Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Sabry Nasif Soliman.
Strigolactones (SLs) are carotenoid derivatives that occur naturally in plants and are defined as novel phytohormones that regulate plant metabolism, growth, and development. Strigolactone assists plants in the acquisition of defensive characteristics against drought stress by initiating physiological responses and mediating the interaction with soil microorganisms.
- strigolactones
- abiotic stress
- ecological microbiome
1. Introduction
Abiotic stresses are the most significant limiting factors of plant survival and growth under the increasing crisis of climatic changes. Numerous studies tried conducted to find a solution for different plant species to cope with various stressors. Plant hormones (phytohormones) are organic molecules that cause signaling effects in plant tissues. Cell elongation, phototropism, stress tolerance, apical dominance, plant growth improvement, senescence, and dormancy are a few of many processes considered as physiological functions of phytohormones. The impacts on plants are substantial despite the minimal concentration of secretion and significantly improve plant tolerance against abiotic stress [1]. In order to adapt to adverse conditions, plants have developed various responses by evoking several signals that cause metabolic and genetic pathways to be reprogrammed [2,3][2][3].
Strigolactones (SLs), was identified as plant hormones that play regulatory roles against abiotic stresses in plants, due to their essential role in regulating plant growth and development [4,5][4][5]. The first naturally occurring germination stimulant for Striga was isolated as early as 1966 from root exudates of cotton (Gossypium hirsutum L.), which is neither a host for Striga nor Orobanche [6,7,8][6][7][8]. SLs are a class of terpenoid-derived compounds that were first discovered as (+)-strigol, which stimulates seeds germination from the parasitic plant Striga [6]. Many researchers reported strigolactone (SL) as a newly identified phytohormone [9,10,11][9][10][11]. Strigolactones improve control the development patterns and interactions between nearby colonies in moss [12,13][12][13] Furthermore, SLs participate in metabolic processes acting against biotic and abiotic stress [4,14,15][4][14][15]. The production of SLs in plants is strongly controlled and influenced by the various kinds of stressors that they experience at different growth phases. Recent evidence of interactions between SLs and other phytohormones, such as abscisic acid, in plant responses to abiotic stressors, implies that SLs actively engage in phytohormone-controlled regulatory networks of plant stress adaption [16].
Several studies were focused on SLs since they strongly demonstrate many internal and external responses to plant growth and development [5,8,17][5][8][17]. They regulate lateral roots and root hairs, gravitropism, soil microbes, vasculature development, nutrient and photoassimilate capture and allocation, light responses, leaf shape, leaf senescence, drought, and salinity tolerance [18]. Crosstalk with other known hormones was discussed by several investigations, and the signaling between SLs and other plant hormones may demonstrate their physiological function. Plants regulate their growth by simultaneously sensing and responding to both the external environmental signals and the internal developmental signals [1,4][1][4]. For instance, plant phytohormones may have responses to the signaling pathways of photoreceptor phytochromes for improving the photosynthesis processes. The phytohormones also affect photoreceptor signal transduction at cellular levels [18]. The crosstalk of strigolactone with other phytohormones is widely investigated. Phenomena caused by these interactions, such as defense against abiotic stress and attraction of microorganisms, have been explored. Plant morphological adaptability in response to changes in environmental factors is largely influenced by phytohormones. Strigolactones (SLs) are carotenoid-derived hormones that affect various aspects of development and interaction with microorganisms. They have been proposed as mediators of environmental stimuli in resource allocation processes; as a result, their pathways must be responsive to environmental cues in order to contribute to adaptive adjustments [19,20][19][20]. The research studies indicate that plants make efficient use of their secondary metabolites to defend themselves and make their environment suitable. For instance, plants actively release volatile compounds to repel herbivores and, at the same time, to draw in natural enemies that are specifically adapted to fight the herbivores [21,22][21][22].
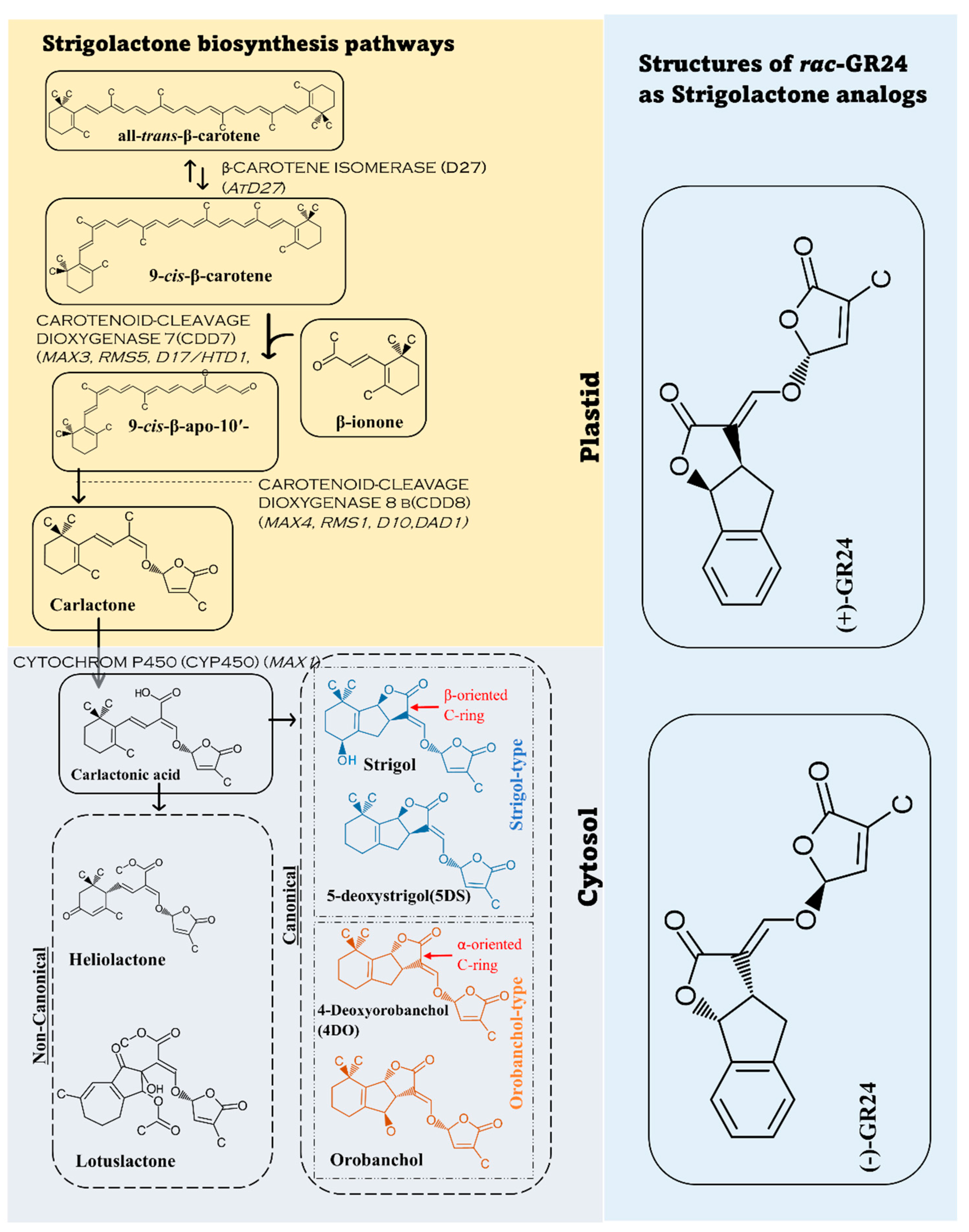
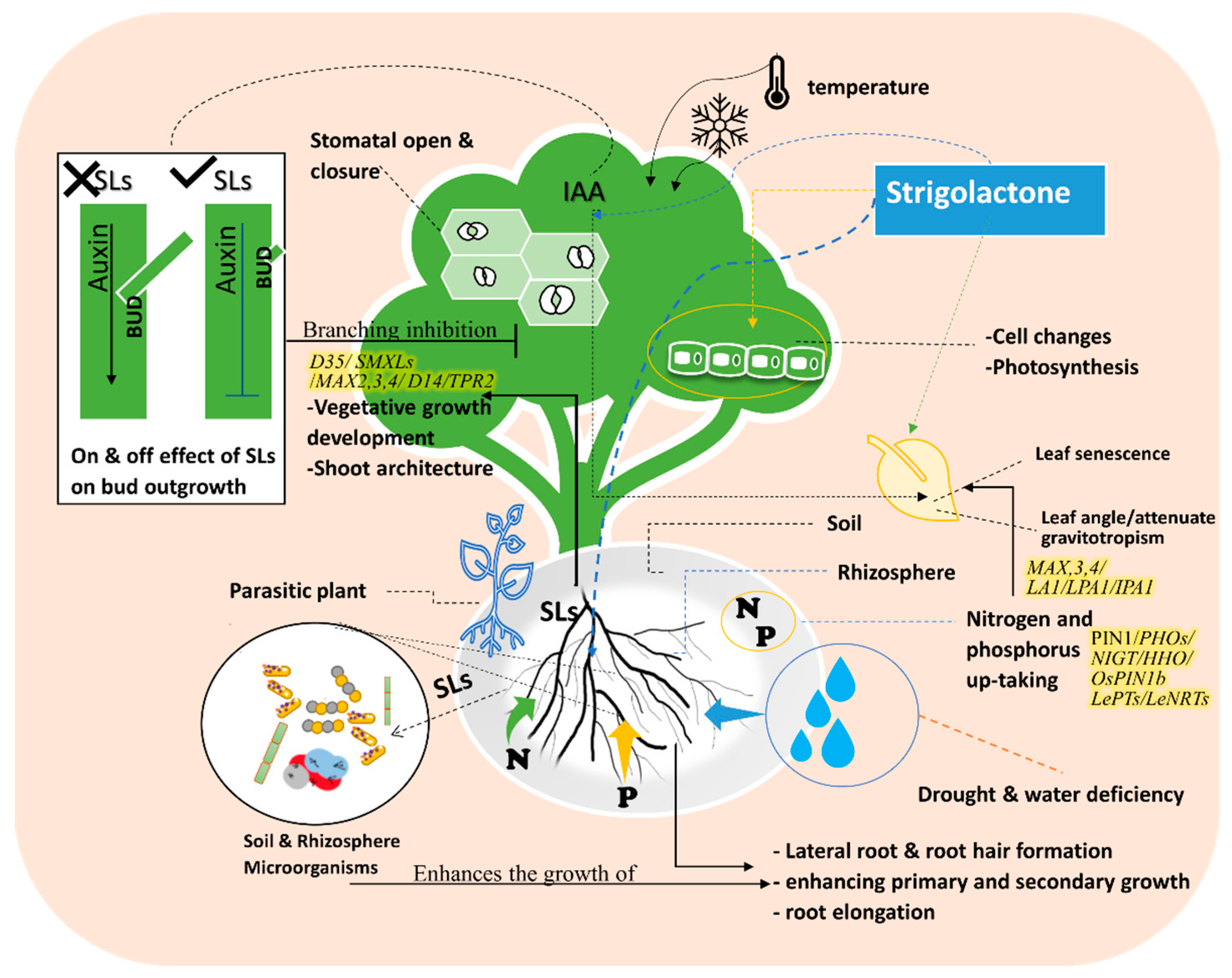
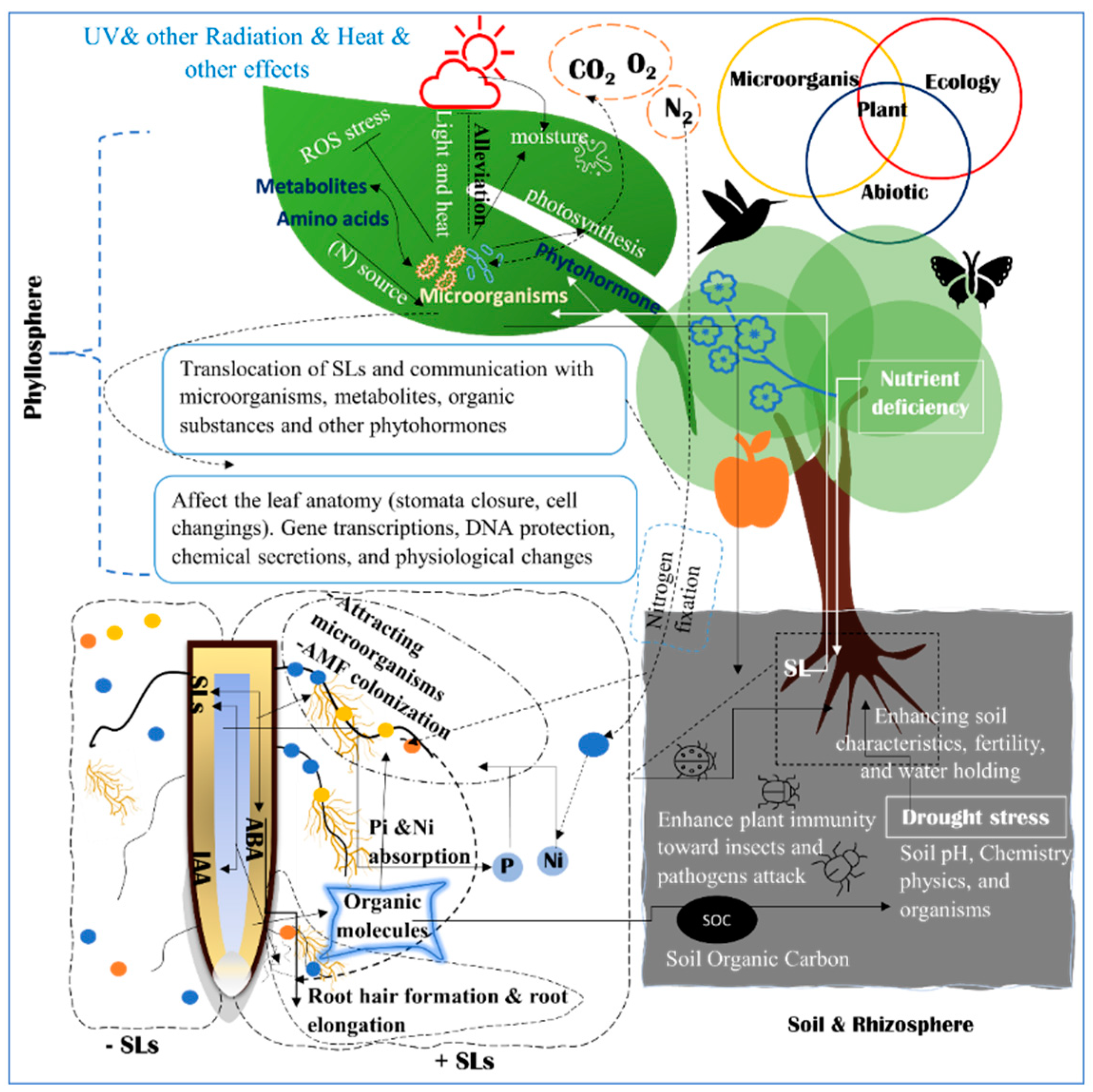
2. Strigolactone Biosynthesis, and Signaling Pathways
2.1. Biosynthesis Pathway
Biosynthesis of the strigolactone molecules and SL-like compounds is carried out by β-CAROTENE ISOMERASE (D27) and a group of other enzymes known as CAROTENOID-CLEAVAGE DIOXYGENASE (CCDs group). There are two main dioxygenases groups including CCD7 and CCD8 [30][23]. In the biosynthetic pathway, D27 has 2 ways of dissociation by converting all-trans-β-carotene to 9-cis-β-carotene and vice versa. 9-cis-β- carotene is converted to 9-cis-β-apo-10′carotenal as CCD7 attacks the 9′ and 10′ bonds of β -carotene, and CCD8 cleaves 9-cis-β-apo-10′carotene and forms carlactone. Carlactone is transformed into carlactonic acid (CLA) in the cytoplasm by sub family CYTOCHROM P450 (CYP450). Carlactonic acid leads to the formation of 5-deoxystrigol(5DS),4-deoxyorobanchol(4DO), and other forms of SLs [31,32,33][24][25][26]. Although the reactions of converting all-trans-β-carotene happen in plastids, the formation of strigolactones are carried out in the cytoplasm. This happens when carlactone (CL), the precursor of strigolactones, is transported to the cytoplasm where strigolactone and SL-like compounds are formed [31][24]. Different plants have different enzyme members from the families wresearchers mentioned. [15,23,33,34,35][15][26][27][28][29]. Many synthetic analogs such as GR24 have been developed and studied. Further studies about synthetic analogs and their characteristics and effects need to occur. The biosynthetic pathway and the common analogs GR24 are explained in Figure 1 [23,34,35,36,37][27][28][29][30][31].
Figure 1. Strigolactone biosynthesis from carotenoids. In plastids all–trans–β–carotene goes through different enzymatic reactions to carlactone. Then, the carlactone transforms to form carlactonic acid in the cytosol (cytoplasm) to form various forms of strigolactones. The figure demonstrates two common examples of SLs in non-canonical form, four common ones of canonical form and the most common strigolactone analog, GR24.
2.2. Signaling Pathways
2.2.1. Impact on Branching and Leaf-Stem Angle with Relatioship to Gravitropism
D53-like SMXLs regulate leaf morphology and SL-induced SMXL6 degradation requires D14 and MAX2 [38,39][32][33]. D53-like SMXLs interact with MAX2 and D14. D53-like SMXLs interact with TPR2 and exhibit transcriptional repression [39][33]. It is believed that D53 regulates the expression of genes essential for the development of secondary shoots in the nucleus [40][34]. In the signaling activation of branching, auxin regulates “MORE AXILLARY GROWTH” MAX3 and MAX4 gene expression, these two genes are also influenced by strigolactone during branching inhibition control [41][35]. MAX3 and MAX4 participate in shoot branching and architecture signaling through the gravitropism isolation of LAZY1(LA1) suppressors, which revealed the involvement of SLs in shoot gravitropism/rice tiller angle Figure 2. SLs attenuate shoot gravitropic response in rice by modifying trilling angles. SL regulation of rice shoot gravitropism is dependent on indigenous auxin levels [42][36]. SL-mediated shoot gravitropism is conserved in Arabidopsis and many other plant species. For instance, it was reported in rice that SLs is a moderator of some genes such as LAZY1(LA1), LOOSE PLANT ARCHITECTURE1(LPA1), and IDEAL PLANT ARCHITECTURE1 (IPA1), which are involved in shoot branching inhibition and shoot gravitropism. Thus, SLs regulate tiller/branch angle in different plant species, indicating that shoot gravitropism is the key component dictating the proper positioning of shoot branches (Figure 2) [42,43][36][37].
Figure 2. Effects of Strigolactone analogs (e.g., GR24) on the plant’s vegetative growth and signaling pathway under abiotic stress in relation to plant biological characteristics and soil & rhizosphere microbiome. The figure shows the role of SLs on nitrogen and phosphorus uptake. Root and Shoot architecture changes identify the effects of SLs in modifying the plant structure to cope with the abiotic stress. The figure demonstrates that SLs impact shoot branching. The plant utilizes SLs to preform mechanisms that defend itself against different abiotic stresses such as drought, senescence, temperature, light, and nutrient deficiency.
2.2.2. Impact on Low-Light Stress
GR24 application increased the activity and gene expression of antioxidant enzymes, and it reduced malonaldehyde (MDA) and hydrogen peroxide (H2O2) content in Low Light stressed plants (LL-stressed plants) [44,45][38][39]. These results suggested that exogenous application of GR24 enhances plant tolerance to LL and improving photosynthesis by promoting utilization of light energy to alleviate photosystem injuries induced by excess light energy and ROS as well as enhancing photosynthesis efficiency to improve plant growth [45,46][39][40]. Exogenous GR24 application on tomato seedlings reduces the negative effects of low light exposure in at least three ways: by reducing growth inhibition, improving photosynthetic efficiency, and relieving oxidative stress [45,47,48][39][41][42]. In addition, the application of GR24 effectively alleviates the photoinhibition of photosystems I and II (PSII and PSI) under high light stress mainly by balancing excitation energy and promoting the electron transfer chain between two photosystems, thus enhancing CEF, PQ pools, and quantum yield of PSII and PSI photochemistry [45][39]. Furthermore, plants growing in LL exhibit reduced levels of the enzymes photosystem II (PS II), ATP synthase, cytochrome b/f, and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), as well as poorer electron transport (ETR) and CO2 consumption [45,49][39][43].2.2.3. Impact on Nitrogen and Phosphorus Deficiency
SLs consider the modulating expression factor of regulatory genes as a signaling pathway for nitrogen (N) and phosphorus (P) starvation defense mechanisms [50][44]. Therefore, many regulatory genes are involved in N and P regulations as well as signaling pathways of N–P integrators PHOs family [50,51,52][44][45][46]. SLs also regulate NIGT/HHO involved in the phosphorus deficiency signaling pathway [50][44]. LePTs and LeNRTs families are responsible for improving phosphorus and nitrogen absorption efficiency [50,52,53,54][44][46][47][48]. A signaling impact of D3-dependent and SLs biosynthesis in the suppression of tiller bud outgrowth under Pi deficit was identified in several SL signaling pathways [26,53][47][49]. OsPIN1b responds to low levels of N and P and regulates the activities in the root apical meristem, which leads to the rice seminal root elongation (Figure 2 and Figure 3) [55][50].
Figure 3. The complex relations between plants, ecology, and microorganisms. The rhizosphere, soil, and root system interact together to alleviate or eliminate the effects of abiotic stress. The phyllosphere ecosystem in the top portion of the figure has a different microbiome. Phyllosphere is composed of all parts above the ground. There are different mechanisms to alleviate abiotic stress with the cooperation of the surrounding ecosystems, physiological processes, and participation of phytohormones and organic substances. The relationship between plants and organisms in the rhizosphere, phyllosphere, and endosphere is demonstrated.
2.2.4. Impact on Other Pathways
Moreover, the expression of several CAB genes induced by auxin-SLs may increase the activation of photosynthesis. Several auxin-activated metabolic pathways may be decreased by GR24 as SLs analog. A series of downstream auxin genes are utilized by SLs to alter the tomatoes’ response to auxin. Simultaneously, the biosynthesis of SLs is regulated by auxin using different genes in the Carotenoid biosynthesis pathway [56][51]. Strigolactone is produced in the roots but can be transported to the shoots [57][52]. By investigating different PIN1 trafficking dynamics in roots compared to shoots, it was found that strigolactone-triggered PIN1 PM depletion carries a more significant effect in the shoot as opposed to the root [58][53].The chemical stability of SLs depends on experimental conditions such as the solvent, pH, and the presence of nucleophiles. SLs are stable in root exudates which are usually composed of an oily mixture of various chemicals that play a crucial role in the plant’s defense against pathogenic attacks. In contrast, SLs exhibit limited stability in aqueous solutions and degrade when removed via several extraction and chromatographic steps [59][54]. These synthetic analogs are more stable, but they are less active than their natural counterparts. One of the most potent and commonly used SL analogs is GR24, a synthetic analog of strigol synthesized by Gerald Rosebery [59,60][54][55]. SLs contain several stereogenic (chiral) centers; for example, strigol contains three stereogenic (chiral) centers, resulting in eight possible stereoisomers [61][56]. At present, two main families of natural SLs are known, namely the strigol family and the orobanchol family, with (+)-strigol and (−)-orobanchol BC stereochemistry, respectively. Natural SLs have a rather complex structure, therefore synthesizing them involves several stages. For instance, enantiopure (+)-strigol must be synthesized in at least 20 stages [59][54]. Since strigolactone is transported in two separate pathways, from shoot to root and vice versa, further experimental study investigations are required to better understand how this substance is distributed inside plants and transported via various tissues. Transportation from the root as the source of strigolactone showed that high concentrations of synthetic strigolactone increase the export of SLs from the root [8].References
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104.
- Banerjee, A.; Wani, S.H.; Roychoudhury, A. Epigenetic Control of Plant Cold Responses. Front. Plant Sci. 2017, 8, 1643.
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 2021, 254, 28.
- Li, W.; Herrera-Estrella, L.; Tran, L.P. Do Cytokinins and Strigolactones Crosstalk during Drought Adaptation? Trends Plant Sci. 2019, 24, 669–672.
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117.
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190.
- Zwanenburg, B.; Pospisil, T. Structure and Activity of Strigolactones: New Plant Hormones with a Rich Future. Mol. Plant 2013, 6, 38–62.
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344.
- Prandi, C.; Kapulnik, Y.; Koltai, H. Strigolactones: Phytohormones with Promising Biomedical Applications. Eur. J. Org. Chem. 2021, 2021, 4019–4026.
- Wang, B.; Wang, Y.; Li, J. 10-Strigolactones. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 327–359.
- Halouzka, R.; Zeljkovic, S.C.; Klejdus, B.; Tarkowski, P. Analytical methods in strigolactone research. Plant Methods 2020, 16, 76.
- Kapulnik, Y.; Koltai, H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014, 166, 560–569.
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogué, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539.
- Sikder, M.M.; Vestergard, M.; Kyndt, T.; Kudjordjie, E.N.; Nicolaisen, M. Phytohormones selectively affect plant parasitic nematodes associated with Arabidopsis roots. N. Phytol. 2021, 232, 1272–1285.
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging Roles of Strigolactones in Plant Responses to Stress and Development. Front. Plant Sci. 2016, 7, 434.
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243.
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110.
- Brewer, P.B. Emerging Roles of Strigolactones in Plant Responses Toward Biotic Stress. In Emerging Plant Growth Regulators in Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 205–214.
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta 2015, 241, 1435–1451.
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183.
- Yoneyama, K.; Kisugi, T.; Xie, X.; Yoneyama, K. Chemistry of Strigolactones: Why and How do Plants Produce so Many Strigolactones? In Molecular Microbial Ecology of the Rhizosphere; Wiley: Hoboken, NJ, USA, 2013; pp. 373–379.
- Takabayashi, J.; Dicke, M. Plant—Carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996, 1, 109–113.
- Harrison, P.J.; Newgas, S.A.; Descombes, F.; Shepherd, S.A.; Thompson, A.J.; Bugg, T.D. Biochemical characterization and selective inhibition of β-carotene cis-trans isomerase D27 and carotenoid cleavage dioxygenase CCD8 on the strigolactone biosynthetic pathway. FEBS J. 2015, 282, 3986–4000.
- Lopez-Obando, M.; Ligerot, Y.; Bonhomme, S.; Boyer, F.-D.; Rameau, C. Strigolactone biosynthesis and signaling in plant development. Development 2015, 142, 3615–3619.
- Wang, J.Y.; Haider, I.; Jamil, M.; Fiorilli, V.; Saito, Y.; Mi, J.; Baz, L.; Kountche, B.A.; Jia, K.P.; Guo, X.; et al. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 2019, 10, 810.
- Mashiguchi, K.; Seto, Y.; Yamaguchi, S. Strigolactone biosynthesis, transport and perception. Plant J. 2021, 105, 335–350.
- Marzec, M.; Muszynska, A.; Gruszka, D. The Role of Strigolactones in Nutrient-Stress Responses in Plants. Int. J. Mol. Sci. 2013, 14, 9286–9304.
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186.
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012, 335, 1348–1351.
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 2009, 21, 1512–1525.
- van Zeijl, A.; Liu, W.; Xiao, T.T.; Kohlen, W.; Yang, W.C.; Bisseling, T.; Geurts, R. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol. 2015, 15, 260.
- Hu, J.; Ji, Y.; Hu, X.; Sun, S.; Wang, X. BES1 Functions as the Co-regulator of D53-like SMXLs to Inhibit BRC1 Expression in Strigolactone-Regulated Shoot Branching in Arabidopsis. Plant Commun. 2020, 1, 100014.
- Wang, L.; Wang, B.; Jiang, L.; Liu, X.; Li, X.; Lu, Z.; Meng, X.; Wang, Y.; Smith, S.M.; Li, J. Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-Like SMXL Repressor Proteins for Ubiquitination and Degradation. Plant Cell 2015, 27, 3128–3142.
- Smith, S.M. Witchcraft and destruction. Nature 2013, 504, 384–385.
- Hayward, A.; Stirnberg, P.; Beveridge, C.; Leyser, O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009, 151, 400–412.
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204.
- Yu, C.; Guo, H.; Zhang, Y.; Song, Y.; Pi, E.; Yu, C.; Zhang, L.; Dong, M.; Zheng, B.; Wang, H.; et al. Identification of potential genes that contributed to the variation in the taxoid contents between two Taxus species (Taxus media and Taxus mairei). Tree Physiol 2017, 37, 1659–1671.
- Zhou, X.; Tan, Z.; Zhou, Y.; Guo, S.; Sang, T.; Wang, Y.; Shu, S. Physiological mechanism of strigolactone enhancing tolerance to low light stress in cucumber seedlings. BMC Plant Biol. 2022, 22, 30.
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving Plant Growth and Alleviating Photosynthetic Inhibition and Oxidative Stress from Low-Light Stress with Exogenous GR24 in Tomato (Solanum lycopersicum L.) Seedlings. Front. Plant Sci. 2019, 10, 490.
- Wani, K.I.; Zehra, A.; Choudhary, S.; Naeem, M.; Khan, M.M.A.; Khan, R.; Aftab, T. Exogenous Strigolactone (GR24) Positively Regulates Growth, Photosynthesis, and Improves Glandular Trichome Attributes for Enhanced Artemisinin Production in Artemisia annua. J. Plant Growth Regul. 2022, 156, 125–134.
- Bi, H.; Liu, P.; Jiang, Z.; Ai, X. Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus. Physiol. Plant 2017, 161, 224–234.
- Cui, L.; Zou, Z.; Zhang, J.; Zhao, Y.; Yan, F. 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). Funct. Integr. Genom. 2016, 16, 29–35.
- Zivcak, M.; Brestic, M.; Kalaji, H.M.; Govindjee, N. Photosynthetic responses of sun- and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354.
- Marro, N.; Lidoy, J.; Chico, M.A.; Rial, C.; Garcia, J.; Varela, R.M.; Macias, F.A.; Pozo, M.J.; Janouskova, M.; Lopez-Raez, J.A. Strigolactones: New players in the nitrogen-phosphorus signalling interplay. Plant Cell Environ. 2022, 45, 512–527.
- Ren, P.; Meng, Y.; Li, B.; Ma, X.; Si, E.; Lai, Y.; Wang, J.; Yao, L.; Yang, K.; Shang, X.; et al. Molecular Mechanisms of Acclimatization to Phosphorus Starvation and Recovery Underlying Full-Length Transcriptome Profiling in Barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 500.
- Bari, R.; Pant, B.D.; Stitt, M.; Golm, S.P. PHO2, MicroRNA399, and PHR1 Define a Phosphate-Signaling Pathway in Plants. Plant Physiol. 2006, 141, 988–999.
- Santoro, V.; Schiavon, M.; Visentin, I.; Constan-Aguilar, C.; Cardinale, F.; Celi, L. Strigolactones affect phosphorus acquisition strategies in tomato plants. Plant Cell Environ. 2021, 44, 3628–3642.
- Albornoz, F.; Gebauer, M.; Ponce, C.; Cabeza, R.A. LeNRT1.1 Improves Nitrate Uptake in Grafted Tomato Plants under High Nitrogen Demand. Int. J. Mol. Sci. 2018, 19, 3921.
- Umehara, M.; Hanada, A.; Magome, H.; Takeda-Kamiya, N.; Yamaguchi, S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010, 51, 1118–1126.
- Sun, H.; Tao, J.; Bi, Y.; Hou, M.; Lou, J.; Chen, X.; Zhang, X.; Luo, L.; Xie, X.; Yoneyama, K.; et al. OsPIN1b is Involved in Rice Seminal Root Elongation by Regulating Root Apical Meristem Activity in Response to Low Nitrogen and Phosphate. Sci. Rep. 2018, 8, 13014.
- Zhan, Y.; Qu, Y.; Zhu, L.; Shen, C.; Feng, X.; Yu, C. Transcriptome analysis of tomato (Solanum lycopersicum L.) shoots reveals a crosstalk between auxin and strigolactone. PLoS ONE 2018, 13, e0201124.
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011, 155, 974–987.
- Shinohara, N.; Taylor, C.; Leyser, O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013, 11, e1001474.
- Halouzka, R.; Tarkowski, P.; Zwanenburg, B.; Zeljkovic, S.C. Stability of strigolactone analog GR24 toward nucleophiles. Pest Manag. Sci. 2018, 74, 896–904.
- Johnson, A.W.; Gowada, G.; Hassanali, A.; Knox, J.; Monaco, S.; Razavi, Z.; Rosebery, G. The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1981, 1, 1734–1743.
- Yang, T.; Lian, Y.; Wang, C. Comparing and Contrasting the Multiple Roles of Butenolide Plant Growth Regulators: Strigolactones and Karrikins in Plant Development and Adaptation to Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 6270.
More
