You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Mohamed Kettani-Halabi.
Resistance to antimicrobials and particularly multidrug resistance is one of the greatest challenges in the health system nowadays. The continual increase in the rates of antimicrobial resistance worldwide boosted by the ongoing COVID-19 pandemic poses a major public health threat. Different approaches have been employed to minimize the effect of resistance and control this threat.
- antimicrobial resistance (AMR)
- multidrug-resistant (MDR) bacteria
- therapeutic strategies
1. Introduction
Multiple drug resistance (MDR) is identified by the World Health Organization (WHO) as one of the most serious threats to global health, food security and development [1]. It can affect anyone, at any age and in any country. It is now a major global public health challenge that arises for multiple reasons, including overpopulation, increased global migration, and selective pressure from increased use of antibiotics. The WHO has listed antibiotic resistance as one of the three most important public health threats in the 21st century (Figure 1) [2]. It estimates that infections caused by multidrug-resistant (MDR) bacteria (bacteria that are simultaneously resistant to three or more kinds of antibiotics used in a clinic) kill about 700,000 people worldwide each year, and that this number might rise to 10 million fatalities by 2050, exceeding the current yearly number of cancer-related deaths, if no action is taken [3,4,5][3][4][5].
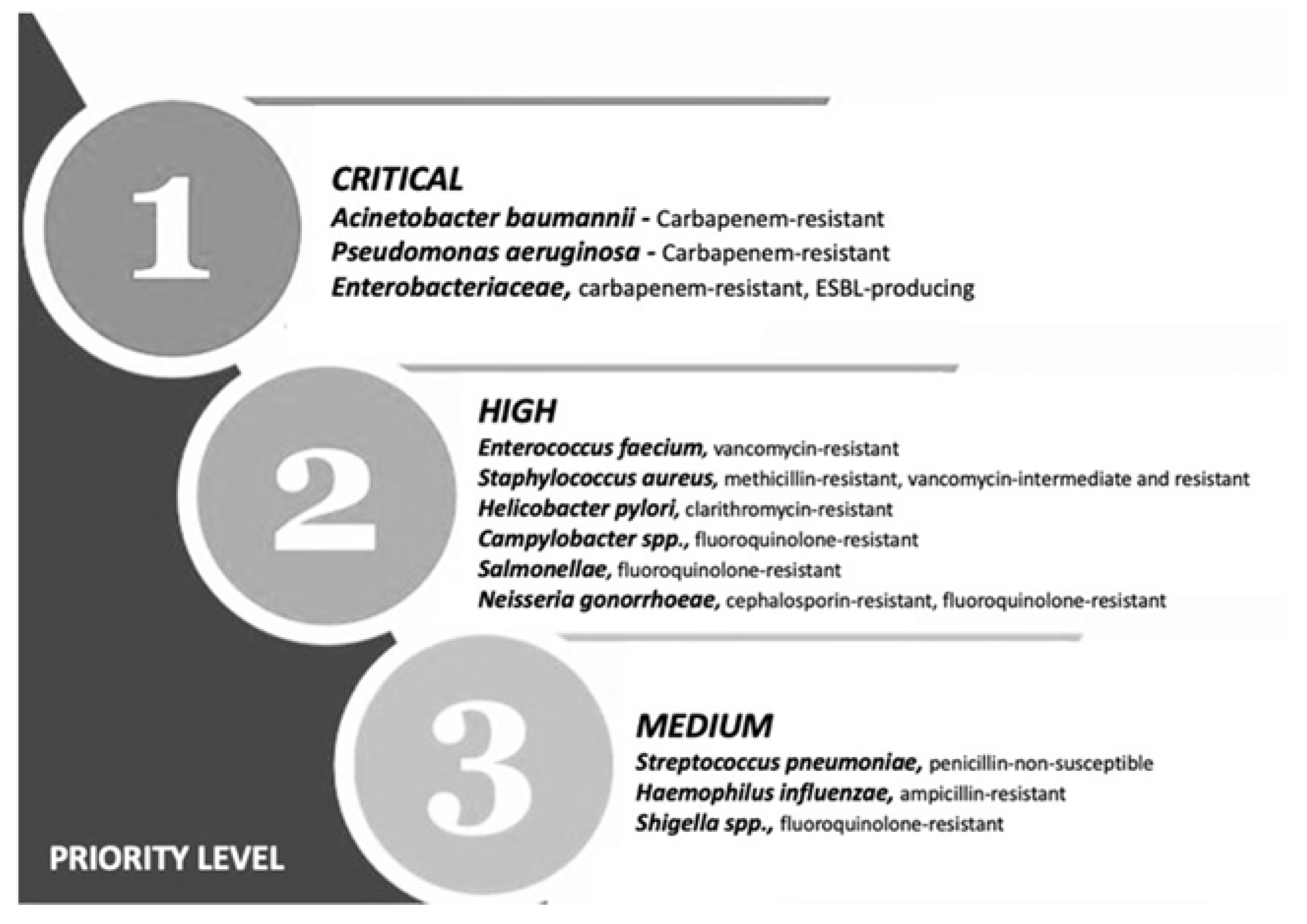
Figure 1. Priority list for development of new antibiotics according to the World Health Organization. Adapted from (Zyman A; et al., 2022) [6].
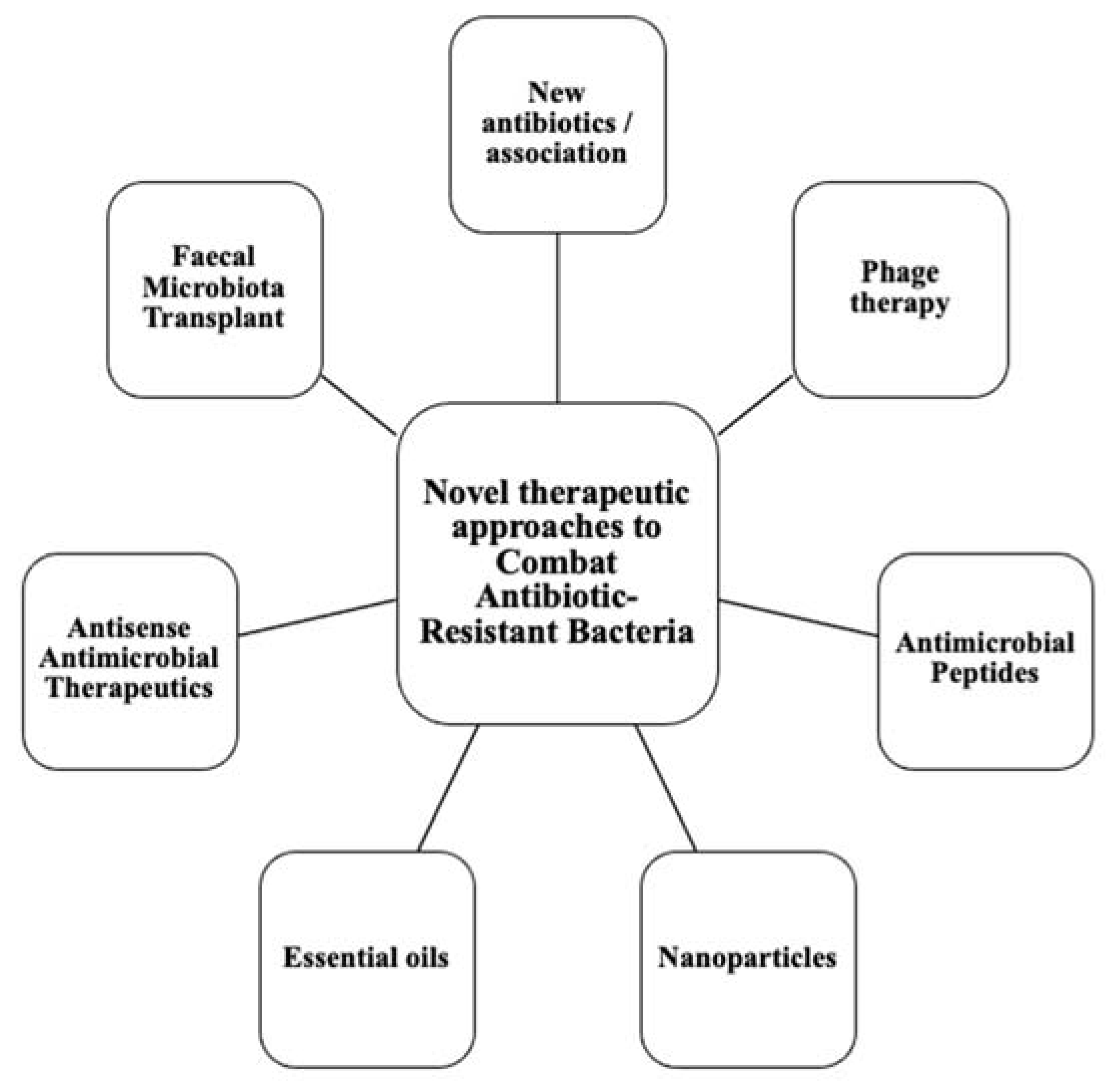
This calls for the scientific community to design new antibiotics or innovative therapeutic approaches for treating critical priority antibiotic-resistant infections [5]. Common bacterial pathogens, such as Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, etc. have evolved and become resistant to multiple antibiotics, and their treatment is now becoming problematic (Figure 1). An increasing number of infections, such as pneumonia, tuberculosis, gonorrhea, or salmonellosis are becoming more difficult to treat as the antibiotics used to treat these infections lose their effectiveness. Unfortunately, the inadequate and irregular administration of antibiotics also contributes significantly to the development of antibiotic resistance, which leads to prolonged hospitalizations and increased medical expenses [7]. Additionally, several studies have reported that widespread use of antibiotics for hospitalized COVID-19 patients without established secondary infection was markedly increased, thereby leading to an increase in antimicrobial resistance through driving selection of MDR organisms [8,9,10,11][8][9][10][11]. The Centers for Disease Control and Prevention (CDC) in its special report of the year 2022 entitled “COVID-19: U.S. Impact on Antimicrobial Resistance” also concluded that the threat of antimicrobial-resistant infections is not only still present but has worsened [12]. Therefore, there is an urgent need for new classes of antimicrobials and other innovative approaches to fight against the emergence of MDR bacteria and escape the therapeutic impasse. In addition to traditional approaches, several new approaches (Figure 2), such as bacteriophages, antimicrobial peptides, essential oils and host-oriented therapies show great potential.
Figure 2. Alternatives therapeutic approaches to conventional antibiotics.
Alternatives therapeutic approaches to conventional antibiotics.
2. Phage Therapeutics
Phage therapy dates back to the beginning of the 20th century, even before Alexander Fleming’s discovery of penicillin in 1928 [68][13]. The first phage activity dated back to 1896, when Ernest Hankin reported that the waters from the rivers Ganges and Yamuna in India possessed antibacterial activity against Vibrio cholerae [69][14]. In the late 1910s, and following initial work by the English bacteriologists Ernest Hankin and Frederick Twort, a French microbiologist from the Pasteur Institute (Felix d’Herelle, 1917) identified viruses that specifically and selectively parasitized bacteria and named them “bacterium eaters” (bacteriophages) [70,71][15][16]. It was d’Herelle who first developed the notion of using phages therapeutically to treat bacterial infection with encouraging results [69][14]. However, since the discovery and development of antibiotics, Phage therapy was largely abandoned in the Western world due to the efficacy and promise held by antibiotics, with the exception of the Soviet Union and some Eastern European countries [69][14]. Recently, and in the face of the rapid emergence of resistant bacteria, phages (estimated to exceed 1031 particles) have re-emerged as alternative and complementary therapies to control bacterial infections [72][17]. Thus, phage therapy has shown to be an interesting alternative in the fight against multi-drug resistant bacteria [73][18]. Phages or bacteriophages are lytic viruses that exclusively and specifically infect bacterial species, showing bactericidal effects against both Gram-positive and Gram-negative bacteria [74,75][19][20]. In contrast, some tend to be specific to a particular species or strain of bacteria [76][21]. The tailed double-stranded DNA phages (order Caudovirales) are the most studied group and are thought to account for 96% of all phages and are easily isolated from various environmental sources (soil, wastewater, and aquatic environments) [73][18]. By adhering, via tail proteins, to specific surface receptors of bacteria, phages insert their genetic material into their bacterial hosts [69][14]. Several types of life cycles can be triggered by bacteriophages, of which the two most frequent are the lytic and lysogenic cycles [68][13]. During the lysogenic cycle, the virion DNA is incorporated in the bacterial genome. The resulting prophage replicates its genetic material within the bacterial cell without damaging it until the lytic cycle is trigger [68,77][13][22]. Undoubtedly, prophages are supposed to be shunned and lytic phages are selected. During the lytic cycle, the phage uses the cellular machinery to yield as many as 20,000 new virions per infected bacterial cell in optimal conditions [68][13]. These phages secrete lytic enzymes (endolysins) that hydrolyze the bacterial cell wall to ensure phage release [77,78][22][23]. Since then, it has become clear that phage therapy and the application of its endolysins offer the possibility to apply more specific antibacterial treatments and propose a potential solution to the problem of antibiotic resistance [79][24]. The table below (Table 1) shows some advantages of applying phage therapy to fight bacterial infections.
Table 1.
Main advantages of bacteriophages for infections control.

| Criteria | Advantages |
|---|---|
| Specificity to bacteria | Highly specific to bacteria (by specific and targeted endolysin mode of action) [24][25] Have no effect on the human host microbiota [25][26] |
| Effect on the immune system | Circumvent the dysbiosis and subsequent overgrowth of pathogenic species often associated with antibiotic treatment [25] |
| Resistance | Phage mixture minimizes the likelihood that bacteria will acquire phage resistance and kill their bacterial host quickly [27] |
| Effectivity on bacterial biofilms | Eradicate biofilms due to the presence of EPS-degrading enzymes like endolysins and depolymerase in their tails [24][28] |
| Dose | Harmless entities showing no ill effects to eukaryotic cells even at high titers (targeted therapy) [29][30][31] Capacity to naturally control bacterial populations (self-dosing property) [31][32] |
| Genetics | Genetic exchange between phages rarely happens [25][33] |
| Environmental impact | Rapid elimination from the environment [24] |
Applications in Medicine
2.1. Applications in Medicine
Before the dawn of antibiotics, phage therapy had been used to treat a broad range of bacterial infection diseases, including cholera [89,90[34][35][36],91], pediatric dysentery [92][37], bubonic plague [93][38], typhoid fever, skin and surgical site infections, peritonitis, septicemia, and external otitis [92,93][37][38]. However, in 1934, the failed attempts to reproduce positive findings had inspired the opposition from the Council on Pharmacy and Chemistry of the American Medical Association [91][36]. This opposition has not prevented parts of Eastern Europe (such as Georgia, Poland and Russia) from continuing to use phages in routine medical practice and today provide uresearchers with a rich source of empirical data [94][39]. For example, The Eliava Institute of Bacteriophage, Microbiology, and Virology in Georgia is one of the longest-running institutions where phage therapy has been provided to frequent bacterial diseases related to urology, pediatrics, internal medicine, and gynecology [71][16]. Recently, phage therapy has been re-employed in the United States and Europe, for the treatment of infections related to burn injuries or soft tissue and skin trauma, osteomyelitis, sepsis, bacteremia, and otitis media as well as urinary tract, pulmonary, and prosthetic device-associated infections, especially when mono- or multi-infected patients with multi-resistant bacteria are without effective treatment options or are terminally ill [71,95][16][40]. In Table 2 below, wresearchers quote the references of the main applications of phage therapy (as an adjuvant or alternative therapy to antibiotics) conducted on human patients infected with various types of MDR bacteria [2]. Results from these studies indicate that this therapy has immense potential with applications in human medicine.
Table 2.
List of references of the main applications of phage therapy against multidrug-resistant bacteria classified according to the WHO priorities.

| Priority | Pathogen Species | Antibiotic-Resistant Bacterium | References | ||||
|---|---|---|---|---|---|---|---|
| Critical | Acinetobacter baumannii, | Carbapenem-resistant | [41][42][43][44][45] | 1[46][47] | |||
| Dosage of bacteriophages, duration of treatment and routes of administration, is poorly controlled, involving the safety and effectiveness of treatment. | Pseudomonas aeruginosa, | Carbapenem-resistant | [48][49][50] | ||||
| [ | 13] | ||||||
| 2 | Inability to replicate the in vitro results in the actual situations. | [86] | Enterobacteriaceae: | ||||
| 3 | Results of experimentation in small animal models does not consistently translate into clinical success, just as in vitro phage activity often fails to correlate with in vivo efficacy. | [81][87][88] | Escherichia coli | ESBL-producing Carbapenem-resistant, |
][ | ||
| 4 | As same as antibiotics, bacteria also develop resistance to phages by specific defense mechanisms. | [25][[51][52][53] | |||||
| 89 | ] | Klebsiella pneumoniae, | Multidrug-resistant Carbapenem-resistant |
[54][55][56 | |||
| 5 | Phages display a short circulation time due to clearance by the spleen. | [90]][57] [57][58] |
|||||
| Enterobacter spp., | Carbapenem-resistant | [59] | |||||
| 6 | [ | 60] | |||||
| Bacterial remnants in the lysate produced from mass production of phages are difficult to be completely eliminated, leading to health risks. | [89][90 | High | Enterococcus faecium, | Vancomycin-resistant | [61][62][63] | ||
| 67 | ] | ||||||
| ] | |||||||
| 7 | Strain specificity of phages hinders mass production. | [21] | Staphylococcus aureus, | Methicillin-resistant, | |||
| 8 | vancomycin-resistant, | [64][65][ | Possibility to contribute to the antimicrobial resistance development through transduction “phage conversion”. | [26]66 | Helicobacter pylori, | Clarithromycin-resistant, | |
| 9 | Key mechanisms that may allow the prediction of in vivo pharmacokinetics and dynamics linked to therapeutic outcome have not yet been fully elucidated. | [13][68][69][70] | |||||
| Campylobacter spp., | Fluoroquinolone-resistant, | ||||||
| 10 | Physicochemical properties of phages in vivo are not fully understood. | [13][71][72] | |||||
| Salmonellae | Fluoroquinolone-resistant, | [ | |||||
| 11 | Lack of regulatory approval for human use. | [29]73][74][75] | |||||
| Neisseria gonorrhoeae, | Cephalosporin-resistant, Fluoroquinolone-resistant, |
[76][77] | |||||
| Medium | Streptococcus pneumoniae, | penicillin-non-susceptible, | [78][79] | ||||
| Haemophilus influenzae, | Ampicillin-resistant, | [80][81][82] | |||||
| Shigella spp., | Fluoroquinolone-resistant, | [83][84][85] |
3.2. Limitations
2.2. Limitations
Although phage therapy has come a long way, and is considered a promising alternative to antimicrobial agents, they have a dark, poorly explored side (Table 3). These limitations complicate the design of clinical protocols, undermine confidence in phage application, and need to be cleared before establishing successful phage therapy on a global scale.
Table 3.
Limits of application of phage therapy in modern human medicine.

| Limits of Application of Phage Therapy | References |
|---|
3. Conclusions
A new era is beginning today with the long-awaited growing research of novel anti-infectious approaches against MDR bacteria. These therapeutic approaches are definably a potent non-antibiotic option to curtail the huge increase in antibiotic resistant bacteria that wresearchers are facing nowadays. It cannot be denied that mechanisms of action of these therapies are complicated to elaborate and that clinical trials are not complete enough to provide valid and concise information. However, all efforts made in making these treatments as a clinical routine must be done with caution taking in consideration the efficacy and safety of each therapy. In the end, weresearchers are convinced that the knowledge and mastery of resistance patterns and mechanisms of action will allow clinicians to increasingly drive antimicrobial treatment towards an individualized and precise medical approach.
References
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic Potentiators Against Multidrug-Resistant Bacteria: Discovery, Development, and Clinical Relevance. Front. Microbiol. 2022, 13, 887251.
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573–3582.
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of Phage Therapy and Corresponding Optimization Strategies: A Review. Molecules 2022, 27, 1857.
- Coates, A.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-infective Ther. 2020, 18, 5–15.
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Zyman, A.; Górski, A.; Międzybrodzki, R. Phage therapy of wound-associated infections. Folia Microbiol. 2022, 67, 193–201.
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. New Engl. J. Med. 2020, 382, 1309–1319.
- Adebisi, Y.A.; Alaran, A.J.; Okereke, M.; Oke, G.I.; Amos, O.A.; Olaoye, O.C.; Oladunjoye, I.; Olanrewaju, A.Y.; Ukor, N.A.; Lucero-Prisno, D.E. COVID-19 and Antimicrobial Resistance: A Review. Infect. Dis. Res. Treat. 2021, 14, 11786337211033870.
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983.
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810.
- Rawson, T.M.; Moore, L.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684.
- Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases; Division of Healthcare Quality Promotion. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. 2022. Available online: https://stacks.cdc.gov/view/cdc/117915.2022 (accessed on 11 October 2022).
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol. Biotechnol. 2021, 63, 1103–1124.
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016, 24, 249–256.
- Venturini, C.; Fabijan, A.P.; Lubian, A.F.; Barbirz, S.; Iredell, J. Biological foundations of successful bacteriophage therapy. EMBO Mol. Med. 2022, 14, e12435.
- Azam, A.H.; Tan, X.-E.; Veeranarayanan, S.; Kiga, K.; Cui, L. Bacteriophage Technology and Modern Medicine. Antibiotics 2021, 10, 999.
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343.
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus Aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314.
- Venhorst, J.; van der Vossen, J.M.B.M.; Agamennone, V. Battling Enteropathogenic Clostridia: Phage Therapy for Clostridioides difficile and Clostridium perfringens. Front. Microbiol. 2022, 13, 891790.
- White, H.E. Bacteriophages: Their Structural Organisation and Function. In Savva EVOE-R; Rijeka, Ed.; IntechOpen: London, UK, 2019; p. 2.
- Bhandari, V.; Suresh, A. Next-Generation Approaches Needed to Tackle Antimicrobial Resistance for the Development of Novel Therapies Against the Deadly Pathogens. Front. Pharmacol. 2022, 13, 1–9.
- Heuler, J.; Fortier, L.-C.; Sun, X. Clostridioides difficile phage biology and application. FEMS Microbiol. Rev. 2021, 45, 1–23.
- Khan, A.; Rao, T.S.; Joshi, H.M. Phage therapy in the Covid-19 era: Advantages over antibiotics. Curr. Res. Microb. Sci. 2022, 3, 100115.
- Bhargava, K.; Nath, G.; Bhargava, A.; Aseri, G.K.; Jain, N. Phage therapeutics: From promises to practices and prospectives. Appl. Microbiol. Biotechnol. 2021, 105, 9047–9067.
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783.
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513.
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666.
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268.
- Kaur, S.; Kumari, A.; Negi, A.K.; Galav, V.; Thakur, S.; Agrawal, M.; Sharma, V. Nanotechnology Based Approaches in Phage Therapy: Overcoming the Pharmacological Barriers. Front. Pharmacol. 2021, 12, 1–18.
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke. Off. J. Int. Stroke. Soc. 2018, 13, 612–632.
- Aranaga, C.; Pantoja, L.D.; Martínez, E.A.; Falco, A. Phage Therapy in the Era of Multidrug Resistance in Bacteria: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4577.
- Kaur, G.; Agarwal, R.; Sharma, R.K. Bacteriophage Therapy for Critical and High-Priority Antibiotic-Resistant Bacteria and Phage Cocktail-Antibiotic Formulation Perspective. Food Environ. Virol. 2021, 13, 433–446.
- Cebriá-Mendoza, M.; Sanjuán, R.; Domingo-Calap, P. Directed Evolution of a Mycobacteriophage. Antibiotics 2019, 8, 46.
- Chanishvili, N. Bacteriophages as Therapeutic and Prophylactic Means: Summary of the Soviet and Post Soviet Experiences. Curr. Drug Deliv. 2016, 13, 309–323.
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85.
- Caflisch, K.; A Suh, G.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev. Anti-Infect. Ther. 2019, 17, 1011–1041.
- Kutter, E.; Sulakvelidze, A. Bacteriophages: Biology and Applications; CRC Press: Boca Raton, FL, USA, 2004; Volume 1.
- Fruciano, D.E.; Bourne, S. Phage as an antimicrobial agent: D’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26.
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2019, 40, 459–463.
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18.
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter baumannii in Mice. Front. Microbiol. 2018, 8, 2659.
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. In Open forum infectious diseases; Oxford University Press: Oxford, UK, 2018; p. ofy064.
- Wu, N.; Chen, L.K.; Zhu, T. Phage therapy for secondary bacterial infections with COVID-19. Curr. Opin. Virol. 2022, 52, 9–14.
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical experience of personalized phage therapy against carbapenem-resistant Acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585.
- Bagińska, N.; Cieślik, M.; Górski, A.; Jończyk-Matysiak, E. The role of antibiotic resistant A. baumannii in the pathogenesis of urinary tract infection and the potential of its treatment with the use of bacteriophage therapy. Antibiotics 2021, 10, 281.
- Anomaly, J. The future of phage: Ethical challenges of using phage therapy to treat bacterial infections. Public Health Ethics. 2020, 13, 82–88.
- Strathdee, S.; Patterson, T. The Perfect Predator: A Scientist’s Race to Save Her Husband from a Deadly Superbug: A Memoir; Hachette Books: New York, NY, USA, 2019.
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45.
- Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.-P. Differential effect of newly isolated phages belonging to PB1-like, phiKZ-like and LUZ24-like viruses against multi-drug resistant Pseudomonas aeruginosa under varying growth conditions. Viruses 2017, 9, 315.
- Chen, P.; Liu, Z.; Tan, X.; Wang, H.; Liang, Y.; Kong, Y.; Sun, W.; Sun, L.; Ma, Y.; Lu, H. Bacteriophage therapy for empyema caused by carbapenem-resistant Pseudomonas aeruginosa. Biosci. Trends 2022, 16, 158–162.
- Terwilliger, A.; Clark, J.; Karris, M.; Hernandez-Santos, H.; Green, S.; Aslam, S.; Maresso, A. Phage Therapy Related Microbial Succession Associated with Successful Clinical Outcome for a Recurrent Urinary Tract Infection. Viruses 2021, 13, 2049.
- Easwaran, M.; De Zoysa, M.; Shin, H. Application of phage therapy: Synergistic effect of phage EcSw (ΦEcSw) and antibiotic combination towards antibiotic-resistant Escherichia coli. Transbound. Emerg. Dis. 2020, 67, 2809–2817.
- Amarillas, L.; Rubí-Rangel, L.; Chaidez, C.; González-Robles, A.; Lightbourn-Rojas, L.; León-Félix, J. Isolation and characterization of phiLLS, a novel phage with potential biocontrol agent against multidrug-resistant Escherichia coli. Front. Microbiol. 2017, 8, 1355.
- Taha, O.A.; Connerton, P.L.; Connerton, I.; El-Shibiny, A. Bacteriophage ZCKP1: A Potential Treatment for Klebsiella pneumoniae Isolated From Diabetic Foot Patients. Front. Microbiol. 2018, 9, 2127.
- Tabassum, R.; Shafique, M.; Khawaja, K.A.; Alvi, I.A.; Rehman, Y.; Sheik, C.; Abbas, Z.; Rehman, S.U. Complete genome analysis of a Siphoviridae phage TSK1 showing biofilm removal potential against Klebsiella pneumoniae. Sci. Rep. 2018, 8, 1–11.
- Qin, J.; Wu, N.; Bao, J.; Shi, X.; Ou, H.; Ye, S.; Zhao, W.; Wei, Z.; Cai, J.; Li, L.; et al. Heterogeneous Klebsiella pneumoniae Co-infections Complicate Personalized Bacteriophage Therapy. Front. Cell. Infect. Microbiol. 2021, 10, 608402.
- Li, M.; Wang, H.; Chen, L.; Guo, G.; Li, P.; Ma, J.; Chen, R.; Du, H.; Liu, Y.; Zhang, W. Identification of a phage-derived depolymerase specific for KL47 capsule of Klebsiella pneumoniae and its therapeutic potential in mice. Virol. Sin. 2022, 37, 538–546.
- Tompkins, K.; van Duin, D. Treatment for carbapenem-resistant Enterobacterales infections: Recent advances and future directions. Eur. J. Clin. Microbiol. 2021, 40, 2053–2068.
- Zhao, J.; Zhang, Z.; Tian, C.; Chen, X.; Hu, L.; Wei, X.; Li, H.; Lin, W.; Jiang, A.; Feng, R.; et al. Characterizing the Biology of Lytic Bacteriophage vB_EaeM_φEap-3 Infecting Multidrug-Resistant Enterobacter aerogenes. Front. Microbiol. 2019, 10, 420.
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133.
- Paul, K.; Merabishvili, M.; Hazan, R.; Christner, M.; Herden, U.; Gelman, D.; Khalifa, L.; Yerushalmy, O.; Coppenhagen-Glazer, S.; Harbauer, T.; et al. Bacteriophage Rescue Therapy of a Vancomycin-Resistant Enterococcus faecium Infection in a One-Year-Old Child following a Third Liver Transplantation. Viruses 2021, 13, 1785.
- Soleimani-Delfan, A.; Bouzari, M.; Wang, R. vB_EfaS-DELF1, a novel Siphoviridae bacteriophage with highly effective lytic activity against vancomycin-resistant Enterococcus faecalis. Virus Res. 2021, 298, 198391.
- Topka-Bielecka, G.; Nejman-Faleńczyk, B.; Bloch, S.; Necel, A.; Węgrzyn, A.; Węgrzyn, G. Phage-Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis. Viruses 2021, 13, 318.
- Barros, J.; Melo, L.D.; Poeta, P.; Igrejas, G.; Ferraz, M.P.; Azeredo, J.; Monteiro, F.J. Lytic bacteriophages against multidrug-resistant Staphylococcus aureus, Enterococcus faecalis and Escherichia coli isolates from orthopaedic implant-associated infections. Int. J. Antimicrob. Agents 2019, 54, 329–337.
- Álvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-Resistant Staphylococcus aureus in Hospitals: Latest Trends and Treatments Based on Bacteriophages. J. Clin. Microbiol. 2019, 57, e01006-19.
- Speck, P.G.; Warner, M.S.; Bihari, S.; Bersten, A.D.; Mitchell, J.G.; Tucci, J.; Gordon, D.L. Potential for bacteriophage therapy for Staphylococcus aureus pneumonia with influenza A coinfection. Futur. Microbiol. 2021, 16, 175–184.
- Van Nieuwenhuyse, B.; Galant, C.; Brichard, B.; Docquier, P.-L.; Djebara, S.; Pirnay, J.-P.; Van der Linden, D.; Merabishvili, M.; Chatzis. A Case of In Situ Phage Therapy against Staphylococcus aureus in a Bone Allograft Polymicrobial Biofilm Infection: Outcomes and Phage-Antibiotic Interactions. Viruses 2021, 13, 1898.
- Sousa, C.; Ferreira, R.; Azevedo, N.F.; Oleastro, M.; Azeredo, J.; Figueiredo, C.; Melo, L.D.R. Helicobacter pylori infection: From standard to alternative treatment strategies. Crit. Rev. Microbiol. 2021, 48, 376–396.
- Singh, A.; Mahajan, S. Advancements in Diagnostics and Treatments of Helicobacter Pylori. J. Pharm. Res. Int. 2021, 435, 444.
- Hafez, R.; El-Didamony, G.; Wagih, A.E.E.; Said, E.A.; Mohammed, B.O.; Mohamed, A.M.; Mohammed, H.A. Anti-Helicobacter pylori activity of Egyptian medicinal plants and bacteriophages. Microbes. Infect. Dis. 2020, 1, 168–181.
- Mota-Gutierrez, J.; Lis, L.; Lasagabaster, A.; Nafarrate, I.; Ferrocino, I.; Cocolin, L.; Rantsiou, K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022, 104, 103998. Available online: https://www.sciencedirect.com/science/article/pii/S0740002022000223 (accessed on 11 October 2022).
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88.
- Makalatia, K.; Kakabadze, E.; Wagemans, J.; Grdzelishvili, N.; Bakuradze, N.; Natroshvili, G.; Macharashvili, N.; Sedrakyan, A.; Arakelova, K.; Ktsoyan, Z.; et al. Characterization of Salmonella Isolates from Various Geographical Regions of the Caucasus and Their Susceptibility to Bacteriophages. Viruses 2020, 12, 1418.
- Gambino, M.; Nørgaard, S.A.; Ahern, S.; Smyrlis, G.; Gencay, Y.E.; Hendrix, H.; Neve, H.; Noben, J.P.; Lavigne, R.; Brøndsted, D. Phage S144, a new polyvalent phage infecting Salmonella spp. and Cronobacter sakazakii. Int. J. Mol. Sci. 2020, 21, 5196.
- Makalatia, K.; Kakabadze, E.; Bakuradze, N.; Grdzelishvili, N.; Natroshvili, G.; Kusradze, I.; Goderdzishvili, M.; Sedrakyan, A.; Arakelova, K.; Mkrtchyan, M.; et al. Activity of bacteriophages to multiply resistant strains of salmonella and their various serotypes. Bull. Veterinary Biotechnol. 2018, 32, 500–508.
- Suay-García, B.; Pérez-Gracia, M.T. Future Prospects for Neisseria gonorrhoeae Treatment. Antibiot 2018, 7, 49.
- Cater, K.; Międzybrodzki, R.; Morozova, V.; Letkiewicz, S.; Łusiak-Szelachowska, M.; Rękas, J.; Weber-Dąbrowska, B.; Górski, A. Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections. Antibiotics 2021, 10, 1030.
- Qadir, M.I.; Sajjad, S. Phage Therapy against Streptococcus pneumoniae: Modern Tool to Control Pneumonia. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 289–295.
- Fernández, L.; Cima-Cabal, M.D.; Duarte, A.C.; Rodríguez, A.; García-Suárez, M.D.M.; García, P. Gram-positive pneumonia: Possibilities offered by phage therapy. Antibiotics 2021, 10, 100.
- Jones, J.D.; Varghese, D.; Pabary, R.; Langley, R.J. The potential of bacteriophage therapy in the treatment of paediatric respiratory infections. Paediatr Respir Rev. 2022. ahead of print.
- Melo, L.D.R.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99.
- Szaleniec, J.; Gibała, A.; Pobiega, M.; Parasion, S.; Składzień, J.; Stręk, P.; Gosiewski, T.; Szaleniec, M. Exacerbations of chronic rhinosinusitis—Microbiology and perspectives of phage therapy. Antibiotics 2019, 8, 175.
- Ranjbar, R.; Farahani, A. Shigella: Antibiotic-Resistance Mechanisms And New Horizons For Treatment. Infect. Drug Resist. 2019, 12, 3137–3167.
- Tang, S.-S.; Biswas, S.K.; Tan, W.S.; Saha, A.K.; Leo, B.-F. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. Peer J. 2019, 7, e6225.
- Jamal, M.; Chaudhry, W.N.; Hussain, T.; Das, C.R.; Andleeb, S. Characterization of new Myoviridae bacteriophage WZ1 against multi-drug resistant (MDR) Shigella dysenteriae. J. Basic Microbiol. 2015, 55, 420–431.
- Górski, A.; Borysowski, J.; Międzybrodzki, R. Phage Therapy: Towards a Successful Clinical Trial. Antibiotics 2020, 9, 827.
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2013, 5, 226–235.
- Nale, J.Y.; Clokie, M.R. Preclinical data and safety assessment of phage therapy in humans. Curr. Opin. Biotechnol. 2021, 68, 310–317.
- Zhang, Y.; Li, C.-X.; Zhang, X.-Z. Bacteriophage-mediated modulation of microbiota for diseases treatment. Adv. Drug Deliv. Rev. 2021, 176, 113856.
- Abedon, S.T. Ecology of Anti-Biofilm Agents I: Antibiotics versus Bacteriophages. Pharmaceuticals 2015, 8, 525–558.
More