Natural polysaccharides are a type of natural biomacromolecule found in plants, fungi, algae, animals, and bacteria. Due to their nontoxic, stable, biodegradable, biocompatibility, and excellent antioxidant activity, natural polysaccharides contribute to the potential value in treating or preventing disease caused by oxidative stress. Polysaccharides can reduce the damage to the cell structure, regulate the signal pathways related to antioxidation, improve the intracellular antioxidant enzyme system, reduce the substances that easily produce reactive oxygen species (ROS), and protect the body tissue from ROS-induced damage through free radical scavenging activity and immunomodulatory activity. Natural polysaccharides play an irreplaceable therapeutic role and have received more and more attention in recent decadesn. Publications related to natural polysaccharides are also increasing year by year.
- natural polysaccharides
- antioxidant
- reactive oxygen species (ROS)
- drug delivery
1. Antioxidant Activity of Natural Polysaccharides
1.1. Higher Plant Polysaccharides
1.2. Algal Polysaccharides
1.3. Microbial Polysaccharides
1.3.1. Fungal Polysaccharides
1.3.2. Bacterial Polysaccharides
1.4. Animal Polysaccharides

2. Antioxidant Activity of Natural Polysaccharide Derivatives
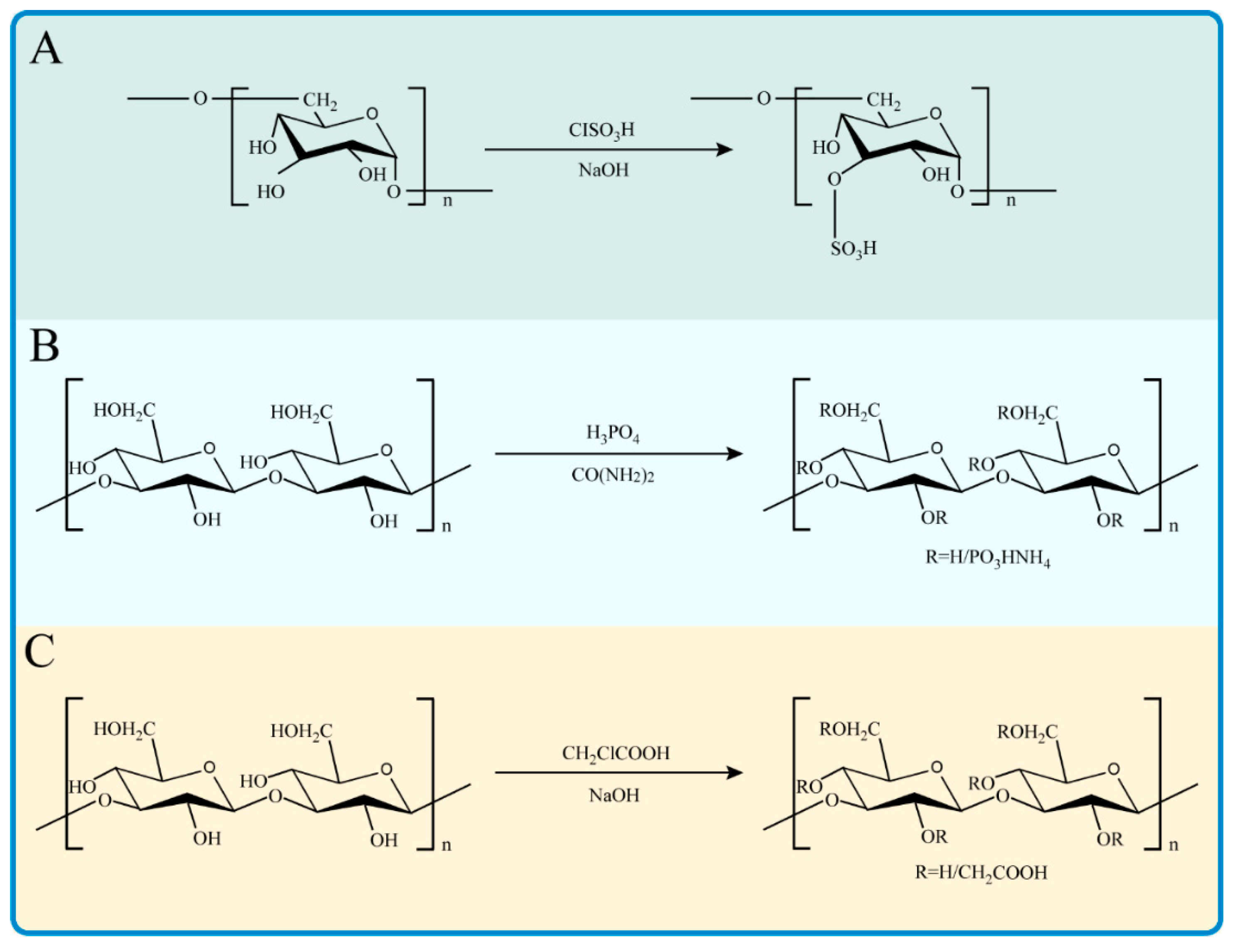
2.1. Sulfated Polysaccharides
2.2. Phosphorylated Polysaccharides
2.3. Carboxymethylated Polysaccharides
Carboxymethylation is a common modification method of polysaccharides to improve water solubility and biological activity. The aqueous medium and organic solvent methods are the most common synthesis methods of carboxymethylation. The aqueous medium method dissolves the polysaccharides with an alkaline solution, adds monochloroacetic acid (MCA) to mix and react for several hours, and adds an acid solution to adjust the pH to a neutral range. Ethanol is the precipitation solvent, and carboxymethyl polysaccharide derivatives can be obtained after dialysis and freeze-drying [81]. This method is unstable and takes a long time, but it is cheap and commonly used. The organic solvent method is to dissolve polysaccharides in organic solvents such as isopropanol or ethanol, and then add MCA for etherification reaction to obtain carboxymethyl polysaccharide derivatives [82]. The reaction is stable and rapid, but the price is relatively expensive with the toxic organic reagent. Carboxymethyl-modified pumpkin polysaccharides prepared by the aqueous medium method were proven to have a good scavenging ability of superoxide anion free radicals and hydroxyl free radicals (Liu & Huang, 2019). It has been reported that the carboxymethylated polysaccharides with a molecular weight of 354 kDa were obtained by the chemical modification of Sargassum fusiforme polysaccharides [83]. Compared with the unmodified Sargassum fusiforme polysaccharides, the modified carboxymethyl polysaccharides have a stronger free radical scavenging ability and total antioxidant activity. Similarly, the crude polysaccharides of Enteromorpha prolifera were degraded with hydrogen peroxide or ascorbic acid and modified by carboxymethyl [84]. The reaction conditions of carboxymethylation were optimized by the response surface method. The product was characterized and analyzed, and carboxymethylated Enteromorpha prolifera polysaccharides with a degree of substitution of 0.849 were obtained. The carboxymethylated Enteromorpha prolifera polysaccharides had stronger antioxidant activity evaluated by DPPH, hydroxyl radical, and superoxide anion radical scavenging ability. Similar conclusions have also been proven in the studies of various carboxymethylated polysaccharides [85].
2.4. Other Modification Methods
References
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z.; Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules. 2019, 9, 389.
- Liu, J.; Pu, Q.; Qiu, H.; Di, D.; Polysaccharides isolated from Lycium barbarum L. by integrated tandem hybrid membrane technology exert antioxidant activities in mitochondria. Ind. Crops Prod. 2021, 168, 113547.
- Li, J.; Shi, M.; Ma, B.; Zheng, Y.; Niu, R.; Li, K.; Protective effects of fraction 4a of polysaccharides isolated from Lycium barbarum against KBrO3-induced renal damage in rats. Food Funct. 2017, 8, 2566–2572.
- Yan, H.; Xie, Y.; Sun, S.; Sun, X.; Ren, F.; Shi, Q.; Wang, S.; Zhang, W.; Li, X.; Zhang, J.; et al. Chemical analysis of Astragalus mongholicus polysaccharides and antioxidant activity of the polysaccharides. Carbohydr. Polym. 2021, 82, 636–640.
- Shang, H.; Chen, S.; Li, R.; Zhou, H.; Wu, H.; Song, H.; Influences of extraction methods on physicochemical characteristics and activities of Astragalus cicer L. polysaccharides. Process Biochem. 2018, 73, 220–227.
- Awad, A.; Khalil, S.R.; Hendam, B.M.; Abd El-Aziz, R.M.; Metwally, M.M.M.; Imam, T.S.; Protective potency of Astragalus polysaccharides against tilmicosin- induced cardiac injury via targeting oxidative stress and cell apoptosis-encoding pathways in rat. Env. Sci. Pollut. Res. Int. 2020, 27, 20861–20875.
- Zhang, Y.; Zhang, L.; Liu, J.; Liang, J.; Si, J.; Wu, S.; Dendrobium officinale leaves as a new antioxidant source. J. Funct. Foods. 2017, 37, 400–415.
- Fan, H.; Meng, Q.; Xiao, T.; Zhang, L.; Partial characterization and antioxidant activities of polysaccharides sequentially extracted from Dendrobium officinale. J. Food Meas. Charact. 2018, 12, 1054–1064.
- Meng, Q.; Fan, H.; Li, Y.; Zhang, L.; Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. J. Food Meas. Charact. 2017, 12, 1-10.
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C.; Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules. 2016, 21, 701.
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N.; Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 2018, 115, 281-286.
- Tian, S.; Hao, C.; Xu, G.; Yang, J.; Sun, R.; Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J. Food Drug Anal. 2017, 25, 766–775.
- Liu, C.Y.; The changes of the polysaccharide and its distribution in the development process of Gastrodia elate. Acta Bot. 1981, 3, 375–380.
- Zhu, Z.Y.; Chen, C.J.; Sun, H.Q.; Chen, L.J.; Structural characterisation and ACE-inhibitory activities of polysaccharide from Gastrodia elata Blume. Nat. Prod. Res. 2019, 33, 1721–1726.
- Zhou, B.; Tan, J.; Zhang, C.; Wu, Y.; Neuroprotective effect of polysaccharides from Gastrodia elata blume against corticosteroneinduced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stressmediated pathway. Mol. Med. Rep. 2018, 17, 1182–1190.
- Huo, J.; Lei, M.; Li, F.; Hou, J.; Zhang, Z.; Long, H.; Zhong, X.; Liu, Y.; Xie, C.; Wu, W.; et al. Structural Characterization of a Polysaccharide from Gastrodia elata and Its Bioactivity on Gut Microbiota. Molecules. 2021, 26, 4443.
- Bao, Q.; Qian, L.; Gong, C.; Shen, X.; Immune-Enhancing Activity of Polysaccharides from Gastrodia elata. J. Food Processing Preserv. 2017, 41, e13016.
- Chen, L.; Zhang, Y.P.; Jin, L.X.; Preparation, characterization and anti-ageing activity of Gastrodia elata blume polysaccharide. Acta Aliment. 2018, 47, 210-219.
- Li, Y.; Hu, Y.; Shi, S.; Jiang, L.; Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. Int. J. Biol. Macromol. 2014, 68, 113–116.
- YouGuo, C.; ZongJi, S.; XiaoPing, C.; Evaluation of free radicals scavenging and immunity-modulatory activities of Purslane polysaccharides. Int. J. Biol. Macromol. 2009, 45, 448–452.
- Cui, X.; Wang, S.; Cao, H.; Guo, H.; Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Han, C.; A Review: The Bioactivities and Pharmacological Applications of Polygonatum sibiricum polysaccharides. Molecules. 2018, 23, 1170.
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G.; Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164.
- Li, L.; Thakur, K.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J.; Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 114, 317–323.
- Yang, H.; Zhang, X.; Polysaccharides from Polygonatum odoratum strengthen antioxidant defense system and attenuate lipid peroxidation against exhaustive exercise-induced oxidative stress in mice. Trop. J. Pharm. Res. 2017, 16, 795.
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q.; Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121.
- Yuan, Y.; Macquarrie, D.; Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr Polym. 2015, 129, 101–107.
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S.; Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants. 2019, 8, 129.
- Mohd Fauziee, N.A.; Chang, L.S.; Wan Mustapha, W.A.; Md Nor, A.R.; Lim, S.J.; Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). Int. J. Biol. Macromol. 2021, 167, 1135–1145.
- Sarithakumari, C.H.; Renju, G.L.; Kurup, G.M.; Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268.
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M.; Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018, 269, 387–395.
- Sen, M.; Effects of molecular weight and ratio of guluronic acid to mannuronic acid on the antioxidant properties of sodium alginate fractions prepared by radiation-induced degradation. Appl. Radiat. Isot. 2011, 69, 126–129.
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B.; Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902.
- Chen, H.; Ju, Y.; Li, J.; Yu, M.; Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218.
- You, R.; Wang, K.; Liu, J.; Liu, M.; Luo, L.; Zhang, Y.; A comparison study between different molecular weight polysaccharides derived from Lentinus edodes and their antioxidant activities in vivo. Pharm Biol. 2011, 49, 1298–1305.
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Xu, F.; Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Charact. 2019, 13, 1382–1389.
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L.; Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531.
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W.; Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77.
- Yan, J.; Zhu, L.; Qu, Y.; Qu, X.; Mu, M.; Zhang, M.; Muneer, G.; Zhou, Y.; Sun, L.; Analyses of active antioxidant polysaccharides from four edible mushrooms. Int. J. Biol. Macromol. 2019, 123, 945–956.
- He, J.Z.; Ru, Q.M.; Dong, D.D.; Sun, P.L.; Chemical characteristics and antioxidant properties of crude water soluble polysaccharides from four common edible mushrooms. Molecules. 2012, 17, 4373–4387.
- Chen, G.T.; Ma, X.M.; Liu, S.T.; Liao, Y.L.; Zhao, G.Q.; Isolation, purification and antioxidant activities of polysaccharides from Grifola frondosa. Carbohydr. Polym. 2012, 89, 61–66.
- Chen, Z.; Tang, Y.; Liu, A.; Jin, X.; Zhu, J.; Lu, X.; Oral administration of Grifola frondosa polysaccharides improves memory impairment in aged rats via antioxidant action. Mol. Nutr. Food Res. 2017, 61, 1700313.
- Cor, D.; Knez, Z.; Knez Hrncic, M.; Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: A Review. Molecules. 2018, 23, 649.
- Fan, L.; Li, J.; Deng, K.; Ai, L.; Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854.
- Ma, C.-w.; Feng, M.; Zhai, X.; Hu, M.; You, L.; Luo, W.; Zhao, M.; Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. Eng. 2013, 44, 886–894.
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J.; Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144.
- Kan, Y.; Chen, T.; Wu, Y.; Wu, J.; Wu, J.; Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 2015, 72, 151–157.
- Liao, B.; Huang, H.; Structural characterization of a novel polysaccharide from Hericium erinaceus and its protective effects against H2O2-induced injury in human gastric epithelium cells. J. Funct. Foods 2019, 56, 265–275.
- Fan, S.; Huang, X.; Wang, S.; Li, C.; Zhang, Z.; Xie, M.; Nie, S.; Combinatorial usage of fungal polysaccharides from Cordyceps sinensis and Ganoderma atrum ameliorate drug-induced liver injury in mice. Food Chem Toxicol. 2018, 119, 66–72.
- Fan, S.T.; Nie, S.P.; Huang, X.J.; Wang, S.; Hu, J.L.; Xie, J.H.; Nie, Q.X.; Xie, M.Y.; Protective properties of combined fungal polysaccharides from Cordyceps sinensis and Ganoderma atrum on colon immune dysfunction. Int. J. Biol. Macromol. 2018, 114, 1049–1055.
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S.; Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962.
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H.; Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb Pathog. 2017, 109, 214–220.
- Asker, M.M.S.; Shawky, B.T.; Structural characterization and antioxidant activity of an extracellular polysaccharide isolated from Brevibacterium otitidis BTS 44. Food Chem. 2010, 123, 315–320.
- Diao, Y.; Xin, Y.; Zhou, Y.; Li, N.; Pan, X.; Qi, S.; Qi, Z.; Xu, Y.; Luo, L.; Wan, H.et al.; et al. Extracellular polysaccharide from Bacillus sp. strain LBP32 prevents LPS-induced inflammation in RAW 264.7 macrophages by inhibiting NF-kappaB and MAPKs activation and ROS production. Int Immunopharmacol. 2014, 18, 12-19.
- Elnahas, M.O.; Amin, M.A.; Hussein, M.M.D.; Shanbhag, V.C.; Ali, A.E.; Wall, J.D.; Isolation, Characterization and Bioactivities of an Extracellular Polysaccharide Produced from Streptomyces sp. MOE6. Molecules. 2017, 22, 1396.
- Manivasagan, P.; Sivasankar, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.K.; Production and characterization of an extracellular polysaccharide from Streptomyces violaceus MM72. Int. J. Biol. Macromol. 2013, 59, 29-38.
- Xiong, Q.; Song, Z.; Hu, W.; Liang, J.; Jing, Y.; He, L.; Huang, S.; Wang, X.; Hou, S.; Xu, T.et al.; et al. Methods of extraction, separation, purification, structural characterization for polysaccharides from aquatic animals and their major pharmacological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 48–63.
- Xue, W.; Zeng, Q.; Lin, S.; Zan, F.; Hao, T.; Lin, Y.; van Loosdrecht, M.C.M.; Chen, G.; Recovery of high-value and scarce resources from biological wastewater treatment: Sulfated polysaccharides. Water Res. 2019, 163, 114889.
- Pomin, V.H.; Review: An overview about the structure-function relationship of marine sulfated homopolysaccharides with regular chemical structures. Biopolymers. 2009, 91, 601–609.
- Zhang, L.S.; Wang, X.; Dong, L.L.; Antioxidation and antiglycation of polysaccharides from Misgurnus anguillicaudatus. Food Chem. 2011, 124, 183–187.
- Zou, S.; Pan, R.; Dong, X.; He, M.; Wang, C.; Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2016, 51, 650–658.
- Wang, Z.; Zhao, Y.; Su, T.; Extraction and antioxidant activity of polysaccharides from Rana chensinensis skin. Carbohydr Polym. 2015, 115, 25–31.
- Wang, Z.; Zhao, Y.; Su, T.; Zhang, J.; Wang, F.; Characterization and antioxidant activity in vitro and in vivo of polysaccharide purified from Rana chensinensis skin. Carbohydr Polym. 2015, 126, 17–22.
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V.; Can fungi compete with marine sources for chitosan production?. Int. J. Biol. Macromol. 2017, 104, 1415–1421.
- Anraku, M.; Kabashima, M.; Namura, H.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Furutani, N.; Tomida, H.; Antioxidant protection of human serum albumin by chitosan. Int. J. Biol. Macromol. 2008, 43, 159–164.
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J.; Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644.
- Chen, L.; Huang, G.; Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746.
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L.; Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447.
- Xu, Y.; Gao, Y.; Liu, F.; Niu, X.; Wang, L.; Li, X.; Chen, H.; Yang, Y.; Sulfated modification of the polysaccharides from blackcurrant and their antioxidant and alpha-amylase inhibitory activities. Int. J. Biol. Macromol. 2018, 109, 1344–1354.
- Zhang, H.; Wang, Z.Y.; Yang, L.; Yang, X.; Wang, X.; Zhang, Z.; In vitro antioxidant activities of sulfated derivatives of polysaccharides extracted from Auricularia auricular. Int. J. Mol. Sci. 2011, 12, 3288–3302.
- Yuan, F.; Gao, Z.; Liu, W.; Li, H.; Zhang, Y.; Feng, Y.; Song, X.; Wang, W.; Zhang, J.; Huang, C. et al.; et al. Characterization, Antioxidant, Anti-Aging and Organ Protective Effects of Sulfated Polysaccharides from Flammulina velutipes. Molecules. 2019, 24, 3517.
- Liu, X.; Chen, T.; Hu, Y.; Li, K.; Yan, L.; Catalytic synthesis and antioxidant activity of sulfated polysaccharide from Momordica charantia L. Biopolymers. 2014, 101, 210–215.
- Chen, L.; Huang, G.; Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019, 126, 743–746.
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y.; Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15.
- Xing, R.; Yu, H.; Liu, S.; Zhang, W.; Zhang, Q.; Li, Z.; Li, P.; Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg Med Chem. 2005, 13, 1387–1392.
- Xia, S.; Zhai, Y.; Wang, X.; Fan, Q.; Dong, X.; Chen, M.; Han, T.; Phosphorylation of polysaccharides: A review on the synthesis and bioactivities. Int. J. Biol. Macromol. 2021, 184, 946–954.
- Chen, L.; Huang, G.; Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261.
- Xiong, X.; Huang, G.; Huang, H.; The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845.
- Chen, J.; Huang, G.; Antioxidant activities of garlic polysaccharide and its phosphorylated derivative. Int. J. Biol. Macromol. 2019, 125, 432–435.
- Chen, F.; Huang, G.; Huang, H.; Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 2021, 252, 117179.
- Duan, Z.; Zhang, Y.; Zhu, C.; Wu, Y.; Du, B.; Ji, H.; Structural characterization of phosphorylated Pleurotus ostreatus polysaccharide and its hepatoprotective effect on carbon tetrachloride-induced liver injury in mice. Int. J. Biol. Macromol. 2020, 162, 533–547.
- Liu, Y.; Huang, G.; The antioxidant activities of carboxymethylated cushaw polysaccharide. Int. J. Biol. Macromol. 2019, 121, 666–670.
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.et al.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250.
- Li, Y.T.; Chen, B.J.; Wu, W.D.; Ge, K.; Wei, X.Y.; Kong, L.M.; Xie, Y.Y.; Gu, J.P.; Zhang, J.C.; Zhou, T.; et al. Antioxidant and antimicrobial evaluation of carboxymethylated and hydroxamated degraded polysaccharides from Sargassum fusiforme. Int. J. Biol. Macromol. 2018, 118, 1550–1557.
- Shi, M.J.; Wei, X.; Xu, J.; Chen, B.J.; Zhao, D.Y.; Cui, S.; Zhou, T.; Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017, 215, 76–83.
- Gao, H.; Huang, G.; Preparation and antioxidant activity of carboxymethylated garlic polysaccharide. Int. J. Biol. Macromol. 2019, 121, 650–654.
- Cao, Y.Y.; Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Hu, F.; Zhang, J.G.; Wei, Z.J.; Effects of sulfated, phosphorylated and carboxymethylated modifications on the antioxidant activities in-vitro of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2020, 146, 887–896.
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y.; Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680.
