1. Eating Disorders in Polycystic Ovary Syndrome (PCOS)
1.1. Classification of Eating Disorders
Eating disorders (EDs) are serious conditions characterized by persistent eating behaviors that negatively impact physical health and disrupt psychosocial functioning
[1][14]. The most common EDs are anorexia nervosa, bulimia nervosa, and binge eating disorder
[2][15]. Based on the DSM-5 classification of mental disorders, there is also a category of other specified feeding or eating disorders (OSFED) that do not meet the full criteria for the diagnosis of major disorders and include atypical anorexia nervosa, bulimia nervosa, and binge eating disorder of low frequency and/or limited duration, purging disorder, and night eating disorder.
1.2. Prevalence of Eating Disorders in PCOS
PCOS is frequently related to an extreme manifestation of disrupted eating behavior, which is highly prevalent in this population
[3][4][16,17].Most of the studies found higher rates of EDs in the PCOS group compared to the control group. The only study that did not demonstrate any differences is the study by Lee et al.
[5][10]. The authors explained that the statistical power of the study was too low to detect these differences. In addition, due to the cross-sectional design, it was difficult to assess the possible confounding role of depression and anxiety on the prevalence of EDs, suggesting that these psychiatric co-morbidities may have a causal role for EDs in the general population. Given that confounding factors in this population may have affected outcomes, replication
of th
ereis study in other populations, including community cohorts, is suggested by the authors. Furthermore, there is a lack of unifying questionnaires for the diagnosis of EDs in all studies. That could be a reason for the variation in the reported prevalence and possibly the potential overreporting of EDs in some studies
[6][21].
Further perspective can be gained from a systematic review and meta-analysis conducted at Mayo Clinic by Thannickal et al. that included 36 studies. They analyzed different EDs and their prevalence in women with PCOS. No significant association between PCOS and anorexia nervosa (three studies) was found, but a significant association was shown between PCOS and bulimia nervosa (five studies), binge eating disorder (four studies), and other not specified eating disorders (four studies)
[7][23].
Morgan et al. studied EDs in 80 women with facial hirsutism. The prevalence of EDs in this population was 36.3% (22.5% eating disorder not otherwise specified (EDNOS), 12.6% bulimia nervosa, and 1.3% anorexia nervosa), and depression, anxiety, low self-esteem, and poor social adjustment were more common in participants suffering from EDs. There was universal co-morbidity of PCOS in women with hirsutism and EDs, proving again the high prevalence of EDs in the PCOS population
[8][24]. This study performed in 2008 used DSM-IV criteria for the categorization of EDs, where binge eating disorder was not recognized as a disorder and is not mentioned separately in this study
[9][25].
The prevalence of EDs in lean PCOS phenotype is less frequently studied. Jeanes et al. used online surveys, including the Bulimia Investigatory Test, Edinburgh, Food Cravings-Trait 26 Questionnaire, and the Three Factor Eating Questionnaire revised−18 to compare EDs in 45 lean women with PCOS and 40 lean women in the control group. A significantly higher mean binge eating score was shown in lean women with PCOS compared with lean, healthy women (10.9 ± 7.8 versus 7.4 ± 6.0,
p = 0.024). Lean women with PCOS had a significantly higher proportion of subclinical disordered eating (36%) compared with lean, healthy women (12%)
[10][20].
The existence of these relationships among physiological and psychological factors strongly suggests that medical management of PCOS would greatly benefit from the inclusion of psychological and behavioral approaches
[11][26]. Different authors have already recommended screening this population for depression and EDs
[6][12][13][21,27,28].
1.3. The Casual Link between Eating Disorders and PCOS
Available literature suggests a causal relationship and significant interplay between physiological and psychological factors affecting women with PCOS
[11][26]. Hyperandrogenism, reproductive and metabolic disturbances, and the high prevalence of obesity contribute to body dissatisfaction as well as psychological aspects such as depression, anxiety, and EDs
[14][29].
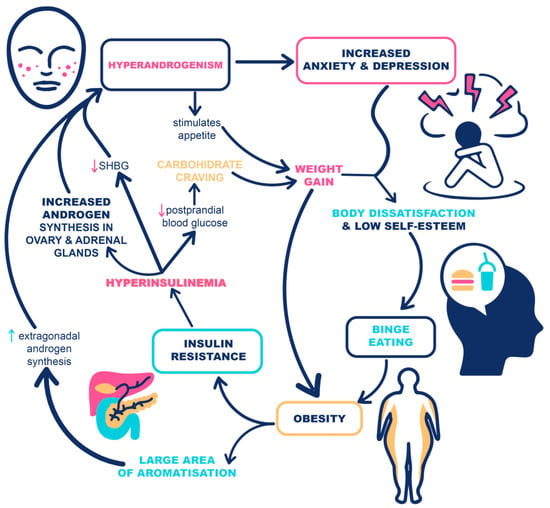
Hyperandrogenism can alter monoamine balance in the central nervous system and has already been linked to depression
[15][30]. In addition, it stimulates appetite and increases anxiety which may confer risk for binge eating behavior
[16][17][31,32]. Indirect evidence for the role of hyperandrogenism in EDs is implied by the observation that an antiandrogen receptor antagonist, flutamide, can lead to a substantial decrease in bulimic symptoms
[18][33].
Moreover, metabolic disbalances, especially hyperinsulinemia, contribute to binge eating behavior. High insulin levels, commonly seen in PCOS, lead to postprandial low blood glucose, another potent appetite stimulant primarily in cravings for carbohydrates. Altered insulin levels contribute to weight gain, with increased distress regarding weight status and a common weight cycling phenomenon, often referred to as Yoyo dieting
[19][34]. Once the obese phenotype is fully developed, there is a high equilibrium body weight set-point and numerous mechanisms preventing weight loss, all causing the maintenance of weight loss following lifestyle or pharmacotherapy intervention challenging. Furthermore, hyperandrogenism and hyperinsulinemia can propagate each other in a vicious cycle
[20][35].
Both mechanisms, through the consequential weight gain, inflammation, and clinical symptoms of hyperandrogenism, can lead to body dissatisfaction with impaired self-appearance, which can also be an additional trigger for EDs. Despite the fact that the body dissatisfaction phenomenon is not limited to PCOS but is frequently observed in overweight women in general, body dissatisfaction is more prominent in PCOS women and can lead to EDs. In a study by Karacan et al., 42 women with PCOS and 52 controls were examined for the associations between PCOS, body dissatisfaction, and eating attitudes. The results revealed that both hirsutism and high BMI in PCOS can contribute to body dissatisfaction and EDs. In addition, another general factor shown to contribute to EDs was the socio-cultural internalization of the perceived ideal thin body figure, which has lowered body dissatisfaction and self-esteem in the general population. However, there was no significant difference in BMI, socio-cultural internalization of ideal appearance, body dissatisfaction, and eating attitudes between PCOS and non-PCOS groups
[21][36].
1.4. The Vicious Cycle of PCOS, Eating Disorders, and Obesity
Since hyperandrogenism and hyperinsulinemia in PCOS are two interrelated factors contributing to weight gain and body dissatisfaction, leading to the high prevalence of EDs, it is essential to explore their relationship and potential interventions at any step in this chain of events. Furthermore, the identification and assessment of EDs and eating behaviors might improve the treatment response to new anti-obesity medications resulting in significant improvement in the general health of women with PCOS.
Multiple potential mechanisms could explain how obesity can play a significant role in PCOS development and clinical presentation. In adolescence, the nutritional status remains a crucial indicator for puberty and menarche induction. A “critical body weight” is necessary to trigger the onset of puberty
[22][23][24][25][37,38,39,40]. Leptin, a hormone that provides indirect information about an organism’s nutritional and metabolic status to the hypothalamic GnRH neuronal system, appears to regulate GnRH function indirectly through forebrain neurons
[26][41]. Signalization of forebrain Kiss 1 neurons by leptin, which is elevated in children with obesity, acts as a permissive factor in the initiation and progression of puberty
[27][42]. Increased hypothalamic ceramide content, which enhances paraventricular nucleus expression of SPTLC1 (a key enzyme for ceramide synthesis) and sympathetic ovarian innervations seem to be the responsible neuroendocrine mechanisms for the early onset of puberty in obese children
[28][43]. Early puberty and menarche can result in adverse mental health and psychosocial consequences. Additional endocrine mechanisms for early puberty in obese girls include increased extragonadal aromatization of androgens to estrogen in adipose tissue
[29][44]. Therefore, a larger area of aromatization can result in breast development and early thelarche. This premature activation of the GnRH axis seen in obesity is highly associated with hyperandrogenism and increased ovarian volume, two characteristic features of PCOS
[30][45].
Obesity can also cause higher rates of irregular menses, amenorrhea, abnormal uterine bleeding, dysmenorrhea, premenstrual disorders, greater risk for infertility, pregnancy complications, breast and endometrial cancers, and PCOS. The most known mechanisms which explain those consequences are IR, high androgen levels, and low sex-hormone-binding globulin
[31][46].
IR, the critical hormonal disturbance in obesity and metabolic syndrome, has a significant impact on the development and progression of PCOS and its clinical manifestations. In addition to IR related to obesity, PCOS is also related to IR that is intrinsically associated with the syndrome. Current evidence suggests that up to 75% of women with PCOS have impaired insulin action and that the degree of IR is often disproportionate to the BMI
[32][33][47,48]. IR leads to hyperinsulinemia, which directly increases the bioavailability of sex steroids by stimulating the production of androgens by ovaries and adrenal glands and reduces sex-hormone-binding globulin levels (SHBG). In addition, the aromatase activity is also enhanced by hyperinsulinemia
[34][49]. The potential mechanisms linking hyperandrogenism and hyperinsulinemia with EDs are summarized in
Figure 1.
Figure 1. The potential mechanisms linking hyperandrogenism and insulin resistance with eating disorders in women with PCOS and obesity.
Based on the fact that obesity is a common finding in PCOS and aggravates many of its reproductive and metabolic features [
50], a greater focus should be placed on more efficient treatment of obesity and, therefore, potentially stopping this vicious circle with one intervention at multiple steps. The anti-obesity treatment strategy should involve the identification of barriers to weight loss, with mental health being identified as one of the key predictors of the likelihood of successful weight loss intervention.
2. Eating Behavior
2.1. Regulation of Eating Behavior
Obesity is a disease in which the disbalance between energy intake and consumption leads to abnormally distributed and excess body fat that has direct and indirect consequences on whole-body health. Therefore, it is essential to carefully consider eating behavior that causes overeating along with insufficient energy expenditure. Eating behavior is a complex interplay of physiological, psychological, social, and genetic factors that influence meal timing, the quantity of food intake, and food preferences
[35][51]. The brain controls it on three levels. Homeostatic eating is eating based on hunger and is mainly under hypothalamic control. It is a balance between feelings of satiety and hunger. Satiety is mediated by glucagon-like peptide 1 (GLP-1), peptide YY, oxyntomodulin, pancreatic polypeptide, and amylin, and hunger is mediated by ghrelin. Hedonic eating is eating based on pleasure and is mainly under mesolimbic control, mediated by feelings of wanting or liking to eat. A “want” to eat is mediated by dopamine, a hormone involved in the reward-behavior system. “Liking” to eat is associated with pleasure derived from eating and is mediated through opioid and cannabinoid receptors. Finally, executive function is the decision to eat, mediated primarily by the prefrontal cortex. Those brain regions are the site of action for behavioral interventions
[36][37][38][39][40][18,52,53,54,55].
Neuroendocrine regulation between the brain, gut, and adipose tissue could be affected by PCOS. Insulin displays an inhibitory action on brain reactivity to food cues, but this effect is compromised by IR, frequently found in the PCOS population. Leptin, which was reported higher in women with PCOS
[41][56], seems to have a controversial role in food intake regulation. It conveys an afferent signal to the central nervous system on body fat status, and in individuals with normal weight, it suppresses appetite and promotes energy expenditure. In obese patients, leptin is incapable of decreasing food intake and body weight due to leptin resistance
[42][57]. Insulin and estradiol as stimulants and androgens as inhibitors of leptin production were demonstrated as hormonal regulators of leptin levels in some studies
[43][44][58,59]. However, well-designed studies failed to find a significant correlation between serum leptin levels and Homeostasis Model Assessment (HOMA) or the degree of hyperinsulinemia in women with PCOS after adjusting for BMI
[45][60], and even the results from interventional studies were controversial. In earlier studies, metformin decreased leptin levels both in obese and lean patients with PCOS
[46][61], while, more recently, the studies did not confirm this evidence, suggesting that the effect of metformin on leptin levels could be mediated by its impact on body weight
[47][62]. More data are necessary to explain the role of leptin in PCOS and its effect on eating behavior. The action of ghrelin, a potent orexigenic peripheral peptide, and neuropeptide Y, a potent orexigenic hypothalamic peptide, are deranged partially by the influence of insulin
[48][63]. That might explain why excessive food intake due to affected neuroendocrine transmitters might account for the high rate of obesity in this population.
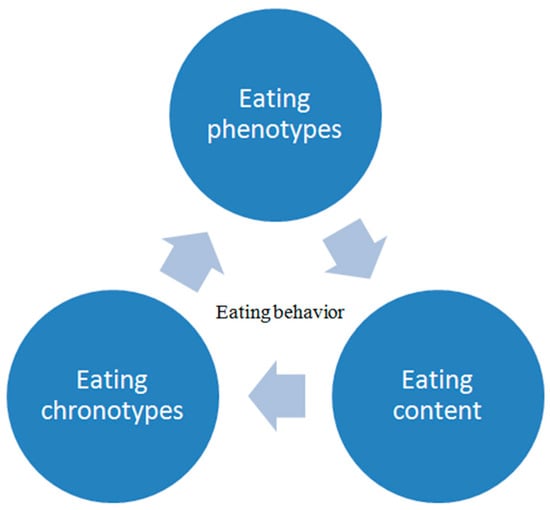
Besides mechanisms that try to explain how neuroendocrine regulation can lead to obesity in the PCOS population, another clinically useful concept is the characterization of obesity phenotype based on pathophysiological classification and eating behaviors. There are three measurable components that have an impact on eating behavior: quantity of food in every meal, time of different activities during the day, and specific content of the meal
[35][51]. Their evaluation enables a clearer picture and suggests the potential individual intervention targeting one component in specific eating behavior.
2.2. Assessment of Eating Behavior
A study by Acosta et al. included 450 obese participants and performed different validated tests after an 8-h fasting period. They stratified obesity into four phenotypes based on eating behavior and energy expenditure: (1) Homeostatic eating behavior, which includes hungry brain (abnormal satiation), (2) hungry gut (abnormal satiety), (3) hedonic eating behavior, which is emotional eating, and (4) low energy expenditure (resting energy expenditure, non-exercise physical activity and thermogenic effect of food and exercise). Available methods which were performed to reveal those phenotypes were: nutrient drink test for satiation test; gastric emptying by scintigraphy for satiety test; validated questionnaires for emotional hunger; and indirect calorimetry for energy expenditure. Hedonic and homeostatic eating behaviors are two nutritional behaviors that tilt the energy balance towards increased intake
[49][64]. This classification can be useful in the selection of personalized treatment for obesity, which is a very common feature of PCOS.
Eating chronotypes, referring to the time of different activities during the day, seem to be another key component in determining the risk of developing obesity. A cross-sectional study of 112 women with PCOS assessed participants’ chronotypes and their risk for obesity. Evening chronotypes showed significantly higher percentages of grade I and II obesity; most of them were smokers and exercised less regularly compared to those with neither or morning chronotypes
[50][65]. Evening and late meals have also been more prevalent in PCOS women than controls in the study by Eleftheriadou et al.
[51][66]. Furthermore, a recent study by Kulshreshtha et al. revealed that PCOS women differed significantly from non-PCOS women of the same weight in the timing of their breakfast and lunch intake rather than macronutrient distribution or caloric intake. Delayed breakfast and lunch were associated with PCOS independent of BMI, and late/missed breakfast was present in 40% of PCOS women
[52][67]. These data suggest that chronotype assessment could be an effective tool to screen and change eating habits and general lifestyle in women with PCOS.
Assessment of eating content has also been recognized as an important determinant of eating behavior. Women with PCOS have low consumption of olive oil, legumes, fish, and nuts compared to healthy women, but they consume higher levels of simple carbohydrates, saturated fatty acids, and total fats. Women with PCOS also consume fewer complex carbohydrates, fibers, and monounsaturated fatty acids, which makes their eating content imbalanced
[51][66]. Based on that evidence, diet and nutritional habits are very useful tools for PCOS management. Another relevant issue to take into consideration is the phenomenon of energy intake underreporting. This phenomenon has been studied by Giuseppe et al. in a pilot study. The study concluded that women with PCOS underreport foods rich in simple sugars rather than underreport their total dietary intake
[53][68]. These results may have implications for the interpretation of diet and health correlations in this population since simple sugars are very involved in the weight gain process.
Altogether, the risk assessment process for eating behavior associated with weight gain and obesity is illustrated in Figure 2.
Figure 2.
The process of assessment of eating behavior.
Moreover, the taste function as a component of eating behavior in PCOS women has been addressed by some authors. Cetik et al. assessed gustatory function by taste strips (sweet, sour, salty, bitter) and three different questionnaires. PCOS group had a lower sour and salty taste, whereas sweet and bitter taste scores were similar. They also concluded that hyperandrogenism was associated with lower total taste strip test score; however, the use of combined oral contraceptives did not alter taste function
[54][69]. In preclinical studies, it was demonstrated that GLP-1 is locally synthesized in taste bud cells and its receptors exist on the gustatory nerves in close proximity to GLP-1-containing taste bud cells. This local paracrine GLP-1 signalizing seems to be involved in the perception of sweetness. Based on this finding, Jensterle et al. designed a randomized, single-blinded, placebo-controlled clinical trial in 30 women with obesity and PCOS to investigate if semaglutide modulates the perception of sweet taste in this population.
Here,This itstudy may identify tongue and taste perception as a novel target for GLP-1 receptor agonists (GLP-1RAs)
[55][70].