.; Wen, S.; Shen, M.; Zhang, G.; Shi, X. Synthesis and Characterization of PEGylated Polyethylenimine-Entrapped Gold Nanoparticles for Blood Pool and Tumor CT Imaging. ACS Appl. Mater. Interfaces 2014, 6, 17190–17199.
- Zhou, B.; Yang, J.; Peng, C.; Zhu, J.; Tang, Y.; Zhu, X.; Shen, M.; Zhang, G.; Shi, X. PEGylated polyethylenimine-entrapped gold nanoparticles modified with folic acid for targeted tumor CT imaging. Colloids Surf. B Biointerfaces 2016, 140, 489–496.
- Santos, A.S.; Oliveira, L.F.S.; Marques, A.M.T.; Silva, D.C.A.; Mansur, C.R.E. Evaluation of the efficiency of polyethylenimine as flocculants in the removal of oil present in produced water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 200–210.
- Vatanpour, V.; Jouyandeh, M.; Akhi, H.; Mousavi Khadem, S.S.; Ganjali, M.R.; Moradi, H.; Mirsadeghi, S.; Badiei, A.; Esmaeili, A.; Rabiee, N.; et al. Hyperbranched polyethylenimine functionalized silica/polysulfone nanocomposite membranes for water purification. Chemosphere 2022, 290, 133363.
- Virgen-Ortiz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, A.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490.
- Tiliket, G.; Ladam, G.; Nguyen, Q.T.; Lebrun, L. Polyethylenimine surface layer for enhanced virus immobilization on cellulose. Appl. Surf. Sci. 2016, 370, 193–200.
- Ye, X.; Li, S.; Chen, X.; Zhan, Y.; Li, X. Polyethylenimine/silk fibroin multilayers deposited nanofibrics for cell culture. Int. J. Biol. Macromol. 2017, 94, 492–499.
- Zhang, H.; Chen, Z.; Du, M.; Li, Y.; Chen, Y. Enhanced gene transfection efficiency by low-dose 25 kDa polyethylenimine by the assistance of 1.8 kDa polyethylenimine. Drug Deliv. 2018, 25, 1740–1745.
- Shirakura, T.; Ray, A.; Kopelman, R. Polyethylenimine incorporation into hydrogel nanomatrices for enhancing nanoparticle-assisted chemotherapy. RSC Adv. 2016, 6, 48016–48024.
- Zhuk, D.S.; Gembitskii, P.A.; Kargin, V.A. Advances in the chemistry of polyethyleneimine (polyaziridine). Russ. Chem. Rev. 1965, 34, 515–527.
- Zou, L.; Lee, S.Y.; Wu, Q.; Zhang, H.; Bastian, A.; Orji, C.; Payne, G.; Galvez, A.; Thomas, T.; Zhang, Z.; et al. Facile Gene Delivery Derived from Branched Low Molecular Weight Polyethylenimine by High Efficient Chemistry. J. Biomed. Nanotechnol. 2018, 14, 1785–1795.
- Jiang, S.N.; Li, S.R.; Mei, W.K.; Zhang, J.Y.; Wu, Y.J.; Liu, S.R.; Yu, X.F. Interlock Protective System from Hyperbranched Polyethyleneimine and Choline Phosphate Liposome for Targeted In Vivo Gene Delivery. Adv. Mater. Interfaces 2022, 9, 2201390.
- Cheng, D.; Theivendran, S.; Tang, J.; Cai, L.; Zhang, J.; Song, H.; Yu, C.Z. Surface chemistry of spiky silica nanoparticles tailors polyethyleneimine binding and intracellular DNA delivery. J. Colloid Interface Sci. 2022, 628, 297–305.
- Wang, H.; Xiong, J.; Liu, G.; Wang, Y. A pH-Sensitive Phospholipid Polymeric Prodrug Based on Branched Polyethylenimine for Intracellular Drug Delivery. Macromol. Chem. Phys. 2016, 217, 2049–2055.
- Duan, Q.-Y.; Zhu, Y.-X.; Jia, H.-R.; Guo, Y.; Zhang, X.; Gu, R.; Li, C.; Wu, F.-G. Platinum-Coordinated Dual-Responsive Nanogels for Universal Drug Delivery and Combination Cancer Therapy. Small 2022, 18, 2203260.
- Chen, X.M.; Feng, W.J.; Bisoyi, H.K.; Zhang, S.; Chen, X.; Yang, H.; Li, Q. Light-activated photodeformable supramolecular dissipative self-assemblies. Nat. Commun. 2022, 13, 3216.
- Fox, S.J.; Fazil, M.H.; Dhand, C.; Venkatesh, M.; Goh, E.T.; Harini, S.; Eugene, C.; Lim, R.R.; Ramakrishna, S.; Chaurasia, S.S.; et al. Insight into membrane selectivity of linear and branched polyethylenimines and their potential as biocides for advanced wound dressings. Acta Biomater. 2016, 37, 155–164.
- Mayandi, V.; Sridhar, S.; Fazil, M.; Goh, E.T.L.; Htoon, H.M.; Orive, G.; Choong, Y.K.; Saravanan, R.; Beuerman, R.W.; Barkham, T.M.S.; et al. Protective Action of Linear Polyethylenimine against Staphylococcus aureus Colonization and Exaggerated Inflammation in Vitro and in Vivo. ACS Infect. Dis. 2019, 5, 1411–1422.
- Socia, A.; Liu, Y.; Zhao, Y.; Abend, A.; Wuelfing, W.P. Development of an ultra-high-performance liquid chromatography-charged aerosol detection/UV method for the quantitation of linear polyethylenimines in oligonucleotide polyplexes. J. Sep. Sci. 2020, 43, 3876–3884.
- Kim, H.; Bae, Y.M.; Kim, H.A.; Hyun, H.; Yu, G.S.; Choi, J.S.; Lee, M. Synthesis and characterization of dexamethasone-conjugated linear polyethylenimine as a gene carrier. J. Cell. Biochem. 2010, 110, 743–751.
- Wagner, E.; Kloeckner, J. Gene delivery using polymer therapeutics. In Polymer Therapeutics I: Polymers as Drugs, Conjugates and Gene Delivery Systems; SatchiFainaro, R., Duncan, R., Eds.; Spinger: Berlin/Heidelberg, Germany, 2006; Volume 192, pp. 135–173.
- Xu, P.; Quick, G.K.; Yeo, Y. Gene delivery through the use of a hyaluronate-associated intracellularly degradable crosslinked polyethyleneimine. Biomaterials 2009, 30, 5834–5843.
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995.
- Hunter, A.C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 2006, 58, 1523–1531.
- Li, J.; Hu, Y.; Yang, J.; Sun, W.; Cai, H.; Wei, P.; Sun, Y.; Zhang, G.; Shi, X.; Shen, M. Facile synthesis of folic acid-functionalized iron oxide nanoparticles with ultrahigh relaxivity for targeted tumor MR imaging. J. Mater. Chem. B 2015, 3, 5720–5730.
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Facile one-pot synthesis of composite nanoparticles for dual-mode MR/CT imaging applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366.
- Chen, C.; Zhou, B.; Zhu, X.; Shen, M.; Shi, X. Branched polyethyleneimine modified with hyaluronic acid via a PEG spacer for targeted anticancer drug delivery. RSC Adv. 2016, 6, 9232–9239.
- Li, A.J.; Qiu, J.R.; Zhou, B.Q.; Xu, B.; Xiong, Z.J.; Hao, X.X.; Shi, X.Y.; Cao, X.Y. The gene transfection and endocytic uptake pathways mediated by PEGylated PEI-entrapped gold nanoparticles. Arab. J. Chem. 2020, 13, 2558–2567.
- Li, A.; Zhou, B.; Alves, C.S.; Xu, B.; Guo, R.; Shi, X.; Cao, X. Mechanistic Studies of Enhanced PCR Using PEGylated PEI-Entrapped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 25808–25817.
- Wang, Y.; Xiong, Z.; He, Y.; Zhou, B.; Qu, J.; Shen, M.; Shi, X.; Xia, J. Optimization of the composition and dosage of PEGylated polyethylenimine-entrapped gold nanoparticles for blood pool, tumor, and lymph node CT imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 9–16.
- Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Mignani, S.; Majoral, J.-P.; Shi, X. Targeted tumor dual mode CT/MR imaging using multifunctional polyethylenimine-entrapped gold nanoparticles loaded with gadolinium. Drug Deliv. 2018, 25, 178–186.
- Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Shi, X. Acetylated Polyethylenimine-Entrapped Gold Nanoparticles Enable Negative Computed Tomography Imaging of Orthotopic Hepatic Carcinoma. Langmuir 2018, 34, 8701–8707.
- Zhou, B.; Xiong, Z.; Zhu, J.; Shen, M.; Tang, G.; Peng, C.; Shi, X. PEGylated polyethylenimine-entrapped gold nanoparticles loaded with gadolinium for dual-mode CT/MR imaging applications. Nanomedicine 2016, 11, 1639–1652.
- Zhou, B.; Zhao, L.; Shen, M.; Zhao, J.; Shi, X. A multifunctional polyethylenimine-based nanoplatform for targeted anticancer drug delivery to tumors in vivo. J. Mater. Chem. B 2017, 5, 1542–1550.
- Zhou, B.; Wang, R.; Chen, F.; Zhao, L.; Wang, P.; Li, X.; Banyai, I.; Ouyang, Q.; Shi, X.; Shen, M. 99mTc-Labeled RGD-Polyethylenimine Conjugates with Entrapped Gold Nanoparticles in the Cavities for Dual-Mode SPECT/CT Imaging of Hepatic Carcinoma. ACS Appl. Mater. Interfaces 2018, 10, 6146–6154.
- Nakamura, Y.; Kim, C.W.; Tsuchiya, A.; Kushio, S.; Nobori, T.; Li, K.; Lee, E.K.; Zhao, G.X.; Funamoto, D.; Niidome, T.; et al. Branched polyethylenimine-based PKCalpha-responsive gene carriers. J. Biomater. Sci. Polym. Ed. 2013, 24, 1858–1868.
- Ghoul, M.; Bacquet, M.; Morcellet, M. Uptake of heavy metals from synthetic aqueous solutions using modified PEI—Silica gels. Water Res. 2003, 37, 729–734.
- Deng, S.; Ting, Y.P. Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res. 2005, 39, 2167–2177.
- Song, M.-H.; Won, S.W.; Yun, Y.-S. Decarboxylated polyethylenimine-modified bacterial biosorbent for Ru biosorption from Ru-bearing acetic acid wastewater. Chem. Eng. J. 2013, 230, 303–307.
- Forrest, M.L.; Meister, G.E.; Koerber, J.T.; Pack, D.W. Partial Acetylation of Polyethylenimine Enhances In Vitro Gene Delivery. Pharm. Res. 2004, 21, 365–371.
- Calarco, A.; Bosetti, M.; Margarucci, S.; Fusaro, L.; Nicoli, E.; Petillo, O.; Cannas, M.; Galderisi, U.; Peluso, G. The genotoxicity of PEI-based nanoparticles is reduced by acetylation of polyethylenimine amines in human primary cells. Toxicol. Lett. 2013, 218, 10–17.
- Wu, Y.; Liu, C.; Zhao, X.; Xiang, J. A new biodegradable polymer: PEGylated chitosan-g-PEI possessing a hydroxyl group at the PEG end. J. Polym. Res. 2007, 15, 181–185.
- Dong, X.; Lin, L.; Chen, J.; Guo, Z.; Tian, H.; Li, Y.; Wei, Y.; Chen, X. A serum-tolerant hydroxyl-modified polyethylenimine as versatile carriers of pDNA/siRNA. Macromol. Biosci. 2013, 13, 512–522.
- Xin, W.; De Rosa, I.M.; Ye, S.; Zheng, L.; Yin, X.; Carlson, L.; Yang, J.-M.; Kodambaka, S. Effects of electron beam irradiation and hydroxyl ion concentration on morphological stability of polyethylenimine-capped gold nanoparticles. Mater. Res. Express 2019, 6, 125031.
- Sung, S.-J.; Min, S.H.; Cho, K.Y.; Lee, S.; Min, Y.-J.; Yeom, Y.I.; Park, J.-K. Effect of Polyethylene Glycol on Gene Delivery of Polyethylenimine. Biol. Pharm. Bull. 2003, 26, 492–500.
- Luo, X.; Pan, S.; Feng, M.; Wen, Y.; Zhang, W. Stability of poly(ethylene glycol)-graft-polyethylenimine copolymer/DNA complexes: Influences of PEG molecular weight and PEGylation degree. J. Mater. Sci. Mater. Med. 2010, 21, 597–607.
- Craciun, B.F.; Gavril, G.; Peptanariu, D.; Ursu, L.E.; Clima, L.; Pinteala, M. Synergistic Effect of Low Molecular Weight Polyethylenimine and Polyethylene Glycol Components in Dynamic Nonviral Vector Structure, Toxicity, and Transfection Efficiency. Molecules 2019, 24, 1460.
- Yang, S.; Yang, X.; Liu, Y.; Zheng, B.; Meng, L.; Lee, R.J.; Xie, J.; Teng, L. Non-covalent complexes of folic acid and oleic acid conjugated polyethylenimine: An efficient vehicle for antisense oligonucleotide delivery. Colloids Surf. B Biointerfaces 2015, 135, 274–282.
- Seo, S.J.; Lee, S.Y.; Choi, S.J.; Kim, H.W. Tumor-Targeting Co-Delivery of Drug and Gene from Temperature-Triggered Micelles. Macromol. Biosci. 2015, 15, 1198–1204.
- Park, J.S.; Yi, S.W.; Kim, H.J.; Park, K.H. Receptor-mediated gene delivery into human mesenchymal stem cells using hyaluronic acid-shielded polyethylenimine/pDNA nanogels. Carbohydr. Polym. 2016, 136, 791–802.
- Liang, K.; Bae, K.H.; Lee, F.; Xu, K.; Chung, J.E.; Gao, S.J.; Kurisawa, M. Self-assembled ternary complexes stabilized with hyaluronic acid-green tea catechin conjugates for targeted gene delivery. J. Control. Release 2016, 226, 205–216.
- Shi, H.; Han, H.; Xing, Z.; Chen, J.; Wang, Y.; Zhang, A.; Shi, W.; Li, Q. A protein–polymer hybrid gene carrier based on thermophilic histone and polyethylenimine. New J. Chem. 2015, 39, 6718–6721.
- Toita, R.; Kang, J.H.; Kim, C.W.; Shiosaki, S.; Mori, T.; Niidome, T.; Katayama, Y. Effect of peptide content on the regulation of transgene expression by protein kinase Calpha-responsive linear polyethylenimine-peptide conjugates. Colloids Surf. B Biointerfaces 2014, 123, 123–129.
- Murata, H.; Futami, J.; Kitazoe, M.; Yonehara, T.; Nakanishi, H.; Kosaka, M.; Tada, H.; Sakaguchi, M.; Yagi, Y.; Seno, M.; et al. Intracellular delivery of glutathione S-transferase-fused proteins into mammalian cells by polyethylenimine-glutathione conjugates. J. Biochem. 2008, 144, 447–455.
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120.
- Shim, M.K.; Yang, S.; Sun, I.-C.; Kim, K. Tumor-activated carrier-free prodrug nanoparticles for targeted cancer Immunotherapy: Preclinical evidence for safe and effective drug delivery. Adv. Drug Deliv. Rev. 2022, 183, 114177.
- Hadji, H.; Bouchemal, K. Effect of micro- and nanoparticle shape on biological processes. J. Control. Release 2022, 342, 93–110.
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224.
- Tseng, W.C.; Su, L.Y.; Fang, T.Y. pH responsive PEGylation through metal affinity for gene delivery mediated by histidine-grafted polyethylenimine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 375–386.
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M.A. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880.
- Lahrouch, F.; Siberchicot, B.; Fevre, J.; Leost, L.; Aupiais, J.; Solari, P.L.; Den Auwer, C.; Di Giorgio, C. Carboxylate- and Phosphonate-Modified Polyethylenimine: Toward the Design of Actinide Decorporation Agents. Inorg. Chem. 2020, 59, 128–137.
- Zou, Y.; Li, D.; Wang, Y.; Ouyang, Z.; Peng, Y.; Tomas, H.; Xia, J.; Rodrigues, J.; Shen, M.; Shi, X. Polyethylenimine Nanogels Incorporated with Ultrasmall Iron Oxide Nanoparticles and Doxorubicin for MR Imaging-Guided Chemotherapy of Tumors. Bioconjug. Chem. 2020, 31, 907–915.
- Zhao, L.; Wen, S.; Zhu, M.; Li, D.; Xing, Y.; Shen, M.; Shi, X.; Zhao, J. 99mTc-labelled multifunctional polyethylenimine-entrapped gold nanoparticles for dual mode SPECT and CT imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, 488–498.
- Sun, W.; Zhang, X.; Jia, H.R.; Zhu, Y.X.; Guo, Y.; Gao, G.; Li, Y.H.; Wu, F.G. Water-Dispersible Candle Soot-Derived Carbon Nano-Onion Clusters for Imaging-Guided Photothermal Cancer Therapy. Small 2019, 15, 1804575.
- Feng, L.; Yang, X.; Shi, X.; Tan, X.; Peng, R.; Wang, J.; Liu, Z. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small 2013, 9, 1989–1997.
- Sun, X.; Dong, S.; Wang, E. One-step synthesis and characterization of polyelectrolyte-protected gold nanoparticles through a thermal process. Polymer 2004, 45, 2181–2184.
- Wang, S.; Yan, J.; Chen, L. Formation of gold nanoparticles and self-assembly into dimer and trimer aggregates. Mater. Lett. 2005, 59, 1383–1386.
- Kosmella, S.; Koetz, J. Poly(ethyleneimine) as reducing and stabilizing agent for the formation of gold nanoparticles in w/o microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 150–156.
- Wu, J.; Huang, J.; Kuang, S.; Chen, J.; Li, X.; Chen, B.; Wang, J.; Cheng, D.; Shuai, X. Synergistic MicroRNA Therapy in Liver Fibrotic Rat Using MRI-Visible Nanocarrier Targeting Hepatic Stellate Cells. Adv. Sci. 2019, 6, 1801809.
- Peng, Y.; Gao, Y.; Yang, C.; Guo, R.; Shi, X.; Cao, X. Low-Molecular-Weight Poly(ethylenimine) Nanogels Loaded with Ultrasmall Iron Oxide Nanoparticles for T1-Weighted MR Imaging-Guided Gene Therapy of Sarcoma. ACS Appl. Mater. Interfaces 2021, 13, 27806–27813.
- Lin, G.; Huang, J.; Zhang, M.; Chen, S.; Zhang, M. Chitosan-Crosslinked Low Molecular Weight PEI-Conjugated Iron Oxide Nanoparticle for Safe and Effective DNA Delivery to Breast Cancer Cells. Nanomaterials 2022, 12, 584.
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Polyethyleneimine-mediated synthesis of folic acid-targeted iron oxide nanoparticles for in vivo tumor MR imaging. Biomaterials 2013, 34, 8382–8392.
- Hu, Y.; Mignani, S.; Majoral, J.-P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900.
- Yu, W.; Li, X.; He, J.; Chen, Y.; Qi, L.; Yuan, P.; Ou, K.; Liu, F.; Zhou, Y.; Qin, X. Graphene oxide-silver nanocomposites embedded nanofiber core-spun yarns for durable antibacterial textiles. J. Colloid Interface Sci. 2021, 584, 164–173.
- Wang, F.; Yan, B.; Li, Z.; Wang, P.; Zhou, M.; Yu, Y.; Yuan, J.; Cui, L.; Wang, Q. Rapid Antibacterial Effects of Silk Fabric Constructed through Enzymatic Grafting of Modified PEI and AgNP Deposition. ACS Appl. Mater. Interfaces 2021, 13, 33505–33515.
- Rao, S.; Li, Y.; Liu, H.; Gao, S.; Zhao, J.; Rahman, N.; Li, J.; Zhou, Y.; Wang, D.; Zhang, L.; et al. Polyethyleneimine induced highly dispersed Ag nanoparticles over g-C3N4 nanosheets for efficient photocatalytic and antibacterial performance. Ceram. Int. 2021, 47, 8528–8537.
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287.
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822.
- Zhu, J.; Zhao, L.; Zhao, P.; Yang, J.; Shi, J.; Zhao, J. Charge-conversional polyethylenimine-entrapped gold nanoparticles with (131)I-labeling for enhanced dual mode SPECT/CT imaging and radiotherapy of tumors. Biomater. Sci. 2020, 8, 3956–3965.
- Quintana, A.; Raczka, E.; Piehler, L.; Lee, I.; Myc, A.; Majoros, I.; Patri, A.K.; Thomas, T.; Mulé, J.; Baker Jr, J.R. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm. Res. 2002, 19, 1310–1316.
- Stella, B.; Arpicco, S.; Peracchia, M.T.; Desmaële, D.; Hoebeke, J.; Renoir, M.; D’Angelo, J.; Cattel, L.; Couvreur, P. Design of folic acid-conjugated nanoparticles for drug targeting. J. Pharm. Sci. 2000, 89, 1452–1464.
- El-Dakdouki, M.H.; El-Boubbou, K.; Zhu, D.C.; Huang, X. A simple method for the synthesis of hyaluronic acid coated magnetic nanoparticles for highly efficient cell labelling and in vivo imaging. RSC Adv. 2011, 1, 1449–1452.
- Ma, M.; Chen, H.; Chen, Y.; Zhang, K.; Wang, X.; Cui, X.; Shi, J. Hyaluronic acid-conjugated mesoporous silica nanoparticles: Excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 2012, 22, 5615–5621.
- Selim, K.M.K.; Ha, Y.-S.; Kim, S.-J.; Chang, Y.; Kim, T.-J.; Lee, G.H.; Kang, I.-K. Surface modification of magnetite nanoparticles using lactobionic acid and their interaction with hepatocytes. Biomaterials 2007, 28, 710–716.
- Yang, W.; Pan, C.-Y.; Liu, X.-Q.; Wang, J. Multiple Functional Hyperbranched Poly(amido amine) Nanoparticles: Synthesis and Application in Cell Imaging. Biomacromolecules 2011, 12, 1523–1531.
- Liu, C.; Liu, F.; Feng, L.; Li, M.; Zhang, J.; Zhang, N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013, 34, 2547–2564.
- Chiu, S.-J.; Ueno, N.T.; Lee, R.J. Tumor-targeted gene delivery via anti-HER2 antibody (trastuzumab, Herceptin®) conjugated polyethylenimine. J. Control. Release 2004, 97, 357–369.
- Xiao, Y.C.; Fan, Y.; Tu, W.Z.; Ning, Y.S.; Zhu, M.F.; Liu, Y.; Shi, X.Y. Multifunctional PLGA microfibrous rings enable MR imaging-guided tumor chemotherapy and metastasis inhibition through prevention of circulating tumor cell shedding. Nano Today 2021, 38, 101123.
- Ratanajanchai, M.; Soodvilai, S.; Pimpha, N.; Sunintaboon, P. Polyethylenimine-immobilized core-shell nanoparticles: Synthesis, characterization, and biocompatibility test. Mater. Sci. Eng. C 2014, 34, 377–383.
- Ding, Y.; Wang, J.; Wong, C.S.; Halley, P.J.; Guo, Q. Synthesis, Characterization and Biocompatibility of Novel Biodegradable Cross-linked Co-polymers Based on Poly(propylene oxide) Diglycidylether and Polyethylenimine. J. Biomater. Sci. Polym. Ed. 2011, 22, 457–473.
- Pun, S.H.; Bellocq, N.C.; Liu, A.J.; Jensen, G.; Machemer, T.; Quijano, E.; Schluep, T.; Wen, S.F.; Engler, H.; Heidel, J.; et al. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjug. Chem. 2004, 15, 831–840.
- van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007, 24, 1405–1414.
- Appelhans, D.; Komber, H.; Quadir, M.A.; Richter, S.; Schwarz, S.; van der Vlist, J.; Aigner, A.; Mueller, M.; Loos, K.; Seidel, J.; et al. Hyperbranched PEI with Various Oligosaccharide Architectures: Synthesis, Characterization, ATP Complexation, and Cellular Uptake Properties. Biomacromolecules 2009, 10, 1114–1124.
- Elfinger, M.; Pfeifer, C.; Uezguen, S.; Golas, M.M.; Sander, B.; Maucksch, C.; Stark, H.; Aneja, M.K.; Rudolph, C. Self-Assembly of Ternary Insulin-Polyethylenimine (PEI)-DNA Nanoparticles for Enhanced Gene Delivery and Expression in Alveolar Epithelial Cells. Biomacromolecules 2009, 10, 2912–2920.
- Liang, S.; Yu, H.; Xiang, J.; Yang, W.; Chen, X.; Liu, Y.; Gao, C.; Yan, G. New naphthalimide modified polyethylenimine nanoparticles as fluorescent probe for DNA detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 359–365.
- Ashwanikumar, N.; Kumar, N.A.; Nair, S.A.; Kumar, G.S.V. Dual drug delivery of 5-fluorouracil (5-FU) and methotrexate (MTX) through random copolymeric nanomicelles of PLGA and polyethylenimine demonstrating enhanced cell uptake and cytotoxicity. Colloids Surf. B Biointerfaces 2014, 122, 520–528.
- Zhang, Y.; Mao, L.; Liu, J.; Liu, T. Self-fluorescent drug delivery vector based on genipin-crosslinked polyethylenimine conjugated globin nanoparticle. Mater. Sci. Eng. C 2017, 71, 17–24.
- Kievit, F.M.; Wang, F.Y.; Fang, C.; Mok, H.; Wang, K.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J. Control. Release 2011, 152, 76–83.
- Dong, D.-W.; Xiang, B.; Gao, W.; Yang, Z.-Z.; Li, J.-Q.; Qi, X.-R. pH-responsive complexes using prefunctionalized polymers for synchronous delivery of doxorubicin and siRNA to cancer cells. Biomaterials 2013, 34, 4849–4859.
- Yan, J.; Su, T.; Cheng, F.; Cao, J.; Zhang, H.; He, B. Multifunctional nanoparticles self-assembled from polyethylenimine-based graft polymers as efficient anticancer drug delivery. Colloids Surf. B Biointerfaces 2017, 155, 118–127.
- Tsai, L.H.; Yen, C.H.; Hsieh, H.Y.; Young, T.H. Doxorubicin Loaded PLGA Nanoparticle with Cationic/Anionic Polyelectrolyte Decoration: Characterization, and Its Therapeutic Potency. Polymers 2021, 13, 693.
- Nehate, C.; Moothedathu Raynold, A.A.; Koul, V. ATRP Fabricated and Short Chain Polyethylenimine Grafted Redox Sensitive Polymeric Nanoparticles for Codelivery of Anticancer Drug and siRNA in Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 39672–39687.
- Cui, N.; Zhu, S.-H. Monoclonal antibody-tagged polyethylenimine (PEI)/poly(lactide) (PLA) nanoparticles for the enhanced delivery of doxorubicin in HER-positive breast cancers. RSC Adv. 2016, 6, 79822–79829.
- Weiss, S.I.; Sieverling, N.; Niclasen, M.; Maucksch, C.; Thunemann, A.F.; Mohwald, H.; Reinhardt, D.; Rosenecker, J.; Rudolph, C. Uronic acids functionalized polyethyleneimine (PEI)-polyethyleneglycol (PEG)-graft-copolymers as novel synthetic gene carriers. Biomaterials 2006, 27, 2302–2312.
- Wang, J.; Meng, F.; Kim, B.K.; Ke, X.; Yeo, Y. In-vitro and in-vivo difference in gene delivery by lithocholic acid-polyethyleneimine conjugate. Biomaterials 2019, 217, 119296.
- Zhang, Y.; Lin, L.; Liu, L.; Liu, F.; Maruyama, A.; Tian, H.; Chen, X. Ionic-crosslinked polysaccharide/PEI/DNA nanoparticles for stabilized gene delivery. Carbohydr. Polym. 2018, 201, 246–256.
- Yin, Y.; Li, X.; Ma, H.; Zhang, J.; Yu, D.; Zhao, R.; Yu, S.; Nie, G.; Wang, H. In Situ Transforming RNA Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy. Nano Lett. 2021, 21, 2224–2231.
- Kordalivand, N.; Li, D.; Beztsinna, N.; Sastre Torano, J.; Mastrobattista, E.; van Nostrum, C.F.; Hennink, W.E.; Vermonden, T. Polyethyleneimine coated nanogels for the intracellular delivery of RNase A for cancer therapy. Chem. Eng. J. 2018, 340, 32–41.
- Meng, Y.; Wang, S.S.; Li, C.Y.; Qian, M.; Yan, X.Y.; Yao, S.C.; Peng, X.Y.; Wang, Y.; Huang, R.Q. Photothermal combined gene therapy achieved by polyethyleneimine-grafted oxidized mesoporous carbon nanospheres. Biomaterials 2016, 100, 134–142.
- Li, G.; Yuan, S.; Deng, D.; Ou, T.; Li, Y.; Sun, R.; Lei, Q.; Wang, X.; Shen, W.; Cheng, Y.; et al. Fluorinated Polyethylenimine to Enable Transmucosal Delivery of Photosensitizer-Conjugated Catalase for Photodynamic Therapy of Orthotopic Bladder Tumors Postintravesical Instillation. Adv. Funct. Mater. 2019, 29, 1901932.
- Chen, W.X.; Li, S.; Shen, Y.X.; Cai, Y.F.; Jin, J.; Yang, Z.Q. Polyethylenimine modified graphene oxide for effective chemo-gene-photothermal triples therapy of triple-negative breast cancer and inhibits metastasis. J. Drug Deliv. Sci. Technol. 2022, 74, 103521.
- Chen, L.; Zhou, L.L.; Wang, C.H.; Han, Y.; Lu, Y.L.; Liu, J.; Hu, X.C.; Yao, T.M.; Lin, Y.; Liang, S.J.; et al. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv. Mater. 2019, 31, 1904997.
- Wang, J.L.; Tang, G.P.; Shen, J.; Hu, Q.L.; Xu, F.J.; Wang, Q.Q.; Li, Z.H.; Yang, W.T. A gene nanocomplex conjugated with monoclonal antibodies for targeted therapy of hepatocellular carcinoma. Biomaterials 2012, 33, 4597–4607.
- Li, J.; Yu, X.; Shi, X.; Shen, M. Cancer nanomedicine based on polyethylenimine-mediated multifunctional nanosystems. Prog. Mater. Sci. 2022, 124, 100871.
- Zou, Y.; Li, D.; Shen, M.W.; Shi, X.Y. Polyethylenimine-Based Nanogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1900272.
- Liu, X.; Gao, C.; Gu, J.; Jiang, Y.; Yang, X.; Li, S.; Gao, W.; An, T.; Duan, H.; Fu, J.; et al. Hyaluronic Acid Stabilized Iodine-Containing Nanoparticles with Au Nanoshell Coating for X-ray CT Imaging and Photothermal Therapy of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 27622–27631.
- Zhuang, Y.; Zhao, L.; Zheng, L.; Hu, Y.; Ding, L.; Li, X.; Liu, C.; Zhao, J.; Shi, X.; Guo, R. LAPONITE-Polyethylenimine Based Theranostic Nanoplatform for Tumor-Targeting CT Imaging and Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 431–442.
- Zhu, J.; Sun, W.; Zhang, J.; Zhou, Y.; Shen, M.; Peng, C.; Shi, X. Facile Formation of Gold-Nanoparticle-Loaded gamma-Polyglutamic Acid Nanogels for Tumor Computed Tomography Imaging. Bioconjug. Chem. 2017, 28, 2692–2697.
- Zhang, P.; Wang, L.; Chen, X.; Li, X.; Yuan, Q. Ultrasmall PEI-Decorated Bi2Se3 Nanodots as a Multifunctional Theranostic Nanoplatform for in vivo CT Imaging-Guided Cancer Photothermal Therapy. Front. Pharmacol. 2021, 12, 795012.
- Zhou, S.Y.; Wu, Z.K.; Chen, X.S.; Jia, L.S.; Zhu, W. PEGylated Polyethylenimine as Enhanced T-1 Contrast Agent for Efficient Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2014, 6, 11459–11469.
- Wang, D.; Su, H.; Liu, Y.; Wu, C.; Xia, C.; Sun, J.; Gao, F.; Gong, Q.; Song, B.; Ai, H. Near-infrared fluorescent amphiphilic polycation wrapped magnetite nanoparticles as multimodality probes. Chin. Sci. Bull. 2012, 57, 4012–4018.
- Liu, G.; Xia, C.; Wang, Z.; Lv, F.; Gao, F.; Gong, Q.; Song, B.; Ai, H.; Gu, Z. Magnetic resonance imaging probes for labeling of chondrocyte cells. J. Mater. Sci. Mater. Med. 2011, 22, 601–606.
- Cai, H.D.; An, X.; Wen, S.H.; Li, J.C.; Zhang, G.X.; Shi, X.Y.; Shen, M.W. Facile Synthesis of Gd(OH)3-Doped Fe3O4 Nanoparticles for Dual-Mode T1- and T2-Weighted Magnetic Resonance Imaging Applications. Part. Part. Syst. Charact. 2015, 32, 934–943.
- Hu, Y.; Wang, R.Z.; Li, J.C.; Ding, L.; Wang, X.L.; Shi, X.Y.; Shen, M.W. Facile Synthesis of Lactobionic Acid-Targeted Iron Oxide Nanoparticles with Ultrahigh Relaxivity for Targeted MR Imaging of an Orthotopic Model of Human Hepatocellular Carcinoma. Part. Part. Syst. Charact. 2017, 34, 1600113.
- Li, J.; He, Y.; Sun, W.; Luo, Y.; Cai, H.; Pan, Y.; Shen, M.; Xia, J.; Shi, X. Hyaluronic acid-modified hydrothermally synthesized iron oxide nanoparticles for targeted tumor MR imaging. Biomaterials 2014, 35, 3666–3677.
- Zhu, W.; Zhao, L.; Fan, Y.; Zhao, J.; Shi, X.; Shen, M. 131I-Labeled Multifunctional Polyphosphazene Nanospheres for SPECT Imaging-Guided Radiotherapy of Tumors. Adv. Healthcare Mater. 2019, 8, 1901299.
- Zhao, L.; Zhu, J.; Gong, J.; Song, N.; Wu, S.; Qiao, W.; Yang, J.; Zhu, M.; Zhao, J. Polyethylenimine-based theranostic nanoplatform for glioma-targeting single-photon emission computed tomography imaging and anticancer drug delivery. J. Nanobiotechnol. 2020, 18, 143.
- Li, D.; Wen, S.H.; Sun, W.J.; Zhang, J.L.; Jin, D.Y.; Peng, C.; Shen, M.W.; Shi, X.Y. One-Step Loading of Gold and Gd2O3 Nanoparticles within PEGylated Polyethylenimine for Dual Mode Computed Tomography/Magnetic Resonance Imaging of Tumors. ACS Appl. Bio Mater. 2018, 1, 221–225.
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21.
- Sun, W.J.; Zhang, J.L.; Zhang, C.C.; Zhou, Y.W.; Zhu, J.Z.; Peng, C.; Shen, M.W.; Shi, X.Y. A unique nanogel-based platform for enhanced dual mode tumor MR/CT imaging. J. Mater. Chem. B 2018, 6, 4835–4842.
- Zhang, C.; Sun, W.; Wang, Y.; Xu, F.; Qu, J.; Xia, J.; Shen, M.; Shi, X. Gd-/CuS-Loaded Functional Nanogels for MR/PA Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 9107–9117.
- Hu, Y.; Wang, R.Z.; Wang, S.G.; Ding, L.; Li, J.C.; Luo, Y.; Wang, X.L.; Shen, M.W.; Shi, X.Y. Multifunctional Fe3O4 @ Au core/shell nanostars: A unique platform for multimode imaging and photothermal therapy of tumors. Sci. Rep. 2016, 6, 28325.
- Guo, Z.; Gao, M.; Song, M.; Li, Y.; Zhang, D.; Xu, D.; You, L.; Wang, L.; Zhuang, R.; Su, X.; et al. Superfluorinated PEI Derivative Coupled with 99mTc for ASGPR Targeted 19F MRI/SPECT/PA Tri-Modality Imaging. Adv. Mater. 2016, 28, 5898–5906.
- Kuang, G.Z.; Lu, H.T.; He, S.S.; Xiong, H.J.; Yu, J.; Zhang, Q.F.; Huang, Y.B. Near-Infrared Light-Triggered Polyprodrug/siRNA Loaded Upconversion Nanoparticles for Multi-Modality Imaging and Synergistic Cancer Therapy. Adv. Healthc. Mater. 2021, 10, 2100938.
- Wang, M.; Zhou, B.; Wang, L.; Zhou, F.; Smith, N.; Saunders, D.; Towner, R.A.; Song, J.; Qu, J.; Chen, W.R. Biodegradable pH-responsive amorphous calcium carbonate nanoparticles as immunoadjuvants for multimodal imaging and enhanced photoimmunotherapy. J. Mater. Chem. B 2020, 8, 8261–8270.
- Liu, J.; Zhao, C.; Chen, W.R.; Zhou, B. Recent progress in two-dimensional nanomaterials for cancer theranostics. Coord. Chem. Rev. 2022, 469, 214654.
- Brunier, B.; Sheibat-Othman, N.; Chevalier, Y.; Bourgeat-Lami, E. Partitioning of Laponite Clay Platelets in Pickering Emulsion Polymerization. Langmuir 2016, 32, 112–124.
- Mizuhara, T.; Saha, K.; Moyano, D.F.; Kim, C.S.; Yan, B.; Kim, Y.-K.; Rotello, V.M. Acylsulfonamide-Functionalized Zwitterionic Gold Nanoparticles for Enhanced Cellular Uptake at Tumor pH. Angew. Chem. Int. Ed. 2015, 54, 6567–6570.
