Ionic Liquids (ILs) are the molten salts with a melting point lower than 100 °C and behave as a liquid at room temperature. ILs properties can be altered by the careful selection of the anion and cation. ILs have bulky organic cations with low molecular symmetry and small organic/inorganic anions that are in their molten form in their pure states under ambient conditions. Global warming is one of the major problems in the developing world, and one of the major causes of global warming is the generation of carbon dioxide (CO2) because of the burning of fossil fuels. Burning fossil fuels to meet the energy demand of households and industries is unavoidable. The current commercial and experimental techniques used for capturing and storing CO2 have serious operational and environmental constraints. The amine-based absorption technique for CO2 capture has a low absorption and desorption ratio, and the volatile and corrosive nature of the solvent further complicates the situation. To overcome all of these problems, researchers have used ionic liquids (ILs) and deep eutectic solvents (DESs) as a replacement for commercial amine-based solvents. ILs and DESs are tunable solvents that have a very low vapor pressure, thus making them an ideal medium for CO2 capture. Moreover, most ILs and DESs have low toxicity and can be recycled without a significant loss in their CO2 capture capability.
- ionic liquids
- ,CO2 capture
- ,products
- ,Catalytic conversion of CO2
- ,Economics of ionic liquids for CO2 capture
1. Introduction
2. Conversion of CO2 into Valuable Products Using Ionic Liquids
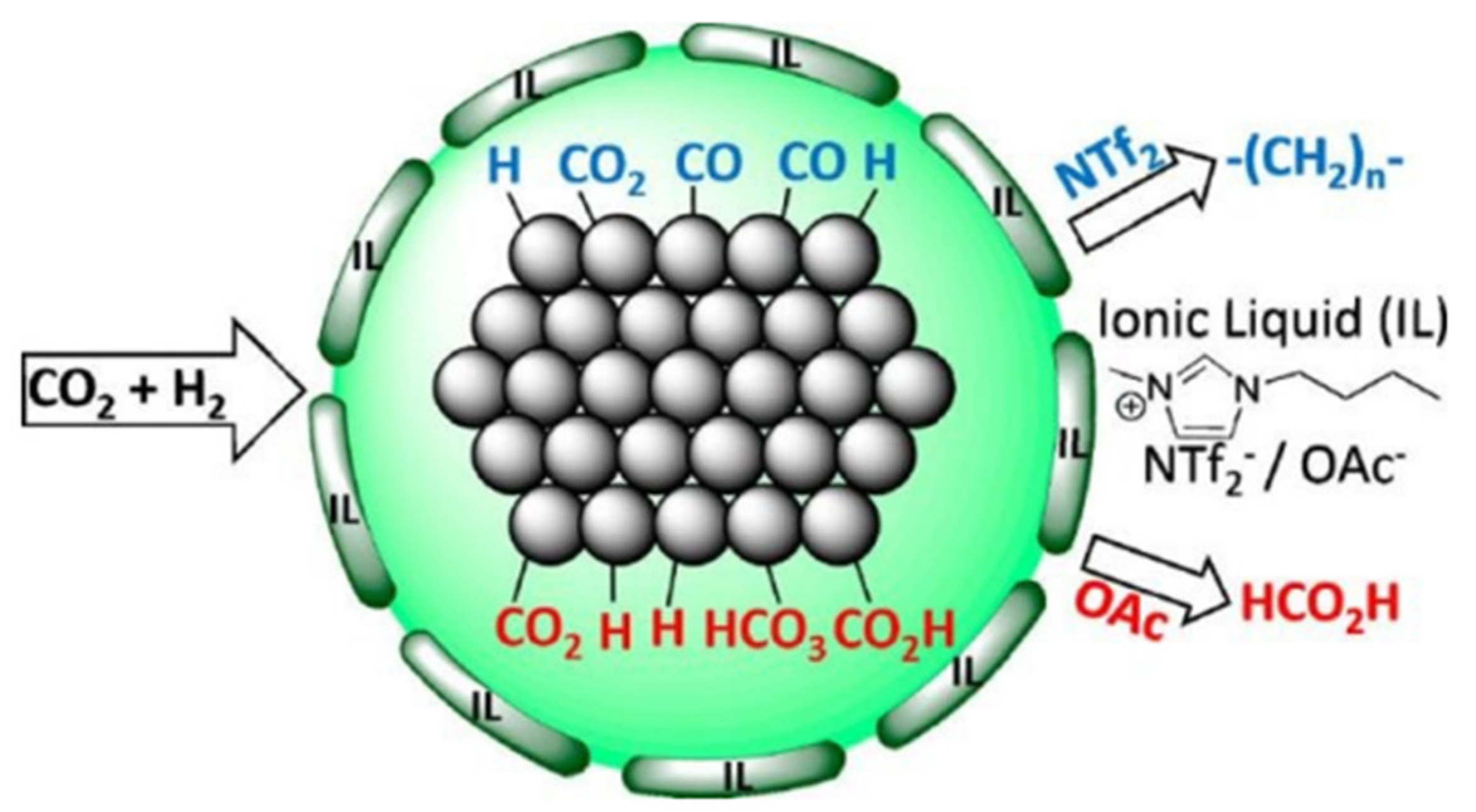
3. Economics of Ionic Liquid-Based CO2 Capture
4. Difficulties and Drawbacks of Using Ionic Liquids to Capture CO2
References
- Treut, H.L.; Somerville, R.; Cubasch, U.; Ding, Y.; Mauritzen, C.; Mokssit, A.; Peterson, T.; Prather, M.; Solomon, S. Historical Overview of Climate Change, the Physical Science Basis; Cambridge University Press: Cambridge, UK, 2007; pp. 93–127.
- Aggarwal, R.K.; Markanda, S. Effect of greenhouse gases and human population in global warming. Huanjing Gongcheng Jishu Xuebao 2013, 2, 13–16.
- Mitchell, J.F.B. The “greenhouse” effect and climate change. Rev. Geophys. 1989, 27, 115–139.
- Wuebbles, D.J.; Jain, A.K. Concerns about climate change and the role of fossil fuel use. Fuel Process. Technol. 2001, 71, 99–119.
- Mendelsohn, R.; Dinar, A.; Williams, L. The distributional impact of climate change on rich and poor countries. Environ. Dev. Econ. 2006, 11, 159–178.
- Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Vyas, P.; Fradera, R.J.C.; et al. Climate Change 2022: Mitigation of Climate Change; Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022.
- Quadrelli, R.; Peterson, S. The energy–climate challenge: Recent trends in CO2 emissions from fuel combustion. Energy Policy 2007, 35, 5938–5952.
- Ali, S.A.; Shah, S.N.; Shah, M.U.H.; Younas, M. Synthesis and performance evaluation of copper and magnesium-based metal organic framework supported ionic liquid membrane for CO2/N2 separation. Chemosphere 2022, 311, 136913.
- Metz, B.; Davidson, O.; De Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005.
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent advances in CO2 capture and utilization. ChemSusChem 2008, 1, 893–899.
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849.
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673.
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633.
- Chen, Y.; Mu, T. Conversion of CO2 to value-added products mediated by ionic liquids. Green Chem. 2019, 21, 2544–2574.
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir 2014, 30, 6302–6308.
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479.
- Peng, J.; Deng, Y. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641.
- Kawanami, H.; Sasaki, A.; Matsui, K.; Ikushima, Y. A rapid and effective synthesis of propylene carbonate using a supercritical CO2–ionic liquid system. Chem. Commun. 2003, 896–897.
- Wang, J.-Q.; Yue, X.-D.; Cai, F.; He, L.-N. Solventless synthesis of cyclic carbonates from carbon dioxide and epoxides catalyzed by silica-supported ionic liquids under supercritical conditions. Catal. Commun. 2007, 8, 167–172.
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Abd Ghani, N. Development and progress of functionalized silica-based adsorbents for CO2 capture. J. Mol. Liq. 2021, 338, 116913.
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20.
- Zhu, J.; He, B.; Huang, J.; Li, C.; Ren, T. Effect of immobilization methods and the pore structure on CO2 separation performance in silica-supported ionic liquids. Microporous Mesoporous Mater. 2018, 260, 190–200.
- Voskian, S.; Brown, P.; Halliday, C.; Rajczykowski, K.; Hatton, T.A. Amine-Based Ionic Liquid for CO2 Capture and Electrochemical or Thermal Regeneration. ACS Sustain. Chem. Eng. 2020, 8, 8356–8361.
- Hu, J.; Ma, J.; Liu, H.; Qian, Q.; Xie, C.; Han, B. Dual-ionic liquid system: An efficient catalyst for chemical fixation of CO2 to cyclic carbonates under mild conditions. Green Chem. 2018, 20, 2990–2994.
- Liu, Z.; Wu, W.; Han, B.; Dong, Z.; Zhao, G.; Wang, J.; Jiang, T.; Yang, G. Study on the Phase Behaviors, Viscosities, and Thermodynamic Properties of CO2/ /Methanol System at Elevated Pressures. Chem.—Eur. J. 2003, 9, 3897–3903.
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, M.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into α-Alkylidene Cyclic Carbonates. ChemSusChem 2017, 10, 1120–1127.
- Zhang, Q.; Yuan, H.-Y.; Fukaya, N.; Yasuda, H.; Choi, J.-C. Direct synthesis of carbamate from CO2 using a task-specific ionic liquid catalyst. Green Chem. 2017, 19, 5614–5624.
- Qadir, M.I.; Weilhard, A.; Fernandes, J.A.; de Pedro, I.; Vieira, B.J.C.; Waerenborgh, J.C.; Dupont, J. Selective Carbon Dioxide Hydrogenation Driven by Ferromagnetic RuFe Nanoparticles in Ionic Liquids. ACS Catal. 2018, 8, 1621–1627.
- Melo, C.I.; Szczepańska, A.; Bogel-Łukasik, E.; Nunes da Ponte, M.; Branco, L.C. Hydrogenation of Carbon Dioxide to Methane by Ruthenium Nanoparticles in Ionic Liquid. ChemSusChem 2016, 9, 1081–1084.
- Shi, F.; Deng, Y.; SiMa, T.; Peng, J.; Gu, Y.; Qiao, B. Alternatives to phosgene and carbon monoxide: Synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids. Angew. Chem. 2003, 115, 3379–3382.
- Jiang, T.; Ma, X.; Zhou, Y.; Liang, S.; Zhang, J.; Han, B. Solvent-free synthesis of substituted ureas from CO2 and amines with a functional ionic liquid as the catalyst. Green Chem. 2008, 10, 465–469.
- Huang, K.; Chen, F.-F.; Tao, D.-J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2017, 5, 67–73.
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Ferro, V.R.; Palomar, J. Techno-economic feasibility of ionic liquids-based CO2 chemical capture processes. Chem. Eng. J. 2021, 407, 127196.
- Xu, C.; Cheng, Z. Dicationic Imizadolium-Based Tetrafluoroborate Ionic Liquids: Synthesis and Hydrothermal Stability Study. ChemistrySelect 2022, 7, e202201799.
- Holbrey, J.D.; Reichert, W.M.; Swatloski, R.P.; Broker, G.A.; Pitner, W.R.; Seddon, K.R.; Rogers, R.D. Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-dialkylimidazolium salts containing methyl- and ethyl-sulfate anions. Green Chem. 2002, 4, 407–413.
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 capture with room temperature ionic liquids; coupled absorption/desorption and single module absorption in membrane contactor. Chem. Eng. Sci. 2020, 223, 115719.
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186.
- Blasucci, V.; Hart, R.; Mestre, V.L.; Hahne, D.J.; Burlager, M.; Huttenhower, H.; Thio, B.J.R.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Single component, reversible ionic liquids for energy applications. Fuel 2010, 89, 1315–1319.
- Hasib-ur-Rahman, M.; Siaj, M.; Larachi, F. Ionic liquids for CO2 capture—Development and progress. Chem. Eng. Process. Process Intensif. 2010, 49, 313–322.
- Altamash, T.; Haimour, T.S.; Tarsad, M.A.; Anaya, B.; Ali, M.H.; Aparicio, S.; Atilhan, M. Carbon Dioxide Solubility in Phosphonium-, Ammonium-, Sulfonyl-, and Pyrrolidinium-Based Ionic Liquids and their Mixtures at Moderate Pressures up to 10 bar. J. Chem. Eng. Data 2017, 62, 1310–1317.
- Vega, F.; Baena-Moreno, F.M.; Gallego Fernández, L.M.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313.
- Ma, T.; Wang, J.; Du, Z.; Abdeltawab, A.A.; Al-Enizi, A.M.; Chen, X.; Yu, G. A process simulation study of CO2 capture by ionic liquids. Int. J. Greenh. Gas Control 2017, 58, 223–231.
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process analysis overview of ionic liquids on CO2 chemical capture. Chem. Eng. J. 2020, 390, 124509.
- de Riva, J.; Suarez-Reyes, J.; Moreno, D.; Díaz, I.; Ferro, V.; Palomar, J. Ionic liquids for post-combustion CO2 capture by physical absorption: Thermodynamic, kinetic and process analysis. Int. J. Greenh. Gas Control 2017, 61, 61–70.
- Mota-Martinez, M.T.; Brandl, P.; Hallett, J.P.; Mac Dowell, N. Challenges and opportunities for the utilisation of ionic liquids as solvents for CO2 capture. Mol. Syst. Des. Eng. 2018, 3, 560–571.
- García-Gutiérrez, P.; Jacquemin, J.; McCrellis, C.; Dimitriou, I.; Taylor, S.F.R.; Hardacre, C.; Allen, R.W.K. Techno-Economic Feasibility of Selective CO2 Capture Processes from Biogas Streams Using Ionic Liquids as Physical Absorbents. Energy Fuels 2016, 30, 5052–5064.
- Shimekit, B.; Mukhtar, H. Natural gas purification technologies-major advances for CO2 separation and future directions. Adv. Nat. Gas Technol. 2012, 2012, 235–270.
- Akinola, T.E.; Oko, E.; Wang, M. Study of CO2 removal in natural gas process using mixture of ionic liquid and MEA through process simulation. Fuel 2019, 236, 135–146.
- Huang, Y.; Zhang, X.; Zhang, X.; Dong, H.; Zhang, S. Thermodynamic Modeling and Assessment of Ionic Liquid-Based CO2 Capture Processes. Ind. Eng. Chem. Res. 2014, 53, 11805–11817.
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-methylimidazolium Acetate. Energy Fuels 2010, 24, 5781–5789.
- Chen, L.; Sharifzadeh, M.; Mac Dowell, N.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids:−-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106.
- Meindersma, G.W.; de Haan, A.B. Conceptual process design for aromatic/aliphatic separation with ionic liquids. Chem. Eng. Res. Des. 2008, 86, 745–752.
- Liu, Y.; Han, W.; Xu, Z.; Fan, W.; Peng, W.; Luo, S. Comparative toxicity of pristine graphene oxide and its carboxyl, imidazole or polyethylene glycol functionalized products to Daphnia magna: A two generation study. Environ. Pollut. 2018, 237, 218–227.
- Wan, R.; Xia, X.; Wang, P.; Huo, W.; Dong, H.; Chang, Z. Toxicity of imidazoles ionic liquid Cl to HepG2 cells. Toxicol. In Vitro 2018, 52, 1–7.
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525.
- Shaikh, A.R.; Ashraf, M.; AlMayef, T.; Chawla, M.; Poater, A.; Cavallo, L. Amino acid ionic liquids as potential candidates for CO2 capture: Combined density functional theory and molecular dynamics simulations. Chem. Phys. Lett. 2020, 745, 137239.
- Baghban, A.; Mohammadi, A.H.; Taleghani, M.S. Rigorous modeling of CO2 equilibrium absorption in ionic liquids. Int. J. Greenh. Gas Control 2017, 58, 19–41.
- Haider, J.; Saeed, S.; Qyyum, M.A.; Kazmi, B.; Ahmad, R.; Muhammad, A.; Lee, M. Simultaneous capture of acid gases from natural gas adopting ionic liquids: Challenges, recent developments, and prospects. Renew. Sustain. Energy Rev. 2020, 123, 109771.
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation. J. Environ. Chem. Eng. 2021, 9, 105860.
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965.
