Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vasile Valeriu Lupu and Version 2 by Sirius Huang.
Bronchial asthma is one of the most common chronic conditions in pediatric practice, with increasing prevalence hampered by poor socioeconomic impacts, leading to major public health issues. Considered as a complex heterogeneous syndrome, not a single disease, the management of the disease is a real challenge, impacting medical staff, patients and caregivers.
- pediatric bronchial asthma
- GINA
- child
- adolescent
- treatment
1. Introduction
Bronchial asthma is one of the most common chronic pathologies in pediatric practice. It affects approximately 5.5 million children in the European Union with an average prevalence of 10% across member countries. Despite progress in understanding the disease and the development of new therapeutic strategies, asthma-related morbidity and mortality continued to increase over the last decades. Although essential, providing optimal asthma management in children encounters a number of challenges, from establishing appropriate diagnosis to early treatment with adjustment and monitoring [1][2][3][4][1,2,3,4].
In recent years, asthma research has focused on severe forms of the disease, achieving encouraging results in the field of biological therapies. However, severe asthma accounts for no more than 2% of all cases of bronchial asthma in the pediatric population and most exacerbations are seen in patients with mild and moderate forms of asthma [5]. Non-severe asthma therefore remains a major health problem, both for patients and their caregivers and for the specialists involved in their care. Recent evidence suggests important benefits from the use of anti-inflammatory medication even for mild forms of the disease that previously only benefited from bronchodilator therapy when needed. Bronchodilator medication has also been shown to produce positive outcomes in treatment combined with inhaled corticosteroids. “Single maintenance and reliever therapy” (SMART or MART) is a new treatment option in moderate disease, and it has been successfully incorporated into current guidelines. Both Global Initiative for Asthma (GINA) and the National Asthma Education and Prevention Program Coordinating Committee (NAEPP) recommend the use of a single inhaler containing the combination of an inhaled corticosteroid (ICS) and formoterol, a specific fast-acting bronchodilator (FABA), for both maintenance and quick relief therapy in steps 3 and 4 of asthma management [6][7][8][6,7,8]. As for uncontrolled, difficult-to-treat asthma and severe asthma, an appropriate evaluation is required in order to assure the right diagnosis and management. Before treatment step up, it is important to exclude an incorrect diagnosis, a poor adherence to treatment, incorrect inhaler technique or the presence of comorbidities. Treating associated comorbidities improves asthma outcomes as well. For example, children with asthma must be evaluated for gastroesophageal reflux in order to treat, as it can help the treatment of asthma or even solve some resistant to standard treatment cases [9].
1.1. Definition
Despite multiple attempts to reach a consensus on the definition of asthma, at least 60 different definitions of pediatric bronchial asthma have been identified in 122 published studies. Although some of those may appear almost identical, the impact they have on the clinical management of the disease is substantially different [4]. The GINA 2022 guidelines define asthma as a heterogeneous condition, typically characterized by chronic airway inflammation and the presence of respiratory symptoms, such as wheezing, dyspnea, chest tightness and cough, the intensity of which may vary over time, associated with variable expiratory airflow limitation, which may become persistent over time [6].
1.2. Diagnosis
Correct diagnosis of the disease is an important first step in its management. In many European countries, diagnosis is still based on history and clinical examination, without additional investigations. As a result, several studies in Europe and North America have shown an increased rate of misdiagnosis with asthma being both under- and over diagnosed [10]. Overdiagnosis resulted in misuse of medication, especially corticosteroids, with possible associated side effects, increased medical bill costs and in some cases delays in establishing the correct diagnosis. On the other hand, under-diagnosis and inappropriate treatment of cases led to decreased quality of life, increased morbidity and mortality, especially in resource-limited healthcare units [1][11][1,11].
1.3. Asthma Phenotypes, Genotypes and Biomarkers
Type 2 (T2) inflammation represents the keystone in asthma pathogenesis. Childhood asthma is known to be frequently characterized by atopy and a T2 high endotype, although current evidence sustains the existence of another T2 low endotype, with different underlying airway immune-mediated inflammation. Type 2-high asthma is described by eosinophilic inflammation, promoted by T2 cytokines as IL-4, IL-5, and IL-13, with IL-3, IL-9, IL-10, and GM-CSF included in broader classification [12][13][12,13]. The secretion of interleukins is triggered by an allergen, germ or pollutant which comes in contact with bronchial epithelial cells and followed by the recruitment of eosinophils, mastocytes and basophils in the respiratory airway as well as an Ig E production [14]. T2-high asthma is also associated with increased CD3+, CD4+, and CD8+ lymphocytes and altered dendritic cells. It denotes a good response to corticosteroid (CS) treatment and due to its biomarker identification, benefits from novel biological therapies, especially the severe cases of asthma [13][15][13,15].
T2-low asthma is represented either by a neutrophilic or a paucigranulocytic inflammatory pattern and it is sustained by IL-8, IL-17A, IL-2 and other T cell-related cytokines, including epithelial cell-derived cytokines. It is a rare endotype, found in patients with severe disease forms. It usually displays an insensitivity to corticotherapy, associated with a corticosteroid resistance [12][13][12,13].
These endotypes are characterized by the presence of some specific biological molecules. A biomarker is a measurable biological indicator which can estimate, in objective approach, the status of health or the disease progression, predict the response to therapy and monitor disease evolution [16]. A valid biomarker needs to be available for the clinicians and be reliable. It can be measured in the sputum, bronchoalveolar lavage (BAL), exhaled breath condensate (EBC), bronchial biopsy, urine and blood. In current practice, the biomarkers that are frequently used are associated with T2 inflammation: blood or sputum eosinophils, serum Ig E and fractional exhaled nitric oxide (FeNO).
Eosinophils, with a blood count cutoff value of 300 cells/microL or a sputum cell count above 2–3% of the total cell count, are usually used to monitor severe forms of the disease [15][16][15,16]. Eosinophil blood count corresponds to asthma severity and airway hyperreactivity (AHR) in pediatric patients and when associated with FeNO, illustrates a good inhaled corticosteroid (ICS) response. Different cutoff values of eosinophilia can be successfully used as a reliable reference, allowing practitioners to identify individuals with specific features of severe asthma that can benefit from varieties of available biological therapies [17]. As for sputum eosinophilia, it does not always correlate with peripheral blood film values. A high number of eosinophils in sputum stands for atopy, AHR, asthma, and has the capacity to predict future severe exacerbations, as well as the response to treatment [12][15][12,15].
Total and allergen-specific IgE levels reflect the severity of clinical symptoms, being a hallmark for atopic status in asthma patients. Total IgE is linked to the risk of developing asthma later in life, for infants with viral-induced wheezing, while asthma severity will follow allergen-specific IgE levels [12][16][12,16].
FeNO is a noninvasive biomarker measured in exhaled breath. Allergen exposure leads to high levels of nitric oxide (NO), making FeNO a marker for eosinophilic inflammation of the airways. It corresponds to AHR, serum IgE levels and blood eosinophils, and the response to ICS administration. It can be assessed as a predictor of control loss [12][15][12,15].
Serum periostin, despite its proven role in diagnosis and correlation to severity of clinical symptoms and prediction of treatment response in adults, in the pediatric department its position is still debated [15].
1.4. Classification of asthma severity
Currently, asthma severity is assessed retrospectively after the treatment over a certain period of time, considering the symptom control and exacerbations. Thus, mild asthma is defined as a disease condition which is well controlled on low dose ICS or as-needed-only ICS-formoterol. Moderate forms are those controlled with low- or moderate-dose ICS in combination with long-acting beta 2-agonists (LABA), and severe forms remain uncontrolled, despite high-dose ICS combined with LABA or other specific drugs, with good patient adherence, correct administration technique and adequate treatment of comorbidities [6][18][19][20][6,18,19,20].
2. Pharmacological Treatment of Asthma
Currently, the treatment of the pediatric patient with asthma comprises two stages: initial treatment, instituted at the patient’s first assessment, and subsequent treatment guided by the course of the disease. The first treatment regimen is chosen according to the presence and frequency of day/night symptoms, limitation of physical activity and the risk of exacerbations. Subsequently, it is based on the progress of the patient who has been put under a treatment program, and asthma may be classified as controlled, partially controlled or uncontrolled [6][21][6,32].
Historically, the treatment of asthma has varied, from chickweed cigarettes, a natural anticholinergic, used until the 1990s, to inhaled adrenaline, used in the first half of the 20th century. This was the first drug administered using a pressurized Metered Dose Inhaler (pMDI) and was replaced a decade later by selective beta2-agonists, such as salbutamol. As for ICS, their benefit and safety have been demonstrated since the early 1970s and they have since become part of standard therapy for bronchial asthma. Over a period of time, it has been shown that the anti-inflammatory effect of ICS is limited and that combining them with a second drug has shown to be significantly more effective than the increase in ICS doses in the chronic treatment of asthma. Thus, for some time the treatment guidelines have been introduced, based on the use of SABA as a crisis medication and ICS as a maintenance drug of choice—a chronic medication [22][23][33,34].
Long-term use of SABA has been associated over the years with a number of adverse reactions, such as reduced ß-receptor numbers, reactivation of bronchial hyper-reactivity (rebound phenomenon), reduced bronchodilator response, and increased allergic response and eosinophilic airway inflammation [24][25][35,36]. An unfavorable clinical outcome has been observed with the daily use of more than three vials of SABA/year, along with an increased risk of referral to the emergency room [26][27][28][37,38,39]. An increased risk of death has also been reported after consumption of 12 vials/year [28][29][39,40].
Studies suggest that these SABAs are used preferentially by patients over the usual ICS or ICS/LABA combinations, thereby masking a worsening clinical picture [29][40]. Since 2019 the GINA guidelines, based on a stepwise, progressive approach to asthma severity, which bring significant updates to the management of mild forms of bronchial asthma, have abandoned the monotherapy use of SABA as needed in adults and children older than 12 [30][41].
GINA 2021 comes with a new bronchial asthma therapeutic regimen, which, depending on the chosen controller medication, divides treatment options into two strategies, a preferred one and an alternative one, each with five steps corresponding to the patient’s symptoms. The recommendations remained unchanged in GINA 2022. Thus, strategy 1, preferably for patients aged 12 years and older, comprises low-dose ICS-formoterol as control therapy at all GINA steps of severity: only as needed in asthma steps 1 and 2 and with daily maintenance dose ICS-formoterol (maintenance and control therapy—MART) in steps 3–5. Strategy 2 (alternative) describes for step 1 the administration of SABA on an as-needed basis together with a low dose of ICS at the same time or immediately after SABA administration. Steps 2–5 recommend maintenance treatment with ICS-LABA and, whenever needed, SABA [6].
In the 2022 GINA report, children aged 6–11 years with symptoms occurring less than twice a month (stage 1) are recommended to take ICS whenever SABA is given as crisis therapy. When symptoms occur at least twice a month—but less frequently than daily (step 2)—ICS is recommended daily as maintenance therapy and SABA whenever needed. In steps 3 and 4 of severity, MART-administration of ICS-formoterol in low or very low doses is recommended, preferably. Finally, the GINA 2022 report recommends that in any severity step of bronchial asthma, crisis treatment should include SABA or ICS-formoterol if needed [6].
To sum up the SMART approach, it is recommended as a preferred treatment for both adolescents 12 years old or older and children 5–11 years old, with moderate disease forms. It significantly reduces the risk for severe exacerbations compared with maintenance ICS or ICS-LABA regimens with a SABA reliever [31][32][42,43]. Step 3 indicates one low dose of budesonide/formoterol given once or twice daily for adolescents aged ≥12 years old and one very low dose of budesonide/formoterol given once daily for children aged 5–11 years old, as maintenance therapy, in association with a dose of budesonide/formoterol as reliever therapy, given one inhalation as needed. Step 4 recommends two medium doses of budesonide/formoterol given twice daily for adolescents aged ≥12 years old and one low dose of budesonide/formoterol given twice daily for children aged 5–11 years old, as maintenance therapy, in association with a dose of budesonide/formoterol as reliever therapy, given one inhalation as needed [6].
For initial wheezing episodes in children younger than 5 years, SABA is recommended as needed. Patients in this age group to be treated according to stage 2 will receive low-dose ICS or LTRA as a secondary option to maintenance treatment, stage 3 will receive double-dose ICS or low-dose ICS and LTRA, and stage 4 patients require specialist assessment for targeted treatment of comorbidities; regardless of severity stage, patients will receive SABA anytime as needed [6].
Although most European countries use the GINA guidelines, globally there is a lack of uniformity in asthma management protocols due partly to national regulations on the use of certain drugs, but also to their low availability [33][34][22,44]
The National Asthma Education and Prevention Program (NAEPP) 2020 envisions, like GINA, a stepwise approach to asthma in severity steps, offering two treatment strategies, one preferred and one alternative. Both guidelines recommend ICS/formoterol combination as maintenance but also control medication as a treatment option starting with step 3 in patients ≥5 years of age. The NAEPP goes further and recommends ICS/LABA also under 5 years of age as maintenance treatment for forms corresponding to stage 4 and above, while GINA restricts the use of this combination to patients aged ≥ 5 years. Differences also occur in the treatment of mild forms of asthma, where the NAEPP still recommends SABA monotherapy in all age groups [35][45]. Nor has the Canadian Thoracic Society abandoned the use of SABA monotherapy as a treatment for mild asthma, but it remains the first-line treatment in patients with bronchial asthma well controlled by SABA when needed and without risk factors, regardless of age. The ICS/formoterol combination is an option for treatment of all forms of asthma only in patients aged 12 years and older [36][46]. The option to administer ICS at each SABA administration which exists in the GINA recommendations is not approved in Canada and is available only for adults with a high risk of exacerbations [36][46].
2.1. Uncontrolled, Difficult-to-Treat Asthma and Severe Asthma
GINA defines uncontrolled asthma as a form of asthma in which poor symptom control is achieved (frequent presence of symptoms or frequent use of crisis medication, limitation of physical activity and night-time awakenings due to asthma) or which is characterized by exacerbations requiring either oral corticosteroids (≥2/year) or hospitalization (≥1/year).
Difficult-to-treat asthma is uncontrolled asthma despite a medium or high dose of ICS and of a second maintenance drug or oral corticosteroid as maintenance treatment (GINA steps 4 and 5). It may be the result of incorrect diagnosis, poor adherence to treatment, incorrect inhaler technique or the presence of comorbidities.
Severe asthma is defined as asthma that remains uncontrolled despite optimized treatment with high dose ICS-LABA, or that requires high dose ICS-LABA to prevent it from becoming uncontrolled LABA in patients with good adherence, correct inhaler technique and optimal treatment of comorbidities [6].
2.2. Management of Severe Asthma
Severe asthma is a complex and heterogeneous condition, and the identification, as precisely as possible, of phenotypes (clinical panels) caused by various endotypes (distinct physio-pathological and immunological mechanisms) is important for choosing the optimal therapy for each patient [36][37][46,47].
2.3. Management of Exacerbations of Bronchial Asthma
Exacerbations are acute or subacute episodes of worsening of respiratory symptoms and lung function (measurable by a decrease in peak expiratory volume in the first second—FEV1, and peak expiratory flow—PEF) compared with the patient’s usual condition. Objective measurement of PEF is used in the classification of asthma severity in children, but this is difficult in patients with deteriorating clinical status or those of very young ages [38][39][48,49].
Acute exacerbations of bronchial asthma are the leading cause of hospitalization of pediatric patients in the United States, with an average length of hospitalization of 1.4 days. Most patients with bronchial asthma are admitted for less than 1 day and can be managed effectively in outpatient settings if time and resources are available [40][50]. A recent meta-analysis revealed that intensive care unit admission for an asthma exacerbation is associated with an increased risk of future admission [41][51].
Analysis of current protocols used in the management of acute exacerbations demonstrates variability in the treatments applied in the emergency room. However, the common goal of treating an acute episode is to prevent progression to acute respiratory failure and to treat respiratory symptoms by combating bronchoconstriction and reducing lung inflammation [3][40][3,50].
Determination of attack severity is mainly based on clinical criteria (respiratory frequency, presence of wheezing and existence of sternocleidomastoid muscle retraction). Although there is no well-validated clinical scale, the lung score (Table 1) is simple to apply at any age [3][14][3,14].
Table 1. Lung score for clinical assessment of asthma exacerbation in children according to the Spanish Guidelines for Asthma Management GEMA 5.0 [3].
| Score * | Respiratory Rate | Wheezing | |
|---|---|---|---|
| <6 Years | ≥6 Years | ||
| 0 | <30 | <20 | Without |
| 1 | 31–35 | 21–35 | At end of breath |
| 2 | 46–60 | 36–50 | Throughout exhalation (with stethoscope) |
| 3 | >60 | >50 | Inhaling and exhaling (without stethoscope) ** |
* Score from 0 to 3 for each section (minimum 0, maximum 9). ** If there is no wheezing and sternocleidomastoid muscle use is increased, the section corresponding to wheezing will receive 3 points.
The severity of the episode can be easily assessed by associating the severity of symptoms with the blood oxygen saturation (SaO2) illustrated in Table 2. Figure 1 represents the therapeutic protocol for asthma exacerbations proposed by the Spanish Pediatric Consensus and GEMA5.0 guidelines according to their severity.
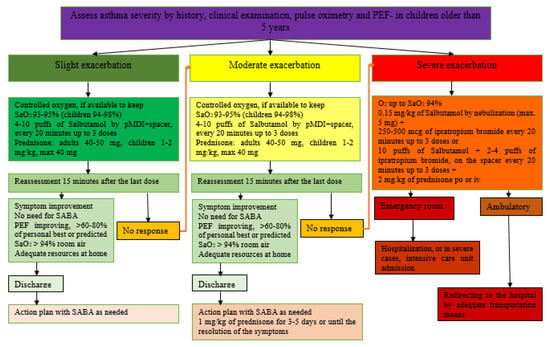
Figure 1.
Table 2. Global assessment of asthma exacerbation in children, including pulmonary score and oxygen saturation based on the Spanish asthma management guide GEMA 5.0 [3].
| Lung Score | SaO2 | |
|---|---|---|
| Mild | 0–3 | >94% |
| Moderate | 4–6 | 91–94% |
| Severe | 7–9 | <91% |
In case of a discrepancy between clinical score and oxygen saturation, the most severe value will be considered.
The basic medications used in the treatment of exacerbations include bronchodilators, oxygen therapy and glucocorticosteroids [3]. Thus, correction of significant hypoxemia will be performed with oxygen supplementation—severe cases with alveolar hypoventilation will require mechanically assisted ventilation; rapid reversal of airflow obstruction will be performed by administration of an inhaled beta2-agonist [6][42][6,52]; their intravenous administration in patients with severe exacerbations is not recommended by GINA. Early administration of systemic corticosteroids is beneficial in patients with bronchial asthma who do not respond promptly and completely to inhaled beta2-agonists [43][53]. In terms of reducing the risk of recurrence of symptoms, a short course of systemic corticosteroids is cited as useful [20].
GINA 2022 does not routinely recommend performing a chest X-ray or administering antibiotics in exacerbations. The decision to hospitalize the patient will be made based on the patient’s clinical status, lung function, response to treatment, history of exacerbations, social support, and ability to comply with therapeutic recommendations at home [6][20][43][44][45][6,20,53,54,55].
