Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
A radiosynovectomy (RS) should be indicated when recurrent articular bleeds related to chronic hemophilia synovitis (CHS) exist, established by clinical examination, and confirmed by imaging techniques that cannot be constrained with hematological prophylaxis. RS can be performed at any point in life, mainly in adolescents (>13–14 years) and adults.
- hemophilia
- synovitis
- complications
1. Introduction
Hemophilic arthropathy happens because of repeated bleeding into articulations resulting in swelling and degeneration of cartilaginous and osseous tissues in the affected joint. Even though hematological prophylaxis averts arthropathy, it is not always appropriate or accessible [1][2][3][4]. The only approach for averting arthropathy in people with hemophilia (PWH) without inhibitors is early primary prophylaxis, although it is not always entirely successful in avoiding articular problems [1][2][3][4]. In infants with inhibitors, prophylaxis with bypassing agents (aPCCs and/or RFVIIa) is also recommended to prevent articular complications [1][2][3][4]. To prevent joint degeneration in the hemophilic joints due to the impact of blood on the synovial membrane and the cartilage cells, early primary prophylaxis (intravenous infusion of the deficient factor) is the gold standard of treatment of hemophilia. In patients with hemophilia A (deficit of factor VIII), emicizumab prophylaxis has led to greater treatment satisfaction compared with FVIII prophylaxis, reflecting in part the low treatment burden of emicizumab associated with its infrequent, subcutaneous administration. Emicizumab can also be used in patients with inhibitors [5][6][7][8].
Articular bleeds cause chondrocyte death and chronic hemophilia synovitis (CHS) resulting in a malicious circle of synovitis-hemartrosis-synovitis. This circle has to be broken quickly to halt or slow the appearance of hemophilic arthropathy. The hypertrophied synovium can be detected through palpation as a hard mass. Removal of the hypertrophied synovial membrane may be performed by using radioisotopes [9][10][11][12][13][14][15][16][17][18][19][20][21]. Figure 1 summarizes the mechanism of action of radioactive materials injected intra-articularly (radiosynovectomy-RS) [21][22][23][24].

Figure 1. Mechanism of action of radiosynovectomy (RS).
The objective of RS is to lower the danger of CHS (Figure 2) and recurrent hemarthroses that eventually degenerate the joint (hemophilic arthropathy). Hemophilia is a polyarticular disease, impacting mainly elbows, knees and ankles.
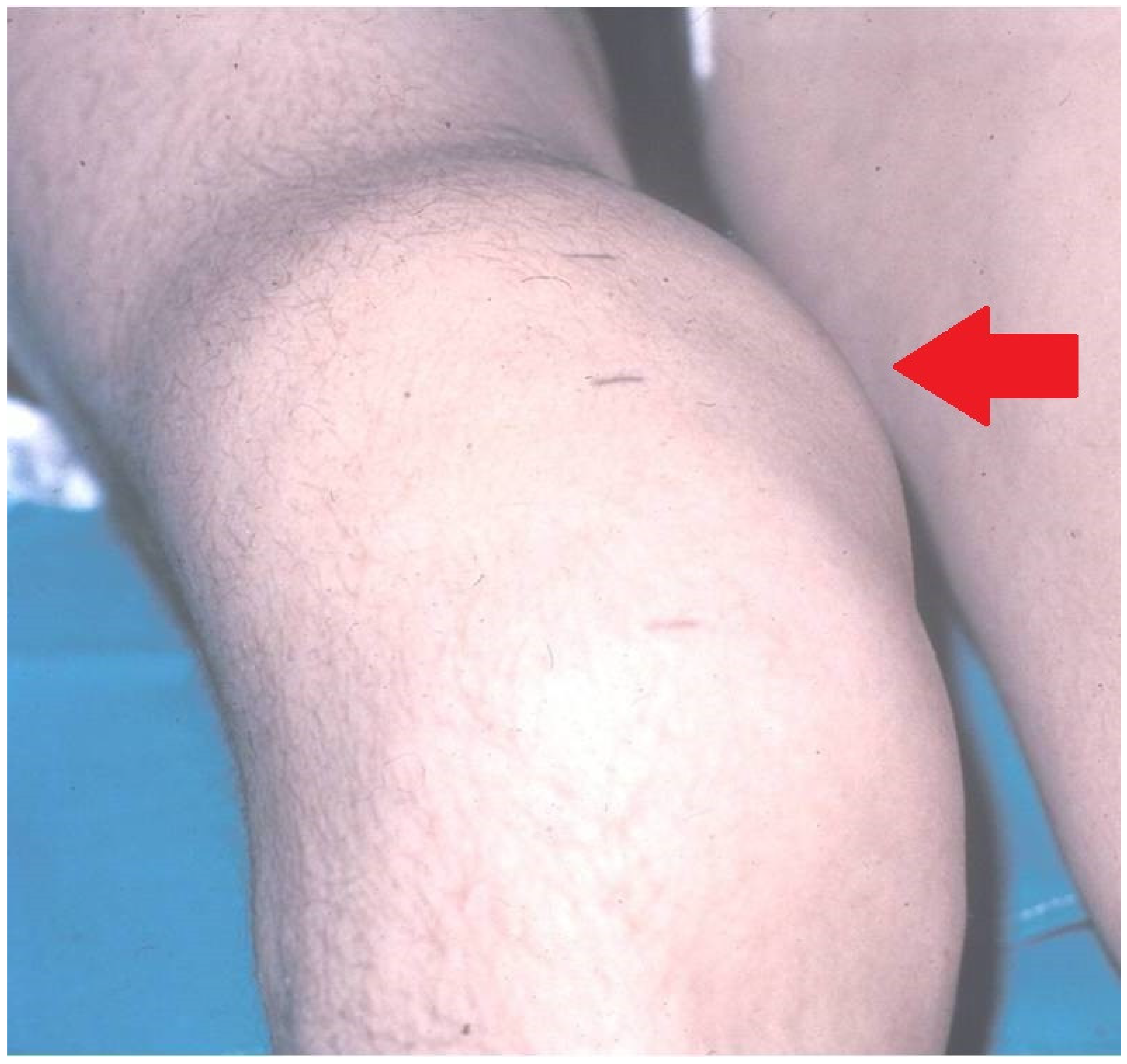
Figure 2. Serious chronic hemophilia synovitis (CHS) in a hemophilic patient (arrow).
Therefore, it is important to remember that wresearchers are facing a multiarticular condition. Confirmation of the problem has to be done with magnetic resonance imaging (MRI) (Figure 3) and/or ultrasonography (US) (Figure 4).
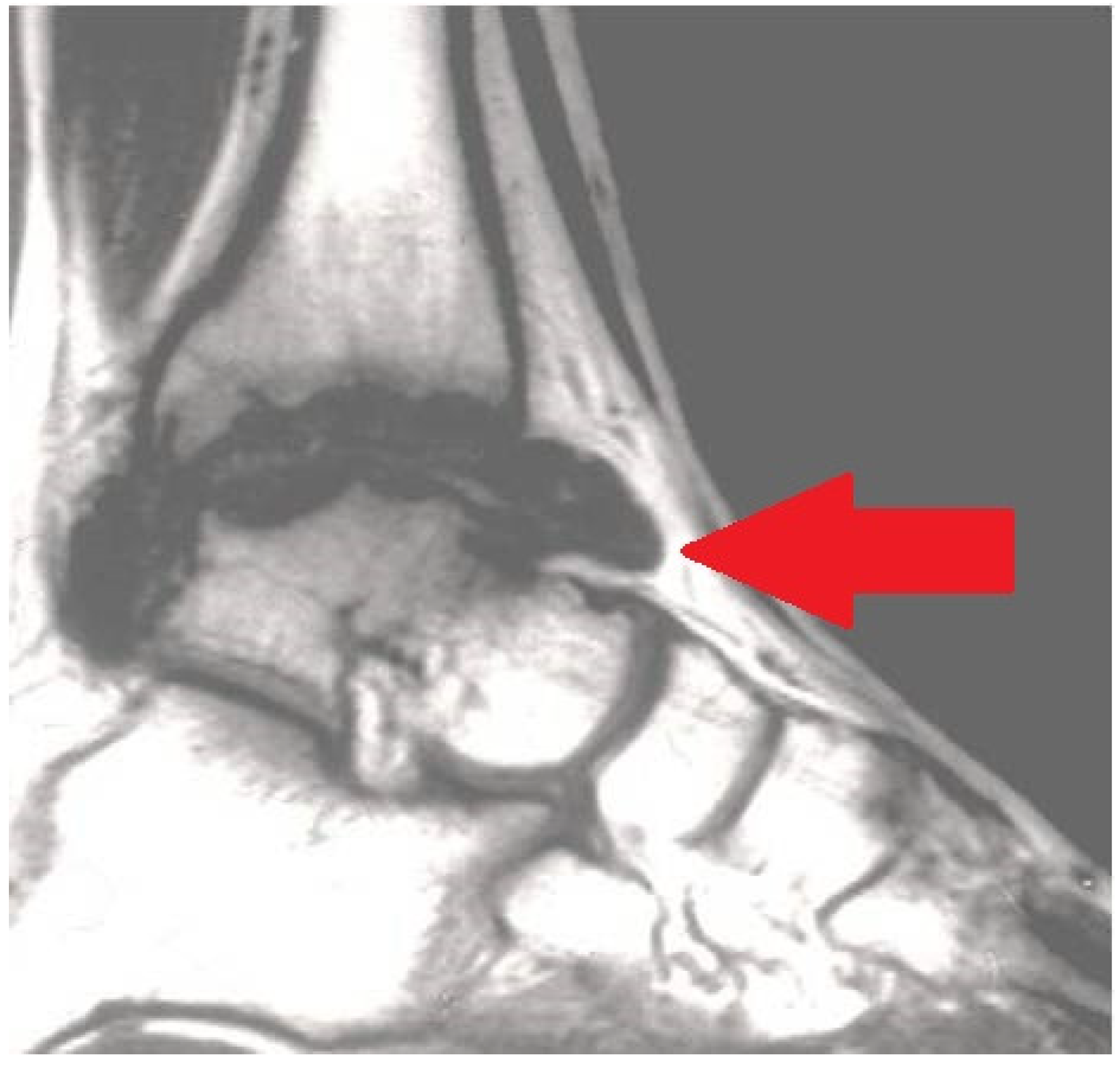
Figure 3. Magnetic resonance imaging (MRI) exhibiting serious chronic hemophilic synovitis (CHS) of the ankle (arrow).
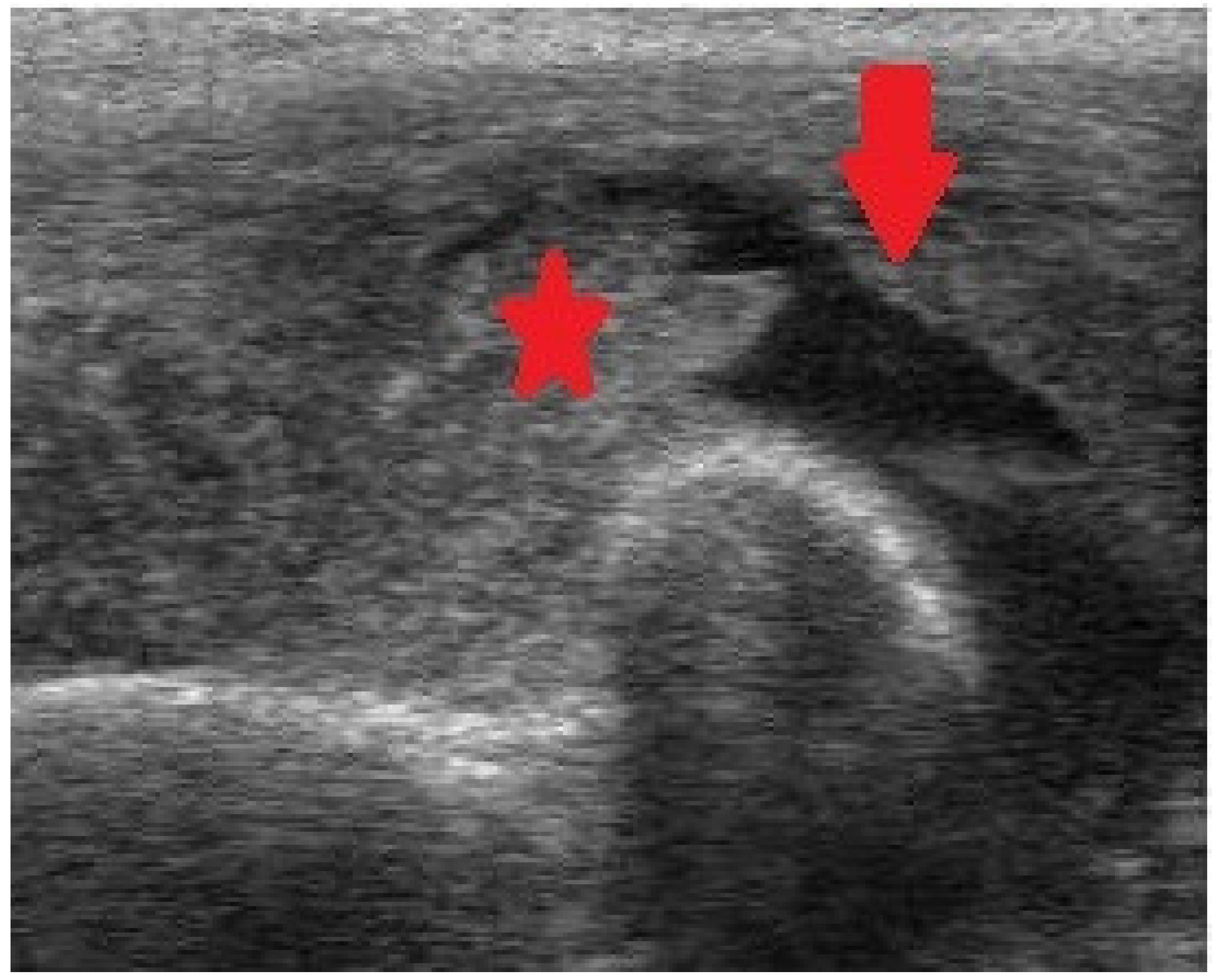
Figure 4. Ultrasonography (US) of the elbow exhibiting intraarticular fluid (arrow) and intense synovial enlargement (asterisk).
2. When Should a Radiosynovectomy (RS) Be Indicated?
RS is the elimination of the hypertrophied synovium using an intraarticular injection of a radioisotope. WResearchers indicate a RS in the following circumstances [15][16][17][18][19][20]: (1) Two or more events of hemarthrosis in the preceding 6 months; hypertrophied synovium must be confirmed by MRI and/or US. (2) An additional RS must be performed in PWH with two or more episodes of articular bleed in the following 6 months. RS must only be done in specialized hemophilia centers.
MRI and/or US may enhance our prompt detection of CHS, and they can be performed at any stage in life. According to Doria et al. [25], even though MRI is the gold standard, US is highly helpful for assessing CHS. MRI can be performed once or twice a year, while US can be carried out as many times as needed. Sierra-Aisa et al. compared US and MRI in PWH [26]. It was found that US was valuable in uncovering joint bleeds, CHS and articular erosions, with results comparable to those of MRI.
When RS has to be repeated, the procedure is identical to that performed for the first procedure. The result measures have to be obtained 6 months after each RS and then every 6 months until the last follow-up evaluation. The most important result measures are the amount of hemarthroses per month (reduction in hemarthroses), factor use, and the clinical outcome [range of motion (ROM) of the involved joint].
Chemical synovectomy with rifampicin has been reported to be efficacious, inexpensive, simple, and especially practical in developing countries where radioactive materials are not easily available [27][28][29][30]. Rifampicin seems to be more efficacious when it is utilized in small joints (elbows and ankles), than when utilized in bigger ones (knees) [27][28]. When RS and/or chemical synovectomy fail, arthroscopic synovectomy (or open synovectomy in some cases) should be indicated. In a study, 6.3% of articulations required arthroscopic synovectomy or total knee arthroplasty (TKA) [20].
3. Technique of Radiosynovectomy
RS must be performed under factor coverage to avoid the risk of hemarthrosis during the procedure. The main radioisotope used in the literature are 90Y (Yttrium-90), 186Rh (Rhenium-186) and 32P (Phosporus-32). All of them give off beta radiation and their therapeutic penetration powers (TPP) in millimeters are 2.8 mm, 1 mm, and 2.2 mm, respectively. In the knee wresearchers use Yttrium-90 [185 Megabecquerels (MBq)]. Rhenium-186 is used for elbows (56–74 MBq) and ankles (74 MBq). A small quantity of 99Tc (technetium) is introduced to perform articular scintigraphy after the procedure (to confirm the right dispersion of the radioisotope into the joint) [15][16][17][18][19][20]. We do not use local anesthetic. An ordinary needle is sufficient. When the joint has been accessed, all the fluid (blood or synovial fluid) is evacuated, and only then the radioisotope is introduced. The needle must be removed gradually whilst simultaneously introducing an anti-inflammatory agent (e.g., betamethasone), so as to not cause skin burn. Figure 5 shows the technique and all the elements required for ankle RS.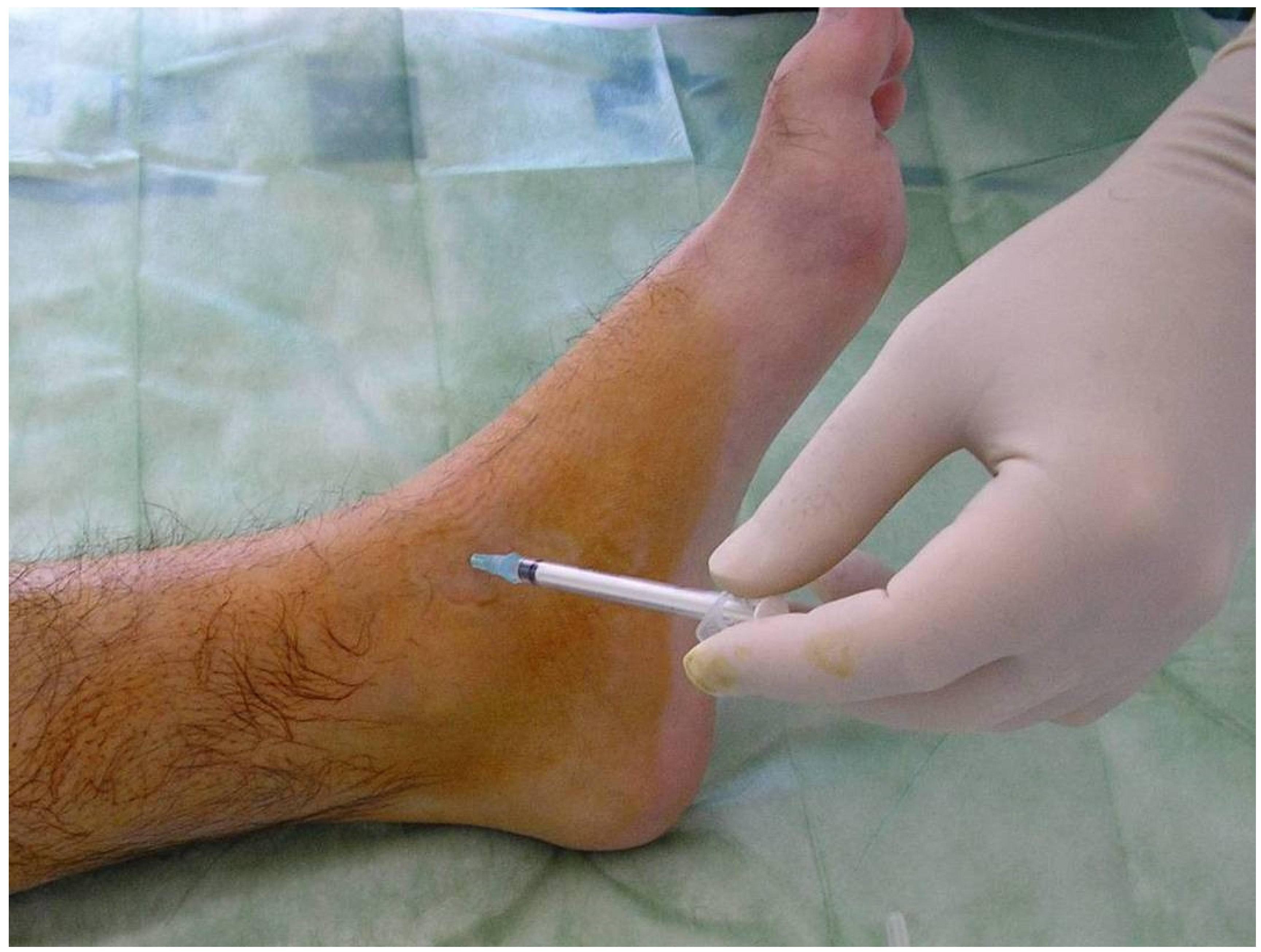
Figure 5. Radiosynovectomy (RS) of the ankle joint with Rhenium-186 in a haemophilic patients. The needle has to be removed gradually while at the same time introducing an anti-inflammatory agent.
4. Effectiveness of Radiosynovectomy
In relevant articles, 40% to 85% of articulations achieve good clinical outcomes; between 30% and 80% of joints exhibit a reduction in hemarthroses; and 35% to 85% of individuals exhibit a reduction in factor use; in other words, the amount of joint bleeds per month decrease from 3 to 6 on average before the procedure to 1 after the procedure [9][10][11][12][13][14][15][16][17][18][19][20][21]. Between 1976 and 2013, wresearchers performed 500 RSs (with Yttrium-90 or Rhenium-186) in 443 joints of 345 individuals suffering from CHS [20]. Their average age was 24 years (range, 6–53) and the follow-up was 18.5 years on average (range: 6 months-38 years). WResearchers performed 1 to 3 procedures with a 6-month interim between them. Articular bleeding frequency declined by 64% on average. In another report, wresearchers encountered that decrease of the number of hemarthoses after RS was 68% on average when RS-1 was used, 62% with RS-2, and 61% with RS-3 [16]. The volume of the synovium declined 31%. World Federation of Hemophilia (WFH) clinical score improved 19%. WFH radiological score did not improve [14][16]. In one of our reports, wresearchers found that knees required more RSs than elbows or ankles, and that the more serious hypertrophied synovial membranes required a greater number of RSs [15]. In another report, wresearchers found that RS was effective in all cohorts of patients, separately from the existence of inhibitor, the kind of articulation affected, the grade of CHS, and the existence of articular destruction (arthropathy) in the radiological examination [17]. In our center, wresearchers have also demonstrated that each RS performs separately in CHS [18]. In another report, wresearchers found that the variables analyzed improved to an equal grade in articulations with joint destruction in simple radiography (AJDSR) and without AJDSR. No joint without AJDSR required RS-3; this was the only dissimilarity our investigation found between joints without AJDSR and those with AJDSR when RS was performed [19]. In 2001, Silva et al. reported 130 RSs utilizing Phosporus-32 with a mean follow-up of 36 months [9]. Excellent and good outcomes (hemarthrosis decrease from 75% to 100%) were obtained in 79.2% of patients at 6 months to 8 years. No correlation between results and age or degree of arthropathy was found. No complications were observed. In another report of 125 RSs, 54% got complete arrest of hemarthroses. 73% of patients reported improved mobility of the injected articulation. 79% of patients had a substantial amelioration in QoL attributable to the treated joint. No complications were observed [10]. The results of RS with 90Y in 163 joints of PWH were published by Heim et al. [11]. The median age at the time of the injection was between 11 and 15 years and the median follow-up period was 11 years. Over 80% of PWH reported a decrease in the amount of articular bleeds and 15% experienced full cessation of hemarthroses. Mortazavi et al. analyzed 66 Phosporus-32 RS in 53 patients [12]. The mean follow-up was 31 months. The mean age of patients at the time of RS was 16 years. It was found that 77% of individuals experienced a 50% decrease in bleeding incidence after RS. In 2009, Calegaro et al. evaluated the effectiveness of RS with 153-Sm-HA (185 Mbq) in 31 patients (30 males). Their mean age was 20 years (8 to 34 years). The use of 153 Sm-HA in the treatment of CHS was effective for elbows and ankles, but less effective for knees [13]. In 2010, Cho et al. analyzed clinical outcomes and radiologic evaluation of 58 RS (53 patients) utilizing Holmium-166-chitosan complex in PWH [14]. The mean age of patients was 14 years. The mean follow-up was 33 months. After the injection, the mean frequency of bleeding of the elbow diminished from 3.76 to 0.47 times a month, the knee from 5.87 to 1.12 times a month, and the ankle from 3.62 to 0.73 times per month, respectively. Turkmen et al. performed 67 Yttrium-90 RS in 67 patients [21]. Their mean age was 17 years. The mean follow-up was 40 months. It was concluded that Yttrium-90 RS in the knee joint is an important resource for the treatment of CHS, markedly reducing joint bleeding.5. Complications of Radiosynovectomy
Our reported percentage of adverse events is 1%. The adverse events that wresearchers have encountered are the following [15][16][17][18][19][20]: (1) Little skin burns repaired in 1–2 weeks just by cleaning them. They occur when the radioactive material is unintentionally introduced out of the joint (Figure 6); (2) infection (septic arthritis) which requires surgical management (arthrotomy and joint debridement) plus intravenous antibiotics; (3) swelling following injections solved with rest and NSAIDS (Nonsteroidal Anti-inflammatory Drugs). WResearchers specifically recommend cyclooxigenase-2 (COX-2) inhibitor inhibitors [31][32].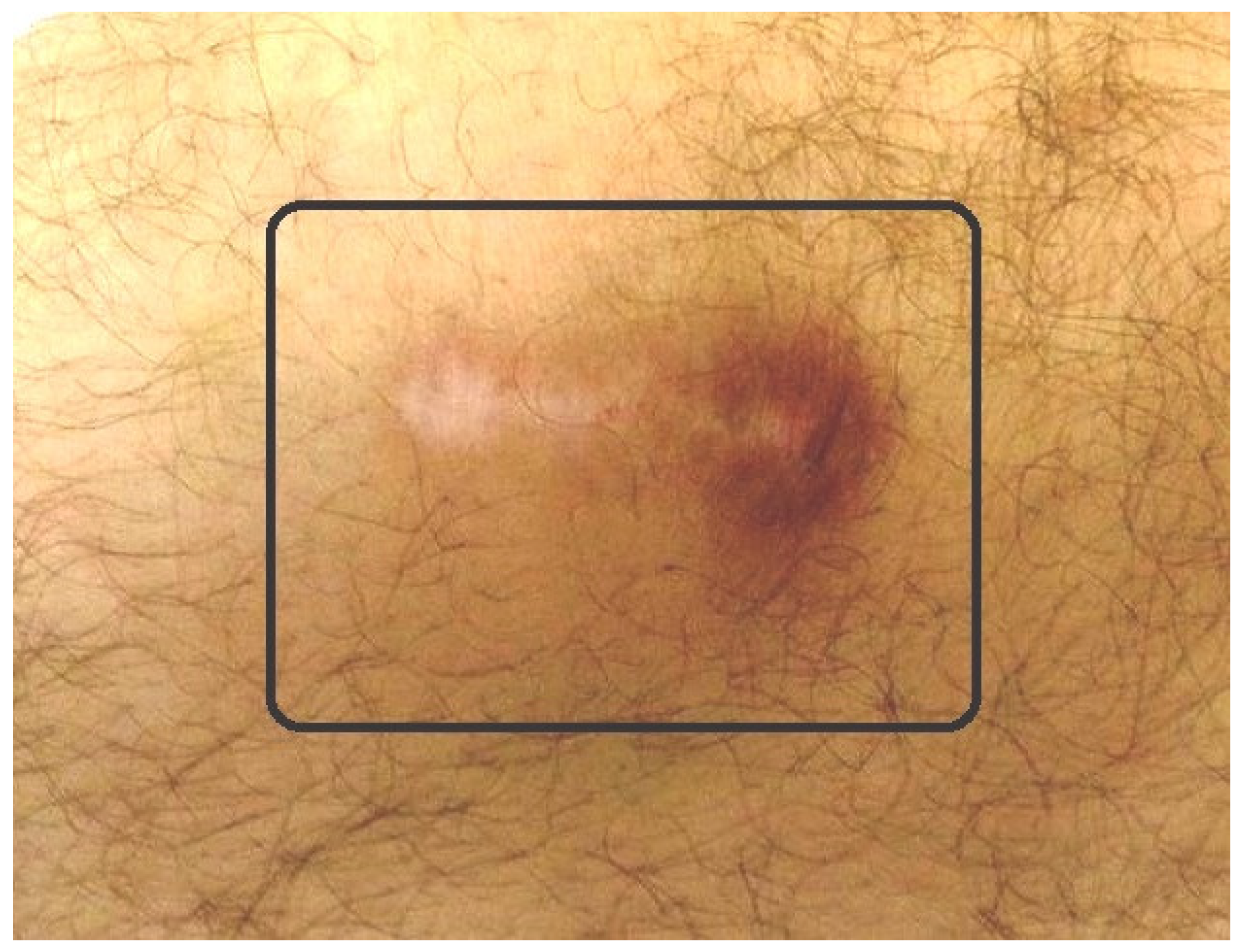
Figure 6. Small radioactive burn of the adjacent skin (square) after knee radiosynovectomy (RS).
References
- Astermark, J. When to start and when to stop primary prophylaxis in patients with severe haemophilia. Haemophilia 2003, 9 (Suppl. S1), 32–36.
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544.
- Berntorp, E.; Fischer, K.; Miners, A. Models of prophylaxis. Haemophilia 2012, 18 (Suppl. S4), 136–140.
- Ljung, R. Hemophilia and prophylaxis. Pediatr. Blood Cancer 2013, 60 (Suppl. S1), S23–S26.
- Rodriguez-Merchan, E.C.; Valentino, L.A. Emicizumab: Review of the literatura and critical appraisal. Haemophilia 2019, 25, 11–20.
- Kempton, C.; Trask, P.; Parnes, A.; Niggli, M.; Campinha-Bacote, A.; Ucallaghan, M.; O’Connell, N.; Paz-Priel, I.; Mahlangu, J.N. Development and testing of the Satisfaction Questionnaire with Intravenous or Subcutaneous Hemophilia Injection and results from the Phase 3 HAVEN 3 study of emicizumab prophylaxis in persons with haemophilia A without FVIII inhibitors. Haemophilia 2021, 27, 221–228.
- Barg, A.A.; Budnik, I.; Avishai, E.; Brutman-Barazani, T.; Bashari, D.; Misgav, M.; Lubetsky, A.; Kuperman, A.A.; Livnat, T.; Kenet, G. Emicizumab prophylaxis: Prospective longitudinal real-world follow-up and monitoring. Haemophilia 2021, 27, 383–391.
- Mazurkiewicz, Ł.; Czernikiewicz, K.; Rupa-Matysek, J.; Gil, L. Emicizumab: A novel drug in hemophilia A prophylaxis—A narrative review. Expert Rev. Hematol. 2022, 15, 933–942.
- Silva, M.; Luck, J.V.; Siegel, M.E., Jr. 32P chromic phosphate radiosynovectomy for chronic haemophilic synovitis. Haemophilia 2001, 7 (Suppl. S2), 40–49.
- Siegel, H.J.; Luck, J.V.; Siegel, M.E., Jr.; Quinones, C. Phosphate-32 colloid radiosynovectomy in hemophilia: Outcome of 125 procedures. Clin. Orthop. Relat. Res. 2001, 392, 409–417.
- Heim, M.; Goshen, E.; Amit, Y.; Martinowitz, U. Synoviorthesis with radioactive Yttrium in haemophilia: Israel experience. Haemophilia 2001, 7 (Suppl. S2), 36–39.
- Mortazavi, S.M.; Asadollahi, S.; Farzan, M.; Shahriaran, S.; Aghili, M.; Izadyar, S.; Lak, M. (32)P colloid radiosynovectomy in treatment of chronic haemophilic synovitis: Iran experience. Haemophilia 2007, 13, 182–188.
- Calegaro, J.U.; Machado, J.; De Paula, J.C.; De Almeida, J.S.; Casulari, L.A. Clinical evaluation after 1 year of 153-samarium hydroxyapatite synovectomy in patients with haemophilic arthropathy. Haemophilia 2009, 15, 240–246.
- Cho, Y.J.; Kim, K.I.; Chun, Y.S.; Rhyu, K.H.; Kwon, B.K.; Kim, D.Y. Radioisotope synoviorthesis with Holmium-166-chitosan complex in haemophilic arthropath. Radioisotope synoviorthesis with Holmium-166-chitosan complex in haemophilic arthropathy. Haemophilia 2010, 16, 640–646.
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. Radiosynovectomy in patients with chronic haemophilic synovitis: When is more than one injection necessary? Eur. J. Haematol. 2011, 86, 430–435.
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. Radiosynovectomy in hemophilia: Quantification of its effectiveness through the assessment of 10 articular parameters. J. Thromb. Haemost. 2011, 9, 928–935.
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. What patient, joint and isotope characteristics influence the response to radiosynovectomy in patients with haemophilia? Haemophilia 2011, 17, e990–e998.
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. Consecutive radiosynovectomy procedures at 6-monthly intervals behave independently in haemophilic synovitis. Blood Transfus. 2013, 11, 254–259.
- Rodriguez-Merchan, E.C.; De la Corte-Rodriguez, H.; Jimenez-Yuste, V. Is radiosynovectomy (RS) effective for joints damaged by haemophilia with articular degeneration in simple radiography (ADSR)? Thromb. Res. 2014, 133, 875–879.
- Rodriguez-Merchan, E.C.; De la Corte-Rodriguez, H.; Jimenez-Yuste, V. Radiosynovectomy in haemophilia: Long-term results of 500 procedures performed in a 38-year period. Thromb. Res. 2014, 134, 985–990.
- Knut, L. Radiosynovectomy in the therapeutic management of arthritis. World J. Nucl. Med. 2015, 14, 10–15.
- Savio, E.; Ures, M.C.; Zeledón, P.; Trindade, V.; Paolino, A.; Mockford, V.; Malanga, A.; Fernández, M.; Gaudiano, J. 188Re radiopharmaceuticals for radiosynovectomy: Evaluation and comparison of tin colloid, hydroxyapatite and tin-ferric hydroxide macroaggregates. BMC Nucl. Med. 2004, 4, 1.
- Uğur, O.; Gedik, G.K.; Atilla, B.; Rubello, D. Radiosynovectomy: Current status in the management of arthritic conditions. Nucl. Med. Commun. 2008, 29, 755–758.
- Chojnowski, M.M.; Felis-Giemza, A.; Kobylecka, M. Radionuclide synovectomy—Essentials for rheumatologists. Reumatologia 2016, 54, 108–116.
- Doria, A.S.; Keshava, S.N.; Mohanta, A.; Jarrin, J.; Blanchette, V.; Srivastava, A.; Moineddin, R.; Kavitha, M.L.; Hilliard, P.; Poonnoose, P.; et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am. J. Roentgenol. 2015, 204, W336–W347.
- Sierra Aisa, C.; Lucía Cuesta, J.F.; Rubio Martínez, A.; Fernández Mosteirín, N.; Iborra Muñoz, A.; Abío Calvete, M.; Guillén Gómez, M.; Moretó Quintana, A.; Rubio Félix, D. Comparison of ultrasound and magnetic resonance imaging for diagnosis and follow-up of joint lesions in patients with haemophilia. Haemophilia 2014, 20, e51–e57.
- Rodriguez-Merchan, E.C.; Caviglia, H.A.; Magallon, M.; Perez-Bianco, R. Chemical synovectomy vs. Radioactive synovectomy for the treatment of chronic haemophilic synovitis: A prospective short-term study. Haemophilia 1997, 3, 118–122.
- Caviglia, H.A.; Fernandez-Palazzi, F.; Galatro, G.; Perez-Bianco, R. Chemical synoviorthesis with rifampicin in haemophilia. Haemophilia 2001, 7 (Suppl. S2), 26–30.
- Rezazadeh, S.; Haghighat, A.; Mahmoodi, M.; Babanezhad, Z.; Karimi, M. Synoviorthesis induced by rifampicin in hemophilic arthropathy: A report of 24 treated joints. Ann. Hematol. 2011, 90, 963–969.
- Suh, H.C.; Kim, D.K.; Kang, S.H.; Seo, K.M.; Kim, H.S.; Lee, J.Y.; Lee, S.Y.; Yoo, K.Y. Clinical and radiological evaluation after chemical synovectomy with rifampicin in hemophilic arthropathy: Korean experience with a 2-week Interval protocol. Ann. Rehabil. Med. 2018, 42, 449–456.
- Rodriguez-Merchan, E.C.; de la Corte-Rodriguez, H.; Jimenez-Yuste, V. Efficacy of celecoxib in the treatment of joint pain caused by advanced haemophilic arthropathy in adult patients with haemophilia A. Haemophilia 2014, 20, e225–e227.
- Rodriguez-Merchan, E.C. Treatment of musculo-skeletal pain in haemophilia. Blood Rev. 2018, 32, 116–121.
More
