The accumulation of cadmium in rice (Oryza sativa L.) and wheat (Triticum aestivum L.) is a serious threat to the safe use of farmland and to the health of the human diet that has attracted extensive attention from researchers. In this review, Ca bibliometric analysis was performed using a VOS viewer (1.6.18, Netherlands) to investigate the status of cadmium contaminationdmium toxicity reduces the uptake and transport of essential elements in rice and wheat growing systems, human health risks, mechanisms of Cd uptake and transport, and the corresponding research hotspots. It has a certain reference value for the prevention and control of c. Cadmium stress seriously affected the growth and morphology of plant roots. In the shoots, Cd toxicity was manifested by a series of physiological injuries, such as decreased photosynthesis, soluble protein, sugar, and antioxidant enzyme activity. Cadmium pollution in rice and wheat planting systems in Chinathat accumulates in the shoots is transferred to grains and then passes up the food chain to people and abroadnimals.
- cadmium pollution status
- hazard quotient
- average daily intake
- pollution mechanism
- hotspots
1. Introduction
2. Cd Pollution in Rice and Wheat Cropping System
2.1. Cd Concentrations in Rice and Wheat Grain
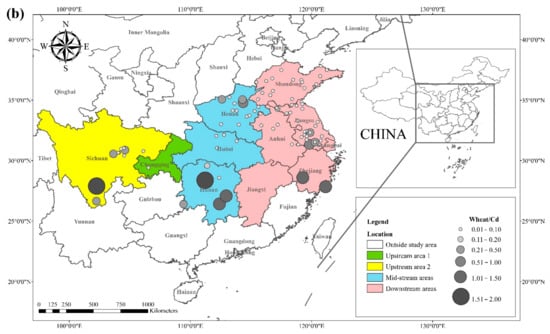
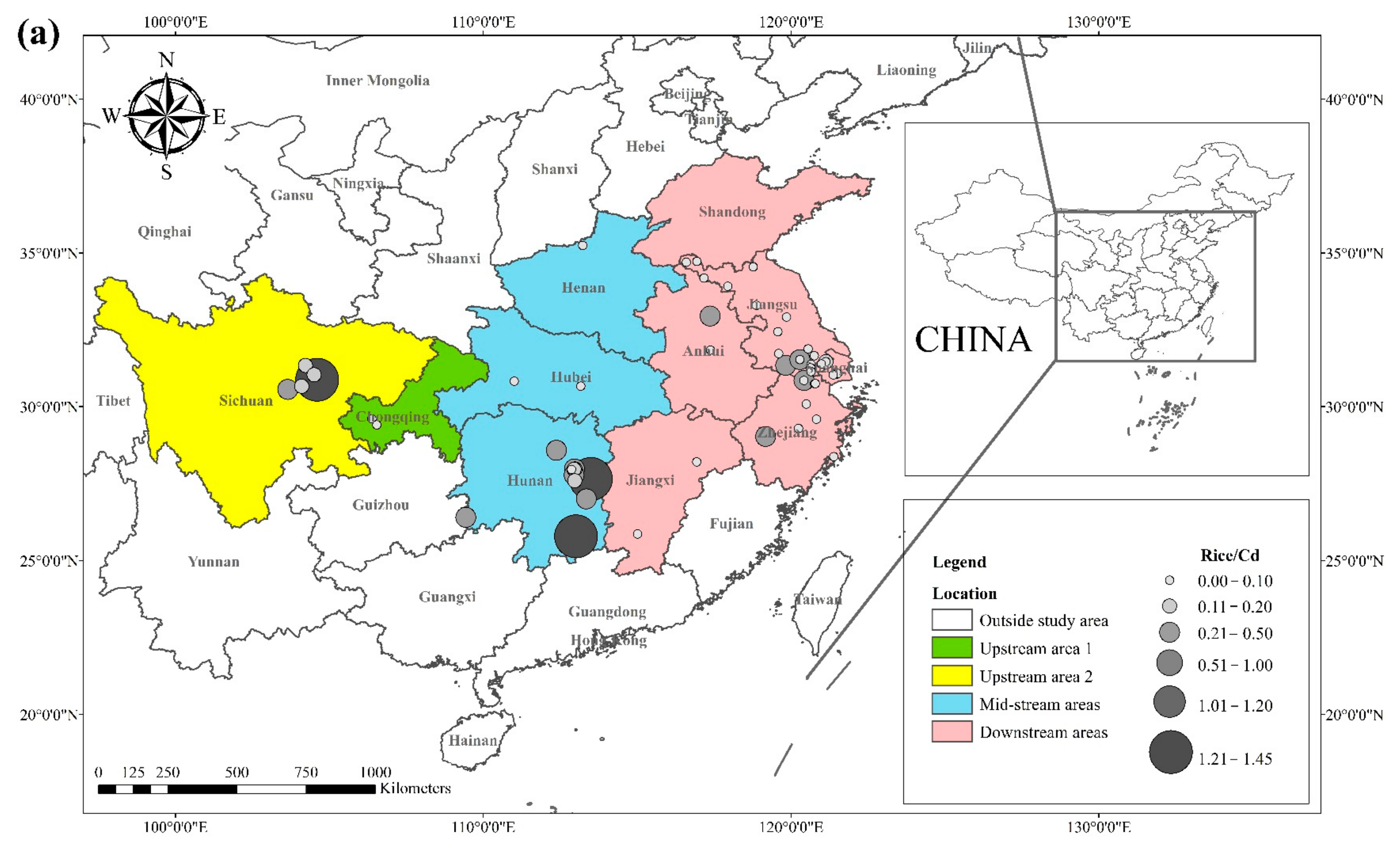
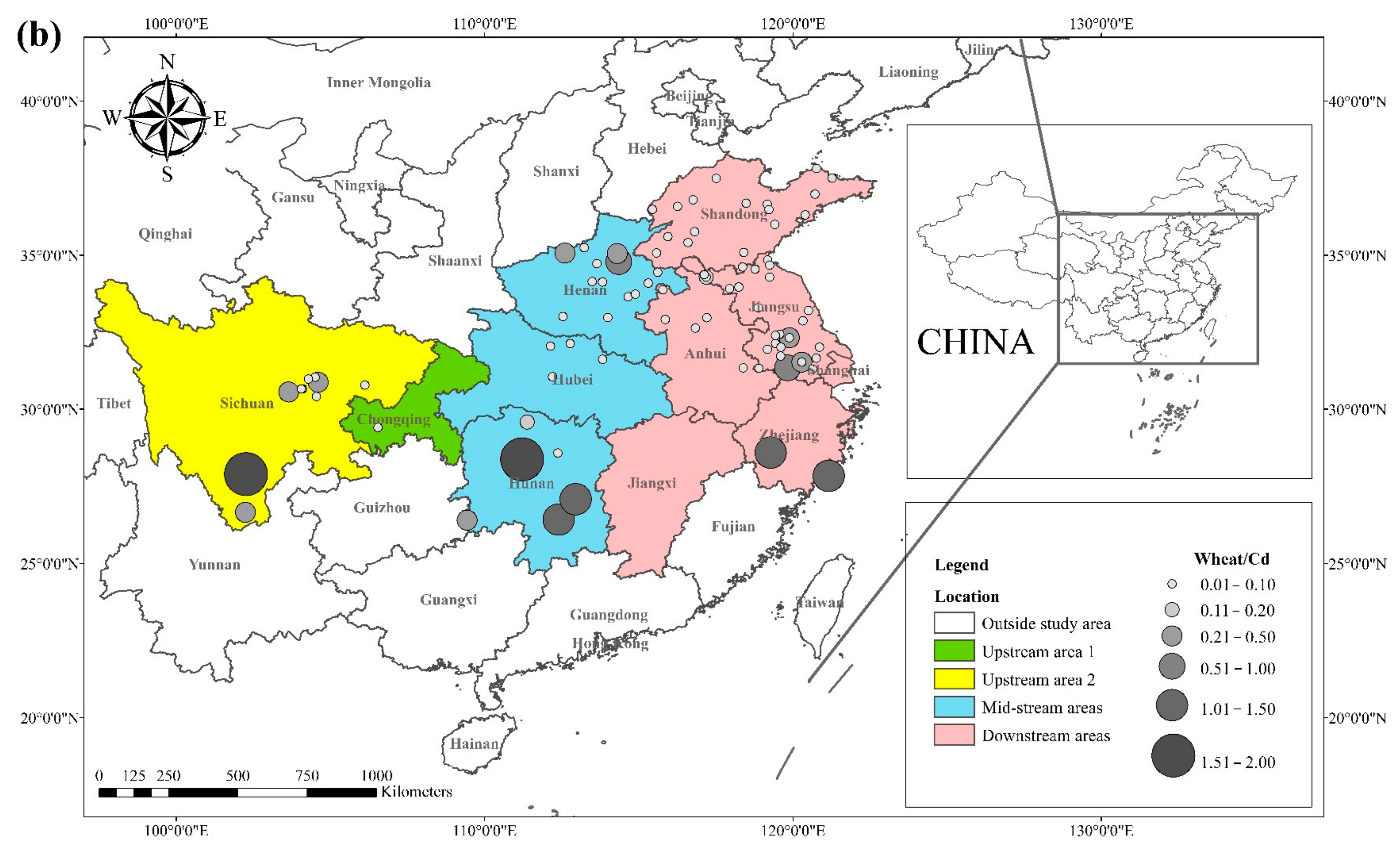
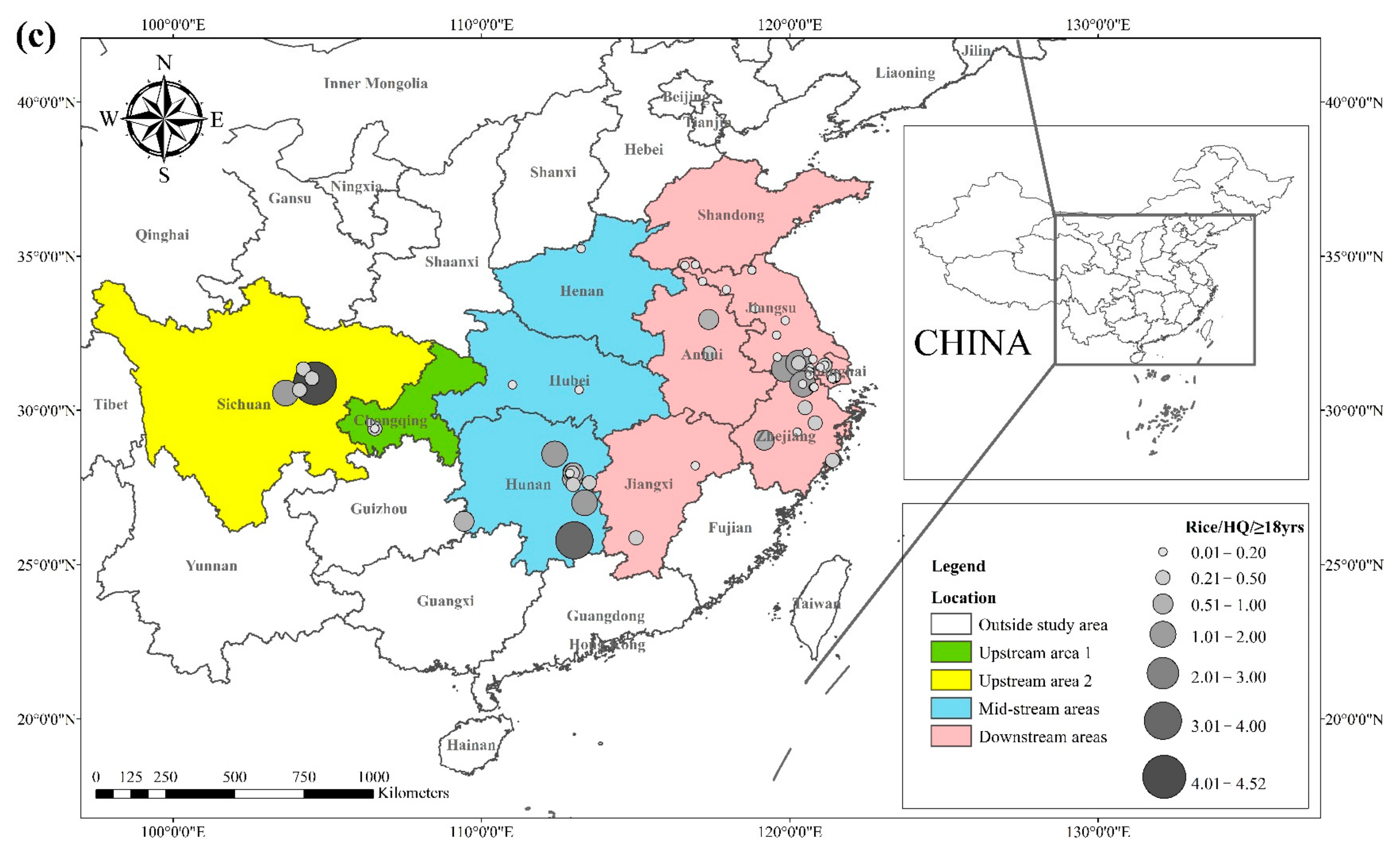
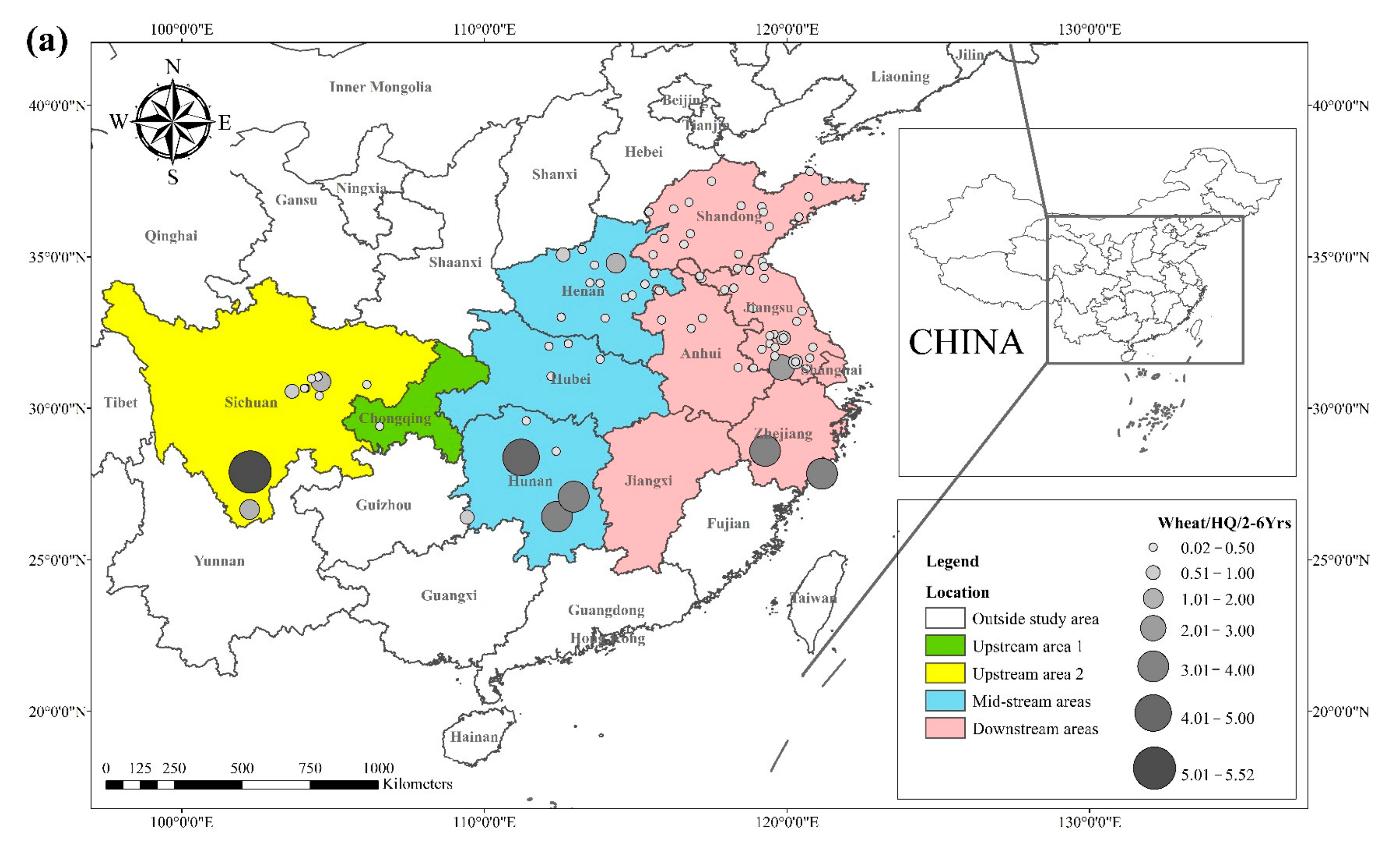
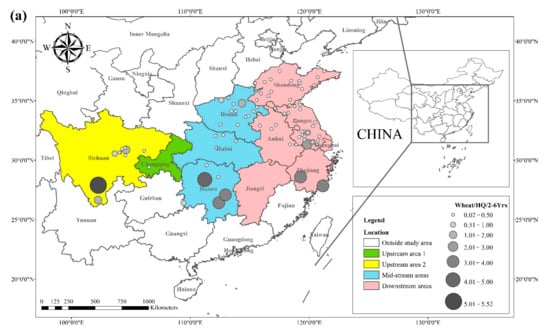
The literature survey results showed that the regions where both rice and wheat were grown were mainly in nine provinces and two municipalities around the Yangtze River Basin and southeast of the Yellow River Basin, including Sichuan, Chongqing, Hubei, Hunan, Jiangxi, Anhui, Zhejiang, Jiangsu, Shanghai, Shandong, and Henan provinces. Table 1 shows the results of other studies on the Cd pollution of rice and wheat cropping systems in China. The retrieved data showed that the average concentration of Cd in rice ranged from 0.0070 mg kg−1 to 1.45 mg kg−1. The proportion of studies where the Cd concentration in rice was lower than the national food safety standard limit (GB 2762-2017) (0.2 mg kg−1) was 79%. The spatial distribution of Cd concentration in rice is presented in Figure 1a, with larger dots representing higher Cd concentrations. Studies on excess Cd content in rice were mainly conducted in the central part of Sichuan province in the upper reaches of the Yangtze River, the southwest of Chongqing, the southeast and northeast of Hunan, as well as the northern part of Anhui, southern Jiangsu, and northern Zhejiang in the Yangtze River Delta region. Studies on Cd accumulation in rice have also been conducted in other major rice-producing countries, including Thailand, Bangladesh, Indonesia, and India. Effective methods should be formulated to remediate Cd-contaminated rice in Punjab (India, 0.99 mg kg−1) and Mae Tao (Thailand, 0.329 mg kg−1), where the average Cd concentration in rice exceeds the required limit. The spatial distribution of Cd concentrations in wheat is shown in Figure 1b. The average concentration of Cd in wheat ranged between 0.0080 mg kg−1 and 2.0 mg kg−1, and the proportion of studies where the Cd concentration in wheat was lower than the national food safety standard (0.1 mg kg−1) was 83%. Studies on excessive Cd concentration in wheat were mainly conducted in the southern part of Sichuan in the upper reaches of the Yangtze River, the southeastern and northern parts of Hunan Province, the southern part of Jiangsu, and the southern part of Zhejiang in the Yangtze River Delta region, as well as Shandong and Henan provinces in the southeast of the Yellow River Basin. The Cd pollution of wheat in the rice and wheat cropping system was relatively low compared with the Cd pollution in rice, and the pollution area was smaller for wheat.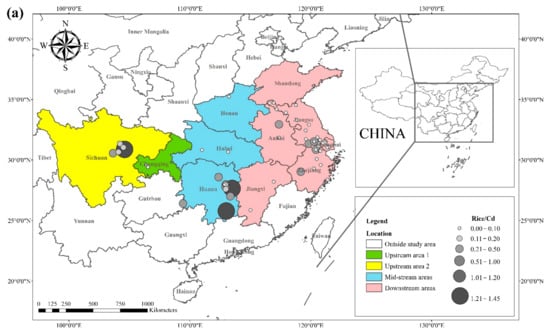
| Province | Mean/Ricecd (mg kg−1) | Range (mg kg−1) | Mean/Wheatcd (mg kg−1) | Range (mg kg−1) | Reference |
|---|---|---|---|---|---|
| Jiangsu | 0.0532 ± 0.0204 | 0.0040~0.4190 | 0.1050 ± 0.0320 | 0.0150~0.8700 | [55,56,57,58,59,60,61,62,63,64,65,66,67][48][49][50][51][52][53][54][55][56][57][58][59][60] |
| Zhejiang | 0.1216 ± 0.0378 | 0.0200~0.3400 | 0.9010 ± 0.3361 | 0.0233~1.30 | [68,69,70,71,72][61][62][63][64][65] |
| Anhui | 0.0860 | 0.0860 | 0.0358 ± 0.0250 | 0.0358~0.0102 | [62,73,74][55][66][67] |
| Hunan | 0.4322 ± 0.1513 | 0.0420~1.415 | 0.8720 ± 0.3185 | 0.0400~1.57 | [62,71,74,[75,76,5577,78]][64][67][68][69][70][71] |
| Jiangxi | 0.0605 ± 0.0185 | 0.0420~0.0790 | — | — | [79,80][72][73] |
| Sichuan | 0.4403 ± 0.2123 | 0.1100~1.4500 | 0.3448 ± 0.1896 | 0.0400~0.4400 | [5,68,81,82,83,84,85][5][61][74][75][76][77][78] |
| Hubei | 0.0460 | 0.0460 | 0.0533 ± 0.0233 | 0.0300~0.100 | [61,86,87][54][79][80] |
| Chongqing | 0.0462 ± 0.0143 | 0.0220~0.0870 | 0.0080 | 0.0080 | [88,89][81][82] |
| Shanghai | 0.0448 ± 0.0111 | 0.0190~0.0820 | — | — | [90][83] |
| Henan | 0.0046 | 0.0046 | 0.0482 ± 0.0174 | 0.0100~0.6300 | [62,73,91,92,93][55][66][84][85][86] |
| Shangdong | — | — | 0.0435 ± 0.0061 | 0.0100~0.0920 | [61,94][54][87] |
2.2. Cd Hazard Quotients in Rice and Wheat
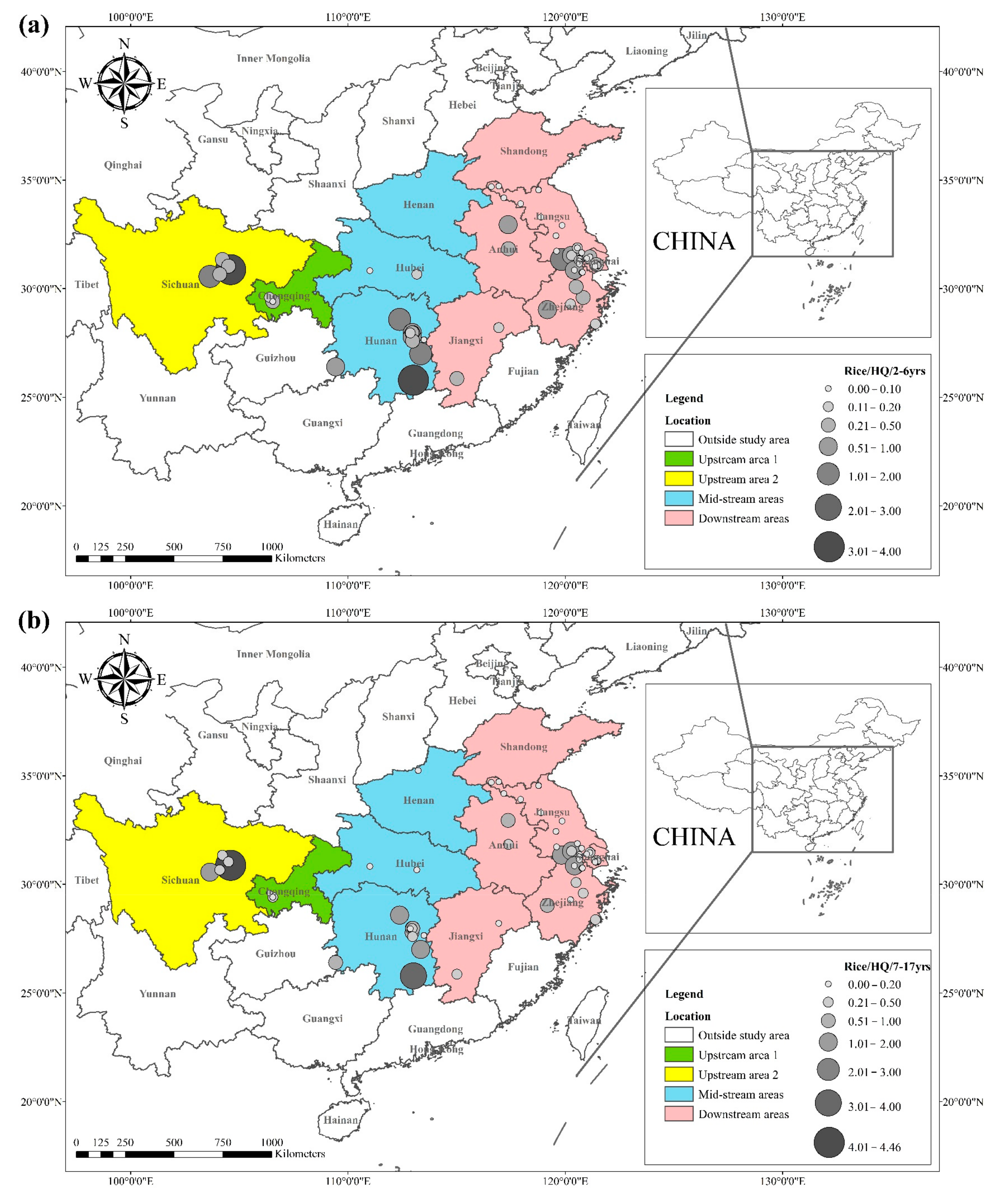
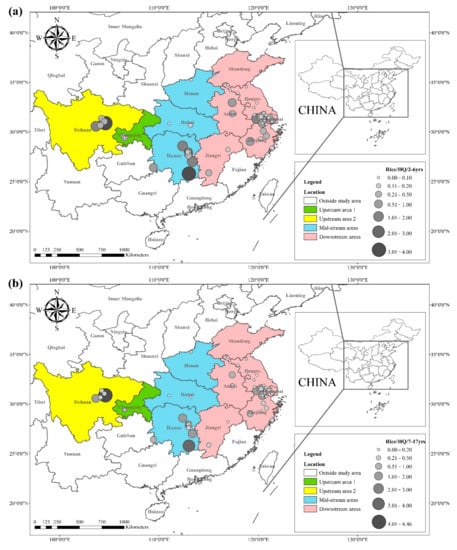
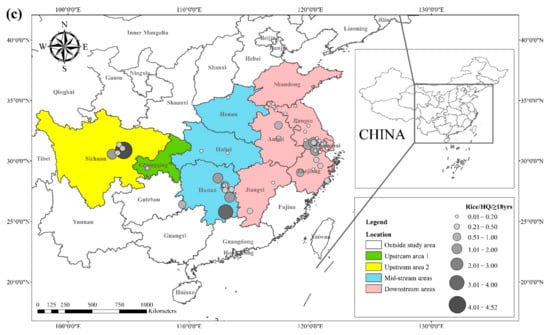
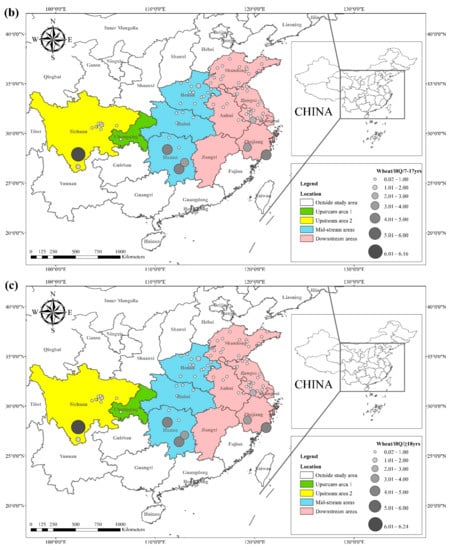
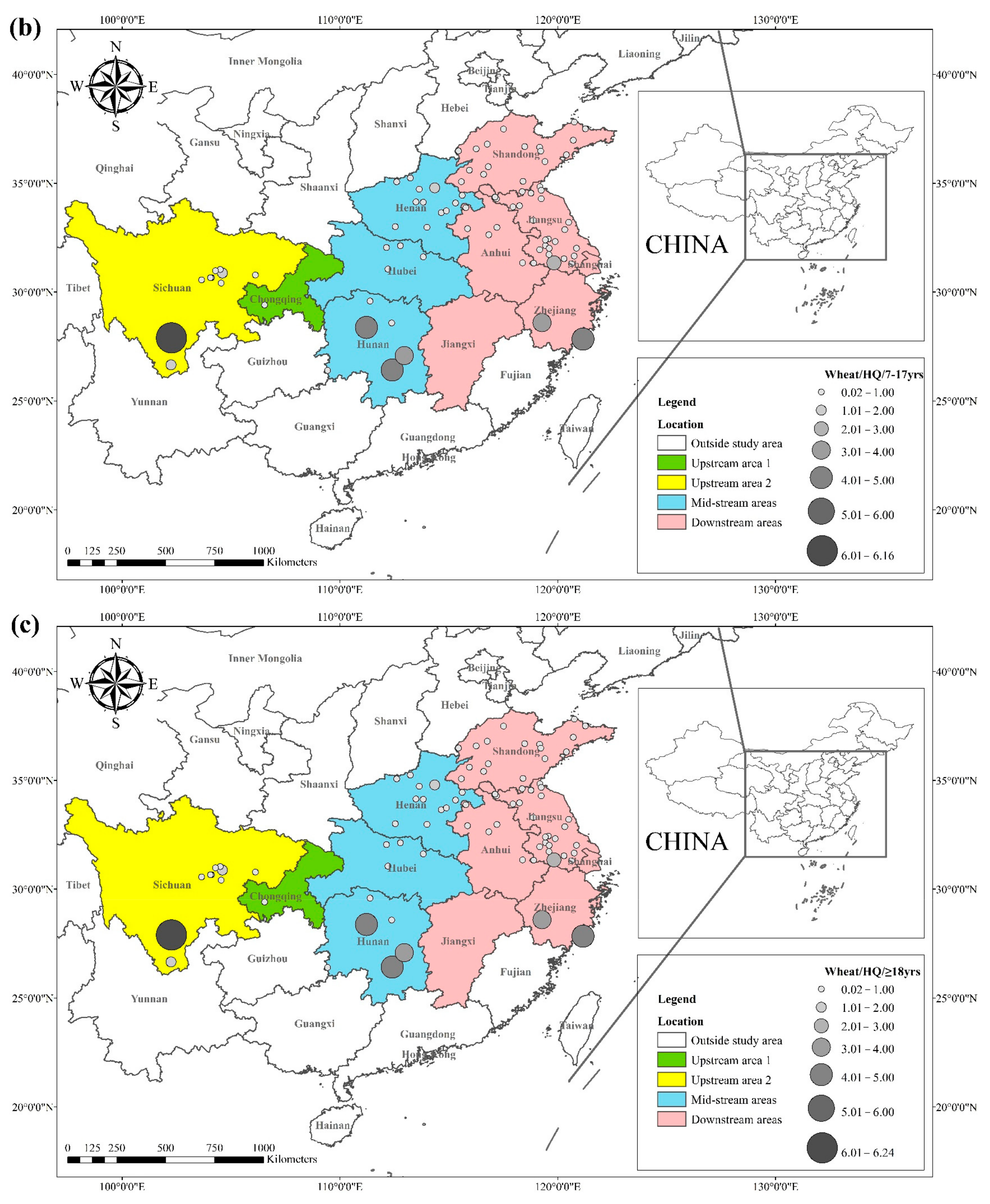
The health risks of exposure to heavy metals through oral, dermal, and inhalation routes can be assessed using a health exposure risk equation. The risk of exposure through dietary Cd intake is expressed in terms of ADI and HQ. The World Health Organization (WHO) has established a provisional tolerable daily intake (PTDI) for Cd of 0.06 mg kg−1 day−1; exposed populations have high health risks when the HQ is greater than 1. Dietary Cd intake poses a threat to local populations due to the combined effect of high grain intake rates and high grain Cd concentrations. The overall Cd concentration in Hunan province is significantly higher than that of other regions due to the extensive industrial activities in this region. The average Cd concentration in rice in Hunan exceeds the recommended limit by approximately 62%, and the average Cd concentration in wheat exceeds the recommended limit by about 81%. The spatial distribution of dietary Cd intake (HQ) in rice and wheat is shown in Figure 2, with a larger point indicating a higher risk factor. Findings from reviewed studies showed that the average risk factor for Cd in rice was 1.20, and the average risk factor for Cd in wheat was 1.54. The areas with a high HQ of Cd in rice were mainly located in the Yangtze River Basin and the southeastern coastal areas, including Sichuan, Hunan, Anhui, Jiangsu, and Zhejiang. Studies conducted in Chongqing, Hubei, and Shandong showed that the risk factor for Cd was low in these regions. The areas with a high HQ of Cd in wheat were primarily located in Sichuan, Hunan, Anhui, Jiangsu, Zhejiang, and Henan, and the risk factors for Cd in Chongqing, Hubei, and Shandong were lower compared with other regions. Consumption of locally produced rice and wheat increased the intake of Cd by the local population.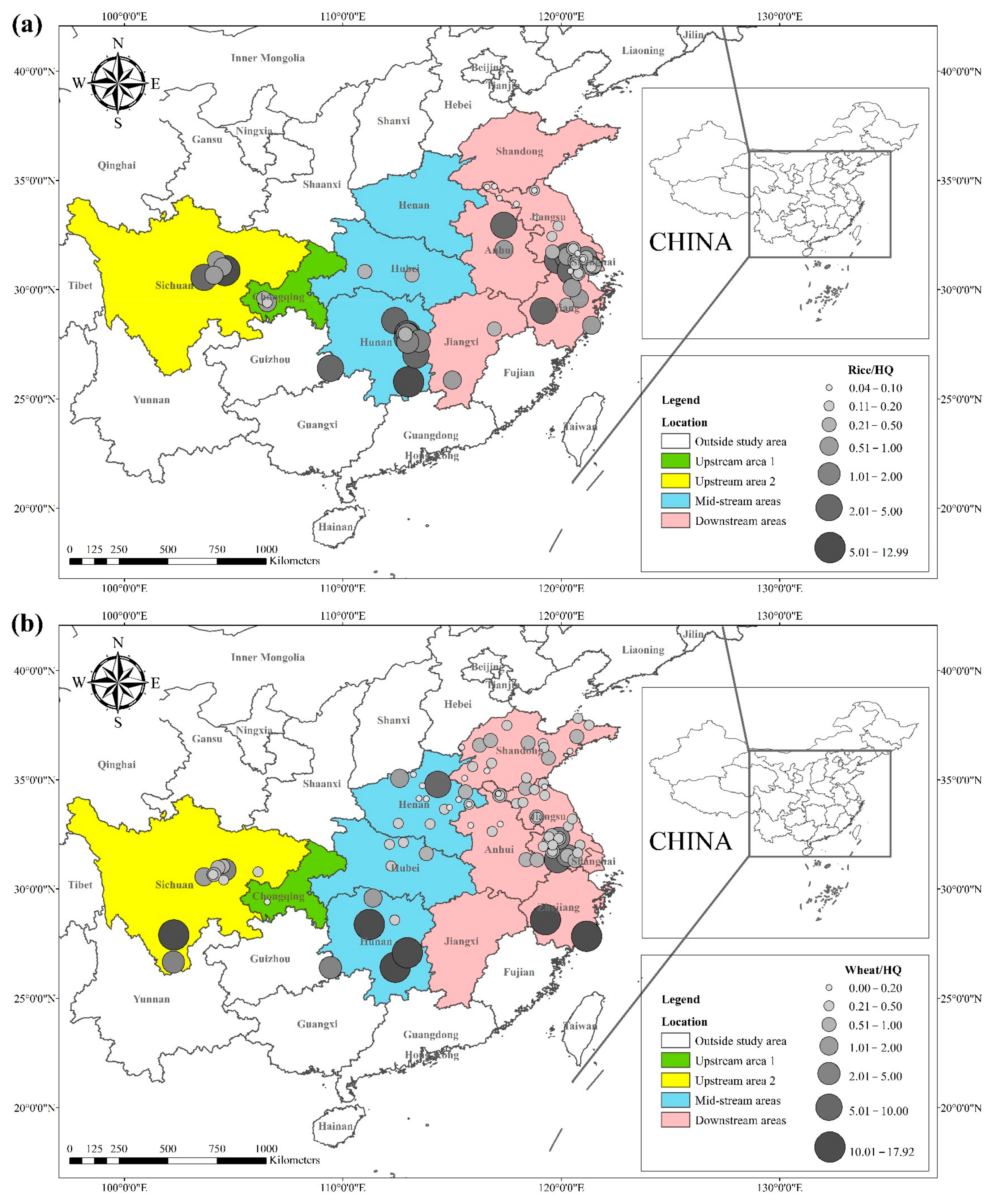
2.3. Cd in Rice-Wheat Cropping Field
A high HQ is positively correlated with a high concentration of Cd in soil. The first step in preventing Cd in grain is to identify and block major sources of contamination in the soil-based rice/wheat system. A high Cd concentration in agricultural soils mainly results from the geological background derived from the parent material and from human activities, such as the deposition of the atmosphere, mining activities, irrigation using wastewater, the application of phosphate fertilizers, livestock manure, organic fertilizer, and sludge application. Cd is a naturally occurring non-essential trace element, and the average concentration of Cd in soils is 0.01~2.00 mg kg−1 globally, whereas the average concentration in China ranges from 0.10~1.80 mg kg−1 [100][93]. Cd mainly exists as a complex with sulfur. Cd is released from this complex into the surface environment during mineral rock weathering. Parent materials determine the Cd content in soil systems. Soils derived from parent materials inherit the characteristics of the parent materials and their trace element content [101][94]. A study conducted in the Khorat Basin of Thailand showed that the different properties of parent materials significantly contributed to the chemical composition of paddy soils [102][95]. In addition, a study in Guizhou, China, reported that dry land topsoil derived from carbonate rocks had higher levels of Cr, Cd, and Hg [103][96]. The soil background value represents the contribution of the parent material, which exhibits a complex and diverse distribution in China. Geographic differences in soil Cd background values are observed, with higher background values reported in some provinces, such as Guizhou and Guangxi. Although there was no notable anthropogenic source of heavy metal pollution in the sampling area, an elevated Cd content was observed in paddy soil samples from the Cd geological anomaly areas in Guangxi and southwest China regions, which can be attributed to the weathering of carbonate rocks [104,105,106,107][97][98][99][100].3. Mechanism of Cd Pollution in Rice and Wheat Cropping System
3.1. Transport of Cd in Rice and Wheat Cropping System
The negative effects of Cd on crop physiological and biochemical processes, such as protein metabolism, mineral absorption, and photosynthesis, have been widely studied in the past [126,127][101][102]. The excessive accumulation of Cd in crops will significantly affect the growth and development of crops and lead to the further accumulation of Cd. Cd accumulation in crops is, therefore, influenced by a variety of soil factors, as well as the bioavailability and environmental availability of the crop to Cd. Environmental availability represents the content of heavy metals in soil, mainly including the proportion dissolved in pore water and the proportion adsorbed on the surface of soil minerals. Bioavailability refers to the portion of heavy metals dissolved in pore water that can be absorbed by the plant, while toxic bioavailability refers to the portion that accumulates in the plant and may be toxic to the plant [128,129][103][104]. The transport of bioavailable Cd from soil to rice grains is via coplastids that absorb roots and via root cells that load or sequester xylem. Cd is then transported through the xylem to the shoots and through the xylem to the nodes. The transport and accumulation of phloem to grain begins. Cd is a transcellular process regulated by a variety of transporters, such as absorption by root isoplasts, loading or isolation by root cells in the xylem, transport to nodes through the xylem, and transport to grains through the phloem. For example, OsIRT1 (IRT, iron-regulated transporter) and OsNramp1 (Nramp, natural resistance-associated macrophage protein) transporters are involved in the uptake of Cd in roots. The OsHMA3 protein regulates Cd sequestration in root cell vacuoles, whereas OsHMA2 (HMA, a P1B-type ATPase) regulates Cd delivery to developing tissues. OsLCT1 (OsLCT1, a low-affinity cation transporter) is a transporter associated with phloem Cd transport [106][99]. In the transport of Cd from soil to grain, xylem loading and transport is the first rate-controlling step, while phloem transport and unloading is the last rate-limiting step. Roots and nodes are the basic barriers to Cd transport to brown rice grains [130,131][105][106]. Cd uptake in wheat plants is mainly through the roots [132,133][107][108]. The rate of Cd uptake by wheat varies with soil type, air pollution, and wheat variety [134,135,136][109][110][111]. In addition, root exudates play an essential role in the accumulation of Cd in wheat [136][111]. A recent study showed that chlorine (Cl− could activate Cd in soil and increase the rate of Cd uptake by wheat. Depending on the wheat variety, the high and low concentrations of Cd accumulated by the root system after Cd uptake are transferred to the shoots [137,138][112][113]. Studies report that the higher retention of Cd in roots can be attributed to the chelation of Cd by organic acids [132][107]. However, another study reported that phytochelation might not be a limiting factor for the differential storage of Cd in wheat roots [139][114]. Shoot Cd accumulation in wheat depends on root-cadmium transport, whereas Cd accumulation in wheat grain mainly depends on root-shoot Cd transfer and the indirect transport of Cd from root to grain through xylem-to-phloem of the stem [140,141][115][116]. Riesen and Feller (2005) reported that Cd could be reactivated in wheat by the phloem and transpiration process [142][117]. Rice exhibits significant differences in Cd transport and accumulation among various genotypes. A study was conducted on 38 rice genotypes (indica vs. japonica), and the results showed that Cd concentrations ranged from 0.06 mg kg−1 to 0.99 mg kg−1, with significantly higher Cd accumulation levels in indica rice than in japonica rice [143][118]. Differences in grain Cd accumulation between different genotypes can be attributed to the differences in the transport of Cd from vegetative organs such as leaves and stems to the reproductive parts [144][119]. Cd transport in the rice-soil ecosystem is regulated by various transporters. Therefore, the expression level of the transporter gene and the content of the transporter affect the level of accumulation of Cd in rice. Overexpression of the OsHMA3 protein limits Cd accumulation in rice grains and enhances Cd tolerance [145][120]. In addition, the co-expression of OsHMA2, OsLCT1, and OsZIP3 effectively inhibits Cd transfer and accumulation in rice grains [146][121]. For safe wheat production, people have tried to screen and plant low-Cd-accumulating wheat varieties in light and moderate Cd-contaminated farmland [147,148][122][123]. Zhang et al. planted 8 wheat varieties in Cd-contaminated soil with a soil Cd content of 1.12 mg kg−1 (pH = 7.15) [148][123], of which 6 wheat varieties had a grain Cd content lower than the national food safety standard (0.10 mg kg−1). Chen et al. (2017) screened out 10 low-Cd accumulation varieties from 261 wheat varieties and planted them in Cd-contaminated farmland with a soil Cd content of 2.4 mg kg−1 (pH = 7.35) [149][124]. Low Cd accumulation in wheat cultivars is related not only to its genetic characteristics, such as low expression of transporters [150][125], smaller root morphological parameters (root length, surface area, and volume), etc. but also to its rhizosphere exudates and microbial communities [151][126]. Variety is an important factor affecting the Cd content of wheat grains. In some densely populated countries such as China and India, the use of heavy metal-contaminated farmland for agricultural production is inevitable, and planting crops with a low accumulation of heavy metals is a cost-effective measure to achieve food security in these regions.References
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15.
- Adnan, M.; Xiao, B.; Xiao, P.W.; Zhao, P.; Li, R.L.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231.
- Wang, P.; Kopittke, P.M.; McGrath, S.P.; Zhao, F.J. Cadmium transfer from soil to plants and its potential risk to human health. Nexus Soils Plants Anim. Hum. Health. 2017, 19, 138–147.
- Qu, M.; Wang, Y.; Huang, B.; Zhao, Y. Spatial uncertainty assessment of the environmental risk of soil copper using auxiliary portable X-ray fluorescence spectrometry data and soil pH. Environ. Pollut. 2018, 240, 184–190.
- Wu, Y.Q.; Yang, H.M.; Wang, M.; Sun, L.; Xu, Y.M.; Sun, G.H. Immobilization of soil Cd by sulfhydryl grafted palygorskite in wheat-rice rotation mode: A field-scale investigation. Sci. Total Environ. 2022, 826, 154156.
- Satarug, S. Editorial to Special Issue Toxic Metals, Chronic Diseases and Related Cancers. Toxics 2022, 10, 125.
- Wang, P.; Chen, H.P.; Kopittke, P.M.; Zhao, F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048.
- Li, Z.; Liang, Y.; Hu, H.; Shaheen, S.M.; Zhong, H.; Tack, F.M.G. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ. Int. 2021, 156, 106749.
- Ministry of Environmental Protection of the People’s Republic of China. Report on the National General Survey of Soil Contamination. 2014. Available online: http://www.gov.cn/govweb/foot/2014-04/17/content_2661768.htm (accessed on 17 April 2014).
- Xiao, J.; Yuan, X.; Li, J. Characteristics and transformation of heavy metal pollution in soil and rice of Yangtze River Delta Region. Agric. Sci. Technol. 2010, 11, 148–163.
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605.
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice. Sci. Total Environ. 2020, 712, 136497.
- Zhang, L.X.; Gao, C.; Chen, C.; Zhang, W.W.; Huang, X.Y.; Zhao, F.J. Overexpression of rice OsHMA3 in wheat greatly decreases cadmium accumulation in wheat grains. Environ. Sci. Technol. 2020, 54, 10100–10108.
- Duan, Y.L.; Li, Q.; Zhang, L.; Huang, Z.P.; Zhao, Z.M.; Zhao, H.; Du, J.; Zhou, J. Toxic Metals in a Paddy Field System: A Review. Toxics 2022, 10, 249.
- Zhao, S.P.; Ye, X.Z.; Chen, D.; Zhang, Q.; Xiao, W.D.; Wu, S.F.; Hu, J.; Gao, N.; Huang, M.J. Multi-Component Passivators Regulate Heavy Metal Accumulation in Paddy Soil and Rice: A Three-Site Field Experiment in South China. Toxics 2022, 10, 259.
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208.
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Rehman, M.Z.; Hannan, F.; Qayyum, M.F.; Hafeez, F. Cadmium stress in rice: Toxic effects, tolerance mechanisms and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879.
- Rizwan, M.; Ali, S.; Abbas, T.; Rehman, M.Z.; Hannan, F.; Keller, C.; Al-Wabel, M.I. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53.
- Ran, J.; Wang, D.; Wang, C.; Zhang, G.; Zhang, H. Heavy metal contents, distribution, and prediction in a regional soil-wheat system. Sci. Total Environ. 2016, 544, 422–431.
- Rehman, M.Z.; Khalid, H.; Akmal, F.; Ali, S.; Rizwan, M.; Qayyum, M.F.; Iqbal, M.; Khalid, M.U.; Azhar, M. Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field. Environ. Pollut. 2017, 227, 560–568.
- Qu, M.; Li, W.; Zhang, C.; Huang, B.; Zhao, Y. Spatially nonstationary relationships between copper accumulation in rice grain and some related soil properties in paddy fields at a regional scale. Soil Sci. Soc. Am. J. 2014, 78, 1765–1774.
- Li, D.; Wang, L.; Wang, Y.; Li, H.; Chen, G. Soil properties and cultivars determine heavy metal accumulation in rice grain and cultivars respond differently to Cd stress. Environ. Sci. Pollut. R. 2019, 26, 14638–14648.
- Khaliq, M.A.; James, B.; Chen, Y.H.; Ahmed Saqib, H.S.; Li, H.H.; Jayasuriya, P.; Guo, W. Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere 2019, 215, 916–924.
- Zhang, Y.; Wang, X.; Ji, X.; Liu, Y.; Lin, Z.; Lin, Z.; Xiao, S.; Peng, B.; Tan, C.; Zhang, X. Effect of a novel Ca-Si composite mineral on Cd bioavailability, transport and accumulation in paddy soil-rice system. J. Environ. Manag. 2019, 233, 802–811.
- Li, Q.S.; Chen, Y.; Fu, H.B.; Cui, Z.H.; Shi, L.; Wang, L.L.; Liu, Z.F. Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J. Hazard Mater. 2012, 227–228, 148–154.
- Wang, Z.W.; Zeng, X.F.; Geng, M.S.; Chen, C.Y.; Cai, J.C.; Yu, X.M.; Hou, Y.Y.; Zhang, H. Health risks of heavy metals uptake by crops grown in a sewage irrigation area in China. Pol. J. Environ. Stud. 2015, 24, 1379–1386.
- Wang, C.; Yuan, X.Y.; Chen, Y.; Ji, J.F.; Xi, B.B. Quantification of contributions from different sources on heavy metals accumulation in the paddy soil from Suzhou area. Acta Sci. Circumst. 2015, 35, 3269–3275.
- Wang, F.J.; Wang, M.; Liu, Z.P.; Shi, Y.; Han, T.Q.; Ye, Y.Y.; Gong, N.; Sun, J.W.; Zhu, C. Different responses of low grain-Cd-accumulating and high grain-Cd accumulating rice cultivars to Cd stress. Plant Physiol. Biochem. 2015, 96, 261–269.
- Yu, R.; Jiang, Q.; Xv, C.; Li, L.; Bu, S.; Shi, G. Comparative proteomics analysis of peanut roots reveals differential mechanisms of cadmium detoxification and translocation between two cultivars differing in cadmium accumulation. BMC Plant Biol. 2019, 19, 137.
- Zhang, Q.; Chen, H.; Xu, C.; Zhu, H.; Zhu, Q. Heavy metal uptake in rice is regulated by pH-dependent iron plaque formation and the expression of the metal transporter genes. Environ. Exp. Bot. 2019, 162, 392–398.
- Kaur, R.; Yadav, P.; Thukral, A.K.; Walia, A.; Bhardwaj, R. Co-application of 6-ketone type brassinosteroid and metal chelator alleviates cadmium toxicity in B. juncea L. Environ. Sci. Pollut. Res. 2017, 24, 685–700.
- Pan, W.; You, Y.; Shentu, J.L.; Weng, Y.N.; Wang, S.T.; Xu, Q.R.; Liu, H.J.; Du, S.T. Abscisic acid (ABA)-importing transporter 1 (AIT1) contributes to the inhibition of Cd accumulation via exogenous ABA application in Arabidopsis. J. Hazard. Mater. 2020, 391, 122189.
- Tudoreanu, L.; Phillips, C.J. Empirical models of cadmium accumulation in maize, rye grass and soya bean plants. J. Sci. Food Agr. 2004, 84, 845–852.
- Bao, A.; Burritt, D.J.; Chen, H.; Zhou, X.; Cao, D.; Tran, L.S.P. The CRISPR/Cas9 system and its applications in crop genome editing. Crit. Rev. Biotechnol. 2019, 39, 321–336.
- Lopez-Luna, J.; Silva-Silva, M.J.; Martinez-Vargas, S.; Mijangos-Ricardez, O.F.; Gon-zález-Chávez, M.C.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.C. Magnetite nanoparticle (NP) uptake by wheat plants and its effect on cadmium and chromium toxicological behavior. Sci. Total Environ. 2016, 565, 941–950.
- Yourtchi, M.S.; Bayat, H.R. Effect of cadmium toxicity on growth, cadmium accumulation and macronutrient content of durum wheat (Dena CV.). Int. J. Agric. Crop Sci. 2013, 6, 1099–1103.
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625.
- Wang, Y.; Qian, Y.; Hu, H.; Xu, Y.; Zhang, H. Comparative proteomic analysis of Cd-responsive proteins in wheat roots. Acta Physiol. Plant. 2011, 33, 349–357.
- Li, Y.; Chen, Z.; Xu, S.; Zhang, L.; Hou, W.; Yu, N. Effect of combined pollution of Cd and BP on photosynthesis and chlorophyll fluorescence characteristics of wheat. Pol. J. Environ. Stud. 2015, 24, 157–163.
- Gondor, O.K.; Szalai, G.; Kovács, V.; Janda, T.; Pál, M. Impact of UV-B on drought-or cadmium-induced changes in the fatty acid composition of membrane lipid fractions in wheat. Ecotoxicol. Environ. Saf. 2014, 108, 129–134.
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 2015, 252, 399–413.
- Huang, Z.Q.; Ye, S.C.; Hu, L.Y.; Hu, K.D.; Yan, H.; Li, W.J.; Jiao, H.; Zhang, H. Hydrogen sulfide promotes wheat grain germination under cadmium stress. Proc. Natl. Acad. Sci. India B Biol. Sci. 2015, 86, 887–895.
- Ishikawa, N.; Ishioka, G.; Yanaka, M.; Takata, K.; Murakami, M. Effects of ammonium chloride fertilizer and its application stage on cadmium concentrations in wheat (Triticum aestivum L.) grain. Plant Prod. Sci. 2015, 18, 137–145.
- Ok, Y.S.; Chang, S.X.; Gao, B.; Chung, H.J. SMART biochar technology-A shifting paradigm towards advanced materials and healthcare research. Environ. Technol. Innov. 2015, 4, 206–209.
- Ahmad, M.T.; Asghar, H.N.; Saleem, M.; Khan, M.Y.; Zahir, Z.A. Synergistic effect of rhizobia and biochar on growth and physiology of maize. Agron. J. 2015, 107, 1–8.
- Greger, M.; Landberg, T. Novel field data on phytoextraction: Pre-cultivation with salix reduces cadmium in wheat grains. Int. J. Phytorem. 2015, 17, 917–924.
- Qiu, Z.; Li, J.; Zhang, Y.; Bi, Z.; Wei, H. Microwave pretreatment can enhance tolerance of wheat seedlings to CdCl2 stress. Ecotoxicol. Environ. Saf. 2011, 74, 820–825.
- Fan, Z.W. Enrichment Characteristic and Rsk Assessment of Heavy Metals in Rice (Oryza.sativa L.) from Suzhou Regions; Suzhou University of Science and Technology: Suzhou, China, 2018.
- Jin, L. Heavy Metal Distribution and Food Safety Risk Analysis and Simulation in Soil-Rice System in Northern Jiangsu Province; Nanjing Agricultural University: Nanjing, China, 2007.
- Qi, Y.B.; Huang, B.; Yang, Y.F.; Darilek, J.L.; Zhao, Y.C.; Sun, W.X.; Wang, Z.G. Heavy metal accumulation characteristics and risk assessment of rice grain in different regions of Suzhou City, China. J. Agro-Environ. Sci. 2010, 29, 659–665.
- Fan, J.; Liao, Q.L.; Xu, H.M.; Ren, J.H.; Chang, Q.; Zhu, B.W.; Li, M. General characteristics of heavy metals absorbed by rice and wheat seeds within topsoil of farmland. J. Geol. 2016, 44, 701–709.
- Li, B.; Lin, Y.S.; Zhang, X.F.; Xu, Y.G.; Yu, F. Heavy metal pollution of the soils and wheat grains alongside the Shanghai-Nanjing Expressway. Rural Eco-Environ. 2005, 21, 50–53.
- Zhang, W.; Jia, J.R.; Dai, B. Distribution of heavy metals in wheat in Jiangsu province. Cereal Food Ind. 2021, 28, 61–64.
- Zhu, H.; Wu, C.F.; Chen, Y. Concentrations of heavy metals in wheat grains and their potential health risk in the central region of Jiangsu. Environ. Monit. Manag. Technol. 2021, 29, 35–56.
- Wan, T.; Li, F.H.; Huang, L.; Qi, H.B.; Xie, F.; Liang, J.; Wang, A.Q. Research on content of harmful metals in wheat grain from different places in China. Mod. Agric. Sci. Technol. 2018, 16, 248–250.
- Pan, Y.M.; Liao, Q.L.; Hua, M.; Gao, M.; Zhu, B.W.; Jin, Y. The evaluation of the heavy metal content in the Plough layer and crop in southern Jiangsu province. Geophys. Geochem. Explor. 2014, 38, 248–250.
- Chen, J.D.; Dai, Q.G.; Xu, X.H.; Zhong, X.C.; Guo, B.W.; Zheng, C.; Zhang, H.C.; Xu, K.; Huo, Z.Y.; Wei, H.Y. Heavy metal contents and evaluation of farmland soil and wheat in typical area of Jiangsu Province. Acta Ecol. Sin. 2012, 32, 3487–3496.
- Dai, W.T. Simulation Study on the Migration Characteristics of Heavy Metals in Soil-Wheat of Mining Area; China University of Mining and Technology: Beijing, China, 2017.
- Yan, L. The Pollution Characteristics of Heavy Metals on Wheat in Mining Area in North of Xuzhou; China University of Mining and Technology: Beijing, China, 2015.
- Zhu, Y.; Liao, S.P.; Liu, Y.; Li, X.K.; Ren, T.; Cong, R.H.; Lu, J.W. Annual nutrient budget differences between oil-rice and wheat-rice rotation systems in the Yangtze River basin. Plant Nutr. Fert. Sci. 2019, 25, 64–73.
- Liu, X.; Fan, Z.X.; Zhang, B.; Bi, Y.P. Study on cadmium pollution and remediation of soil in China. Shandong Agric. Sci. 2007, 6, 94–97.
- Tang, D.D.; Yuan, X.Y.; Wang, Y.M. Enrichment characteristics and risk prediction of heavy metals for rice grains growing in paddy soils with a high geological background. J. Agro-Environ. Sci. 2018, 37, 18–26.
- Ma, J.Y.; Ma, J.W.; Liu, D.; Fu, W.J.; Ye, Z.Q. Survey and risk assessment of soil heavy metals in the main rice producing areas in Hangjiahu Plain. J. Zhejiang A F Univ. 2021, 38, 336–345.
- Zhang, A.B.; Chu, X.Y.; Yin, H.Q.; Xu, M.X.; Huang, C.L.; Song, M.Y. Spatial variation of eight heavy metals in farmland soils and their accumulation in rice grains in Longyou pyrite mine, Zhejiang Province. Soils 2017, 49, 760–769.
- Dong, L.X. Assessment of Heavy Metal Pollution in Farmland Soil in Zhejiang Province; Wenzhou University: Wenzhou, China, 2017.
- Ma, B.J.; Wang, H.L.; Li, X.C.; Zhang, Y.H.; Liu, J.; Li, D.Y. Pollution of heavy metals in typical crops of northern Henan Prov-ince and health risk assessment. Ecol. Environ. Sci. 2014, 23, 1351–1358.
- Li, P.F.; Tan, J.; Lei, D.E.; Tao, C.J.; Du, G.Q.; Liu, C. Characteristics and ecological security assessment of heavy metal on wheat and root soils along Huaihe river commercial grain base in Anhui Province. Southwest China J. Agric. Sci. 2020, 33, 1580–1586.
- Xiao, X.P.; Peng, K.L.; Zhou, M.H. Investigation and evaluation of heavy metal pollution in suburb an paddy soils. Chin. J. Eco-Agric. 2008, 16, 680–685.
- Ruan, J.Z. Characteristics of Heavy Metal Pollution in Paddy Soils and Soil-Rice Heavy Metal Coupling Relationship in Typical Paddy Fields; Nanjing Agricultural University: Nanjing, China, 2020.
- Jiang, C.Y. Heavy Metal Pollution in Farmland Soil and Crops in a Mining Area in Hunan Province and Its Health Risk Assessment; Hunan Agricultural University: Nanjing, China, 2020.
- Zeng, Y.L. Distribution Characteristics of Cadmium-Contaminated Paddy Soil-Rice Elements and Evaluation of Heavy Metal Pollution in Liling, Hunan Province; Chengdu University of Technology: Chengdu, China, 2019.
- Zhou, M.; Tang, Z.M.; Zhang, M.; Liang, X.H.; Zhan, L. Characteristics and health risk assessment of heavy metal in soil-rice system in the Ganzhou area, Jiangxi Province. Geol. Bull. China. 2021, 40, 2149–2158.
- Li, B.Y.; Wang, P.; Wu, X.C.; Li, Z.P.; Zhou, D.M. Effect of long-term fertilization experiment on concention of micronutrients heavy metals in soil and brown rice. Acta Pedol. Sin. 2009, 46, 281–288.
- Lan, Y.S.; Shi, Z.H.; Yang, G.; Si, Y.Y.; Cheng, R. Status of heavy metal pollution in paddy soil and human health risk as-sess-ment of rice around phosphogypsum yard. J. Earth Environ. 2021, 12, 224–231.
- Yang, Y.; Deng, L.J. Effects of heavy metals in the paddy soil in Sichuan province on rice grain. J. Agro-Environ. Sci. 2005, 24, 174–177.
- Liu, Y. The Speciation and Bioavailability Study of Heavy Metals in Paddy Soils under the Rice-Wheat Cultivation Rotation; Sichuan Agricultural University: Ya’an, China, 2008.
- Jia, F.F. Effects of Swine Manure Application on Heavy Metals in Soils and Crops under Rice Wheat Rotation System; Sichuan Agri-cultural University: Ya’an, China, 2017.
- Li, J. Application Study on In-Situ Remediation of Safe Use Farmland Soil with Mild to Moderate Cd Contamination; Beijing Forestry University: Beijing, China, 2020.
- Shen, T.Z.; Zhu, M.X.; Xiao, J. Characteristics of Migration and Accumulation of Heavy Metals in Soil-Rice System of Tianmen and Its Health Risk Assessment. Chin. J. Soil Sci. 2014, 45, 221–226.
- Liu, L.; Peng, M.M.; Xia, H. Investigation and Analysis of Wheat Quality in Hubei Province. Hubei Agric. Sci. 2017, 56, 4872–4874.
- Zou, J.S.; Li, X.; Wan, W.; Liu, K. Study on tracking and verification of heavy metal content in soil and rice in a typical area of Chongqing. Acta Agric. Jiangxi. 2020, 32, 91–96.
- Hao, F. Heavy Metal Correlation between Soil and Grain Crops Characteristics and Pollution Assessment in Chongqing; South-west Agricultural University: Guangzhou, China, 2014.
- Hao, X.J.; Jin, C.Z.; Sun, C.; Liu, S.C.; Yao, H.H.; Zhang, T.; Jiang, J.P. The content and enrichment characteristics of heavy met-als in soil-rice system of paddy field in Minhang District, Shanghai. Acta Agric. Shanghai. 2020, 36, 88–93.
- Sun, H.; Han, J.X.; Ma, J.H. Health risk assessment of wheat seeds heavy metals in the sewage irrigation area of Huafei River, Kaifeng City. J. Agro-Environ. Sci. 2008, 27, 2332–2337.
- Han, H.; Wu, X.J.; Hui, R.Q.; Xia, X.; Chen, Z.J.; Yao, L.G.; Yang, J.J. Synergistic effects of Cd-loving Bacillus sp. N3 and iron oxides on immobilizing Cd and reducing wheat uptake of Cd. Environ. Pollut. 2022, 305, 119303.
- Li, Y.L.; Chen, W.P.; Yang, Y. Heavy metal pollution characteristics and comprehensive risk evaluation of farmland across the eastern plain of Jiyuan city. Acta Sci. Circumst. 2020, 40, 2229–2236.
- Zhang, B.C.; Fan, L.X.; Zhao, P.J.; Chen, L. Contamination status and evaluation of cadmium and chromium in wheat grain in typical areas of Shandong Province. J. Triticeae Crops. 2016, 36, 1396–1401.
- Liu, Q.; Li, M.; Wu, H. Spatial variation and evaluation of Pb and Cd in suburban farmland soils–a case study of Changsha city. Resour. Environ. Yangtze Basin. 2012, 21, 195–203.
- He, P. Study on Safety Control Technology of Soil Cadmium at Typical Pollution Area in Southern China; Guizhou University: Gui-yang, China, 2019.
- Song, Y.; Wang, Y.B.N.; Mao, W.F.; Sui, H.X.; Yong, L.; Yang, D.J.; Jiang, D.G.; Zhang, L.; Gong, Y.Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978.
- EFSA. Cadmium dietary exposure in the European population. EFSA J. 2012, 10, 2551.
- FAO; WHO. Evaluation of certain food additives and contaminants. Seventy-third Report of the Joint FAO/WHO Expert Committee on Food Additives. Indian J. Med. Res. 2012, 135, 446–447.
- Teng, W.; Liu, Q.; Li, Q. Hazard and Risk Assessment of Heavy Metal Pollution to Agricultural Products; South-West Agricultural University: Guangzhou, China, 2010; pp. 38–44.
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140.
- Inboonchuay, T.; Suddhiprakarn, A.; Kheoruenromne, I.; Anusontpornperm, S.; Gilkes, R.J. Amounts and associ ations of heavy metals in paddy soils of the Khorat basin, Thailand. Geoderma. Reg. 2016, 7, 120–131.
- Tu, C.L.; He, T.B.; Liu, C.Q.; Lu, X.H. Effects of land use and parent materials on trace elements accumulation in topsoil. J. Environ. Qual. 2013, 42, 103–110.
- Liu, Y.Z.; Xiao, T.F.; Xiong, Y.; Ning, Z.P.; Shuang, Y.; Li, H.; Ma, L.; Chen, H.Y. Accumulation of heavy metals in agricultural soils and crops from an area with a high geochemical background of cadmium, Southwestern China. Environ. Sci. 2019, 40, 2877–2884.
- Ma, H.H.; Peng, M.; Liu, F.; Guo, F.; Tang, S.Q.; Liu, X.J.; Zhou, Y.L.; Yang, K.; Li, K.; Yang, Z.; et al. Bioavailability, trans-loca-tion, and accumulation characteristic of heavy metals in a soil-crop system from a typical carbonate rock area in Guangxi. China Environ. Sci. 2020, 41, 449–459.
- Chen, T.; Pang, R.; Wang, F. Safety assessment of rice planting in soil cadmium geological anomaly areas in Southwest Guang-xi. Environ. Sci. 2020, 41, 1855–1863.
- Wen, Y.B.; Li, W.; Yang, Z.F.; Zhuo, X.X.; Guan, D.X.; Song, Y.X.; Guo, C.; Ji, J.F. Evaluation of various approaches to predict cadmium bioavailability to rice grown in soils with high geochemical background in the karst region, Southwestern China. Environ. Pollut. 2020, 258, 113645.
- Sebastian, A.; Prasad, M.N.V. Iron- and manganese-assisted cadmium tolerance in Oryza sativa L.: Lowering of rhizotoxicity next to functional photosynthesis. Planta 2015, 241, 1519–1528.
- Saleh, S.R.; Kandeel, M.M.; Ghareeb, D.; Ghoneim, T.M.; Talha, N.I.; Alaoui-Sosse, B.; Aleya, L.; Abdel-Daim, M.M. Wheat bi-ological responses to stress caused by cadmium, nickel and lead. Sci. Total Environ. 2019, 706, 136013.
- Harmsen, J. Measuring bioavailability: From a scientific approach to standard methods. J. Environ. Qual. 2007, 36, 1420–1428.
- Kim, R.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation–a critical review. Environ. Geochem. Health. 2015, 37, 1041–1061.
- Yamaguchi, N.; Ishikawa, S.; Abe, T. Role of the node in controlling traffic of cadmium, zinc, and manganese in rice. J. Exp. Bot. 2012, 63, 2729–2737.
- Feng, X.; Han, L.; Chao, D. Ionomic and transcriptomic analysis provides new insight into the distribution and transport of cadmium and arsenic in rice. J. Hazard Mater. 2017, 331, 246–256.
- Adeniji, B.A.; Budimir-Hussey, M.T.; Macfie, S.M. Production of organic acids and adsorption of Cd on roots of durum wheat (Triticum turgidum L. var. durum). Acta Physiol. Plant. 2010, 32, 1063–1072.
- Black, A.; McLaren, R.G.; Speir, T.W.; Clucas, L.; Condron, L.M. Gradient differences in soil metal solubility and uptake by shoots and roots of wheat (T. aestivum). Biol. Fert. Soils 2014, 50, 685–694.
- Guo, H.; Tian, R.; Zhu, J.; Zhou, H.; Pei, D.; Wang, X. Combined cadmium and elevated ozone affect concentrations of cad-mi-um and antioxidant systems in wheat under fully open-air conditions. J. Hazard. Mater. 2012, 209, 27–33.
- Liu, K.; Lv, J.; He, W.; Zhang, H.; Cao, Y.; Dai, Y. Major factors influencing cadmium uptake from the soil into wheat plants. Ecotoxicol. Environ. Saf. 2015, 113, 207–213.
- Greger, M.; Landberg, T. Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 2008, 312, 195–205.
- Dahlin, A.S.; Eriksson, J.; Campbell, C.D.; Öborn, I. Soil amendment affects Cd uptake by wheat—Are we under estimating the risks from chloride inputs? Sci. Total Environ. 2016, 554, 349–357.
- Ci, D.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Cadmium stress in wheat seedlings: Growth, cadmium accumu-la-tion and photosynthesis. Acta Physiol. Plant. 2010, 32, 365–373.
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Kochian, L.V. Characterization of cadmium uptake, translocation and stor age in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol. 2006, 172, 261–271.
- Harris, N.S.; Taylor, G.J. Cadmium uptake and translocation in seedlings of near isogenic lines of durum wheat that differ in grain cadmium accumulation. BMC Plant Biol. 2004, 4, 1–12.
- Harris, N.S.; Taylor, G.J. Cadmium uptake and partitioning in durum wheat during grain filling. BMC Plant Biol. 2013, 13, 103.
- Riesen, O.; Feller, U. Redistribution of nickel, cobalt, manganese, zinc, and cadmium via the phloem in young and maturing wheat. J. Plant Nutr. 2005, 28, 421–430.
- Van der Vliet, L.; Peterson, C.; Hale, B. Cd accumulation in roots and shoots of durum wheat: The roles of tran spiration rate and apoplastic bypass. J. Exp. Bot. 2007, 58, 2939–2947.
- He, J.Y.; Zhu, C.; Ren, Y.F.; Yan, Y.P.; Jiang, D. Genotypic variation in grain cadmium concentration of lowland rice. J. Plant Nutr. Soil Sci. 2006, 169, 711–716.
- Sasaki, A.; Yamaji, N.; Feng, M.J. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn trans porter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021.
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.H.; Zhang, Y.X.; Chai, T.Y. Co-expression of multiple heavy metal transport ers changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard Mater. 2019, 380, 120853.
- Liu, N.; Huang, X.M.; Sun, L.M.; Li, S.S.; Chen, Y.H.; Cao, X.Y.; Wang, W.X.; Dai, Y.L. Screening stably low cadmium and mod-erately high micronutrients wheat cultivars under three different agricultural environments of China. Chemosphere 2020, 241, 125045.
- Zhang, L.; Zhang, C.; Du, B.Y.; Lu, B.X.; Zhou, J.; Zhou, Y. Effects of node restriction on cadmium accumulation in eight Chi-nese wheat (Triticum turgidum) cultivars. Sci. Total Environ. 2020, 725, 138158.
- Chen, Y.R.; Zhang, Q.F.; Fu, B.S.; Cai, S.; Wu, J.D.; Chen, Y. Differences of lead, cadmium and zinc accumulation among Chi-nese wheat mini-core collections germplasms and screening for low Pb, Cd and Zn accumulative cultivars in grains. J. Nanjing Agric. Univ. 2017, 40, 393–399.
- Qiao, K.; Wang, F.; Liang, S.; Wang, S.; Chai, T. New biofortification tool: Wheat Ta CNR5 enhances zinc and manganese tol-erance and increases zinc and manganese accumulation in rice grains. J. Agric. Food Chem. 2019, 67, 9877–9884.
- Liu, N.; Miao, Y.; Zhou, X.; Gan, Y.; Liu, Y.; Wang, W.; Dai, J. Roles of rhizospheric organic acids and microorgan isms in mer-cury accumulation and translocation to different winter wheat cultivars. Agric. Ecosyst. Environ. 2018, 258, 104–112.
