Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Martina Catalano.
Immunotherapy is an ever-expanding field in lung cancer treatment research. The lung cancer treatment paradigm has been completely changed by immunotherapy; however, less than half of the treated patients obtain a response, and an even smaller proportion achieve a long survival.
- lung cancer
- immunotherapy
- emerging immune checkpoint inhibitors
1. Introduction
The last decade has seen the rapid development of immunotherapy and its role as a crucial strategy in cancer treatment, particularly in the field of lung cancer. The immune system closely interacts with tumors along the entire process of cancer onset and progression. Tumor cells develop numerous ways to escape immune cell recognition and removal by regulating their antigen presentation, through the secretion of immunosuppressive cytokines such as interleukin (IL)-10 and transforming growth factor (TGF)-β, or by affecting their metabolism, causing an alteration in the tumor microenvironment (TME) [1,2,3,4,5,6][1][2][3][4][5][6]. However, the most potent mechanism to limit normal anti-tumor immune responses is the activation of immune checkpoint pathways such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed death 1 (PD-1) and programmed death ligand-1 (PD-L1) [7,8,9][7][8][9]. In fact, the CTLA-4 and PD-1/PD-L1 blockade was capable of restoring the host’s T cell-mediated immune system response, suppressed by the tumour [9]. These findings paved the way to the development of immune checkpoint inhibitors (ICIs), which take advantage of the host’s immune system to enhance anti-tumor activity. Clinical efficacy and durable responses were recorded in several tumour types [10[10][11][12][13],11,12,13], especially in non-small-cell lung cancer (NSCLC) [14]. In patients with NSCLC without a driver mutation and in those with small-cell lung cancer (SCLC), immunotherapy in the form of ICIs is currently the cornerstone of treatment [15,16][15][16]. In NSCLC PD-L1, despite representing to date the most reliable predictive biomarker of response to immunotherapy, it fails to select the right subset of patients who would benefit from this treatment. Indeed, only a limited number of patients respond to ICI, and also in the event of a lasting response they eventually experience disease progression. Moreover, due to the paucity of effective second-line treatments, the mortality rate of this disease remains still high [17,18,19][17][18][19]. In addition, about 15–25% of patients treated with ICIs developed serious immune-related adverse events (irAEs), which can sometimes be fatal [20,21,22][20][21][22]. Therapy strategies which involve the combination of ICI with each other or with other drugs (i.e., chemotherapy, target therapy, agents, poly ADP ribose polymerase (PARP) inhibitors) or local treatment, have been adopted to overcome these hindrances, resulting in increased clinical responses. However, as many patients show primary or acquired resistance to ICIs [23[23][24][25][26],24,25,26], a great interest is addressed to discover novel targets. The next generation immune checkpoints, such as lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin (Ig) and Immunoreceptor Tyrosine-Based Inhibitory Motif (ITIM) domain (TIGIT), T cell immunoglobulin and mucin-domain containing-3 (TIM-3), V-domain Ig suppressor of T cell activation (VISTA), B7 homolog 3 protein (B7-H3), inducible T cell costimulatory (ICOS), and B and T cell lymphocyte attenuator (BTLA), appear to be promising therapeutic strategies with the possibility of future clinical applications [27,28,29,30,31,32,33][27][28][29][30][31][32][33]. Furthermore, the addition of novel ICIs, which do not exhibit overlapping mechanisms of action with those already in use, could improve efficacy and decrease toxicity.
2. Current Role of Immunotherapy in Lung Cancer
In the past seven years, numerous ICIs received approval for lung cancer treatment in different settings of disease, particularly for NSCLC. Indeed, ICIs commenced as a second-line treatment strategy for metastatic NSCLC. The therapeutic indications were then extended to the first-line advanced settings and later also to the earlier stages, including both unresectable and resectable disease (Figure 1).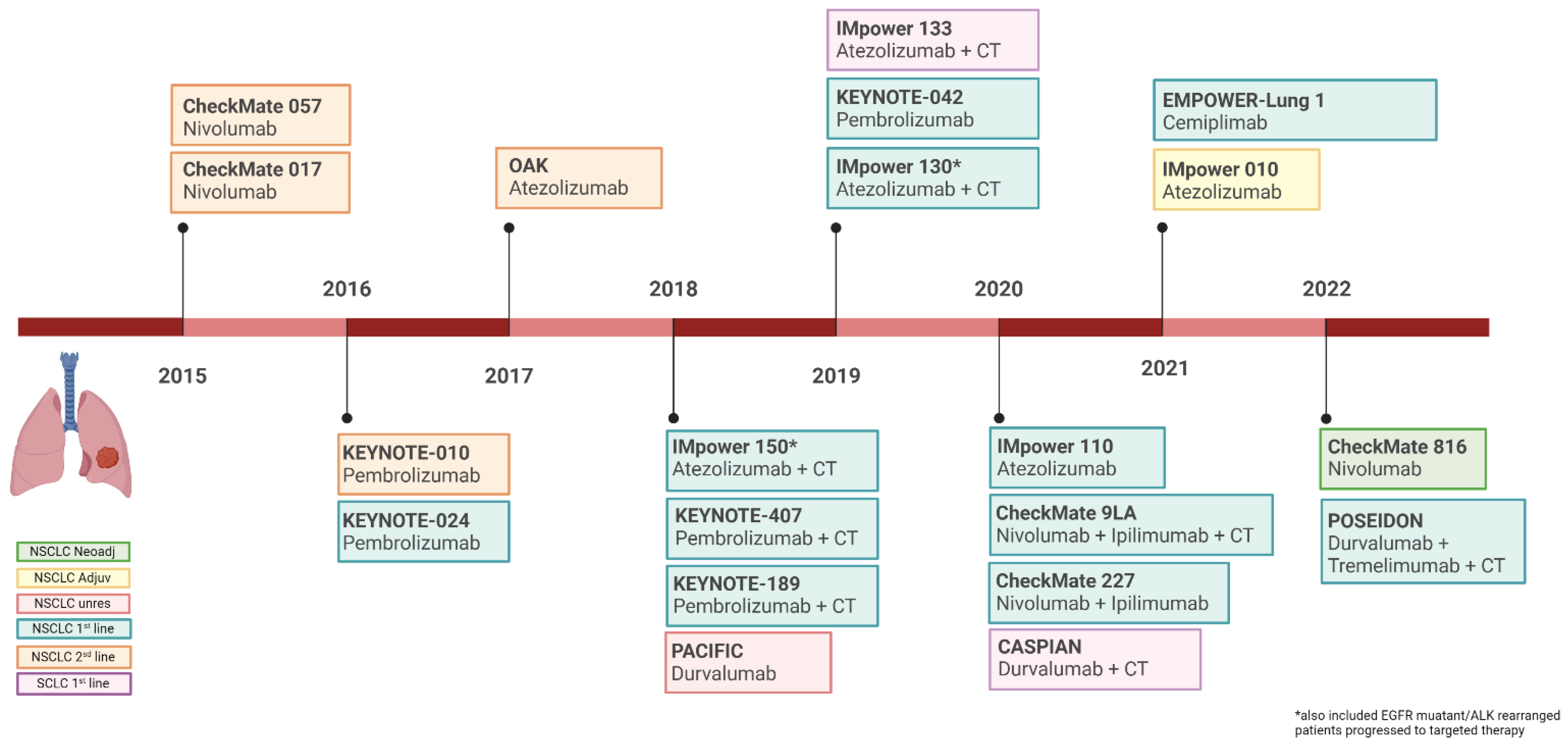
Figure 1. Timeline of Food and Drug Administration approval of immune checkpoints inhibitors in lung cancer. Image created with BioRender.com, accessed on 5 July 2022.
PD-L1 Expression Levels and Outcome Related
PD-L1 is currently one of the few recognized and approved biomarkers predictive of response to immunotherapy. Despite the confirmed benefit of assigning ICIs according to PD-L1 expression, the latter biomarker alone is still inadequate to select the right candidates for immunotherapy [57]. In NSCLC, patients with higher levels of PDL1 expression tend to respond more favorably to the ICIs [58,59][58][59]. Different diagnostic immunohistochemistry test, with variations in cut-off values have been used to establish PD-L1 expression [60,61,62,63][60][61][62][63]. The frequency of PD-L1 expression in lung cancer has been reported by several authors [64,65,66][64][65][66]. In the largest real-world study conducted on 2368 advanced NSCLC patients, 22% had PD-L1 TPS ≥ 50%, 52% PD-L1 TPS ≥ 1%, and 48% PD-L1 TPS < 1%. Prevalence of PD-L1 TPS ≥ 50% and TPS ≥ 1% were similar across geographic regions ranging from 21–24% and 47–55%, respectively [64]. Another study assessed PD-L1 expression in 264 cases of NSCLC showing: high PD-L1 expression (≥50%) in 29.5% of cases, low (1–49%) in 43.9% and absent (<1%) in the 26.5% [66]. Skov et al., in their prospective study, included 819 patients with NSCLC reported a PD-L1 ≥ 1% positive cells in the 63% of NSCLC patients and PD-L1 ≥ 50% in 30% [66]. Unlike NSCLC, in other types of lung cancer such as SCLC PD-L1 expression levels are understudied, with contradictory reports of expression status [67]. Most recently Xu et al. conducted a meta-analysis to evaluate the efficacy of ICI monotherapy or combined with chemotherapy and estimate the predictive value of PD-L1 expression in predicting the response from these treatment [67]. Results showed better OS, PFS and ORR with anti-PD-1/PD-L1 monotherapy compared with chemotherapy in the intention-to-treat population (ITT) and emphasized the value of positive PD-L1 expression in predicting improvement of clinical outcome from anti-PD-1/PD-L1 treatment. Indeed, better efficacy outcomes correlated with higher PD-L1 levels (mainly PD-L1 ≥ 50%), whereas no statistical survival benefit was observed for the PD-L1 < 1% population who received anti-PD-1/PD-L1 monotherapy compared to chemotherapy alone. Subgroup analyzes showed significant improvement in ORR from ICI in patients with PD-L1 ≥ 50%, no difference in patients with PD-L1 < 1%, and better ORR with chemotherapy versus ICI monotherapy in patients with PD-L1 expression ranging from 1 to 49%. Similar results derived from Liu et al.’s metanalysis [67]. In this restudyearch, in patients with PD-L1 ≥ 1%, ten immunotherapy combinations were associated with significantly prolonged OS and PFS (the latter especially with anti-PD-1 plus chemotherapy) compared with chemotherapy. In patients with PD-L1 1–49%, seven immunotherapy combinations also significantly improved OS and PFS compared with chemotherapy. In patients with PD-L1 ≥ 50%, nine immunotherapy combinations (except for durvalumab-tremelimumab), showed significantly higher OS and PFS benefit than standard chemotherapy [67]. Finally, another metanalysis investigated the efficacy and safety of dual ICIs ± other therapies. An improved OS with the combination therapy in the ITT population was shown. However, according to the analysis, no statistically significant difference between the two groups was found for patients with PD-L1 < 1%, thus narrowing the benefit from this combination for PD-L1 ≥ 1% expression [68].References
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296.
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416.
- Wu, A.A.; Drake, V.; Huang, H.S.; Chiu, S.C.; Zheng, L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015, 4, e1016700.
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618.
- Croci, D.O.; Zacarías Fluck, M.F.; Rico, M.J.; Matar, P.; Rabinovich, G.A.; Scharovsky, O.G. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol. Immunother. 2007, 56, 1687–1700.
- Parayath, N.; Padmakumar, S.; Nair, S.V.; Menon, D.; Amiji, M.M. Strategies for Targeting Cancer Immunotherapy Through Modulation of the Tumor Microenvironment. Regen. Eng. Transl. Med. 2020, 6, 29–49.
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 86.
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 8.
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H.; et al. Advances in cancer immunotherapy 2019-Latest trends. J. Exp. Clin. Cancer Res. 2019, 38, 268.
- Smyth, M.J.; Ngiow, S.F.; Ribas, A.; Teng, M.W.L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016, 13, 143–158.
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580.
- Chu, X.; Niu, L.; Xiao, G.; Peng, H.; Deng, F.; Liu, Z.; Wu, H.; Yang, L.; Tan, Z.; Li, Z.; et al. The Long-Term and Short-Term Efficacy of Immunotherapy in Non-Small Cell Lung Cancer Patients With Brain Metastases: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 2297.
- Singh, N.; Temin, S.; Baker, S.; Blanchard, E.; Brahmer, J.R.; Celano, P.; Duma, N.; Ellis, P.M.; Elkins, I.B.; Haddad, R.Y.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO Living Guideline. J. Clin. Oncol. 2022, 40, 3323–3343.
- Zugazagoitia, J.; Paz-Ares, L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J. Clin. Oncol. 2022, 40, 671–680.
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11.
- Trebeschi, S.; Drago, S.G.; Birkbak, N.J.; Kurilova, I.; Cǎlin, A.M.; Delli Pizzi, A.; Lalezari, F.; Lambregts, D.M.J.; Rohaan, M.W.; Parmar, C.; et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann. Oncol. 2019, 30, 998–1004.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723.
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486.
- Altmann, D.M. A Nobel Prize-worthy pursuit: Cancer immunology and harnessing immunity to tumour neoantigens. Immunology 2018, 155, 283–284.
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019.
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47.
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.L.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81.
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532.
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998.
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96.
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541.
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57.
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592.
- Yang, S.; Wei, W.; Zhao, Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int. J. Biol. Sci. 2020, 16, 1767–1773.
- Marinelli, O.; Nabissi, M.; Morelli, M.B.; Torquati, L.; Amantini, C.; Santoni, G. ICOS-L as a Potential Therapeutic Target for Cancer Immunotherapy. Curr. Protein Pept. Sci. 2018, 19, 1107–1113.
- Yu, X.; Zheng, Y.; Mao, R.; Su, Z.; Zhang, J. BTLA/HVEM Signaling: Milestones in Research and Role in Chronic Hepatitis B Virus Infection. Front. Immunol. 2019, 10, 617.
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985.
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357.
- FDA Approves Atezolizumab as Adjuvant Treatment for Non-Small Cell Lung Cancer|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-adjuvant-treatment-non-small-cell-lung-cancer (accessed on 16 May 2022).
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929.
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311.
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639.
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrietac, O.; Frontera, O.A.; Chiari, R.; et al. Five-year outcomes from the randomized, phase iii trials checkmate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J. Clin. Oncol. 2021, 39, 723–733.
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265.
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833.
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339.
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604.
- Awad, M.M.; Gadgeel, S.M.; Borghaei, H.; Patnaik, A.; Yang, J.C.H.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Altan, M.; et al. Long-Term Overall Survival From KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin With or Without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 162–168.
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092.
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051.
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937.
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial. Nat. Med. 2022, 28, 2374–2380.
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031.
- FDA Approves Nivolumab Plus Ipilimumab for First-Line mNSCLC (PD-L1 Tumor Expression ≥1%)|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-first-line-mnsclc-pd-l1-tumor-expression-1 (accessed on 16 May 2022).
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211.
- FDA Approves Nivolumab Plus Ipilimumab and Chemotherapy for First-Line Treatment of Metastatic NSCLC|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-and-chemotherapy-first-line-treatment-metastatic-nsclc (accessed on 16 May 2022).
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.-W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non–Small-Cell Lung Cancer: The Phase III POSEIDON Study. J. Clin. Oncol. 2022.
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229.
- Giunchi, F.; Degiovanni, A.; Daddi, N.; Trisolini, R.; Dell’Amore, A.; Agostinelli, C.; Ardizzoni, A.; Fiorentino, M. Fading with time of PD-L1 immunoreactivity in non-small cells lung cancer tissues: A methodological study. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 489–494.
- Ameratunga, M.; Asadi, K.; Lin, X.; Walkiewicz, M.; Murone, C.; Knight, S.; Mitchell, P.; Boutros, P.; John, T. PD-L1 and Tumor infiltrating lymphocytes as prognostic markers in resected NSCLC. PLoS ONE 2016, 11, e0153954.
- Casadevall, D.; Clavé, S.; Taus, Á.; Hardy-Werbin, M.; Rocha, P.; Lorenzo, M.; Menéndez, S.; Salido, M.; Albanell, J.; Pijuan, L.; et al. Heterogeneity of Tumor and Immune Cell PD-L1 Expression and Lymphocyte Counts in Surgical NSCLC Samples. Clin. Lung Cancer 2017, 18, 682–691.e5.
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028.
- Jaafar, J.; Fernandez, E.; Alwan, H.; Philippe, J. Programmed cell death-1 and programmed cell death ligand-1 antibodies-induced dysthyroidism. Endocr. Connect. 2018, 7, R196–R211.
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846.
- Spira, A.I.; Park, K.; Mazières, J.; Vansteenkiste, J.F.; Rittmeyer, A.; Ballinger, M.; Waterkamp, D.; Kowanetz, M.; Mokatrin, A.; Fehrenbacher, L. Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR). J. Clin. Oncol. 2015, 33, 8010.
- Dietel, M.; Savelov, N.; Salanova, R.; Micke, P.; Bigras, G.; Hida, T.; Antunez, J.; Guldhammer Skov, B.; Hutarew, G.; Sua, L.F.; et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non–small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer 2019, 134, 174–179.
- Skov, B.G.; Rørvig, S.B.; Jensen, T.H.L.; Skov, T. The prevalence of programmed death ligand-1 (PD-L1) expression in non-small cell lung cancer in an unselected, consecutive population. Mod. Pathol. 2020, 33, 109–117.
- Holmes, M.; Mahar, A.; Lum, T.; Boyer, M.; Kao, S.; Cooper, W. P1.09-26 Prevalence of PD-L1 Expression Rates in Different NSCLC Specimens. J. Thorac. Oncol. 2019, 14, S506.
- Ullah, A.; Pulliam, S.; Karki, N.R.; Khan, J.; Jogezai, S.; Sultan, S.; Muhammad, L.; Khan, M.; Jamil, N.; Waheed, A.; et al. PD-L1 Over-Expression Varies in Different Subtypes of Lung Cancer: Will This Affect Future Therapies? Clin. Pract. 2022, 12, 653–671.
- Zhang, P.P.; Wang, J.; Ding, D.Z.; Zhang, L.; Cheng, C.; Chen, D.K. Efficacy and safety of PD-1/PD-L1 inhibitors combined with CTLA-4 inhibitor versus chemotherapy for advanced lung cancer: A meta-analysis. Medicine 2021, 100, e27121.
More
