Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Laura Saule and Version 2 by Vivi Li.
Early diagnosis of recurrent prostate cancer is a cornerstone for further adequate therapy planning. Therefore, clinical practice and research still focuses on diagnostic tools that can detect prostate cancer in early recurrence when it is undetectable in conventional diagnostic imaging. 18F-PSMA-1007 PET/CT is a novel method to evaluate patients with biochemical recurrent PCa.
- F-PSMA
- PET/CT
- prostate cancer
- local recurrence
- lymph nodes
- bone metastases
- biochemical relapse
1. Introduction
Prostate cancer (PCa) is one of the most common malignancies that affect men worldwide. Prostate cancer is the second most commonly occurring cancer in men and the fourth most common cancer overall [1]. A total of 1,414,259 new cases of prostate cancer and 375,304 related deaths were reported in 2020 globally [2]. A systematic review of autopsy studies reported a prevalence of PCa at age <30 years of 5% (95% confidence interval [CI]: 3–8%), increasing by an odds ratio (OR) of 1.7 (1.6–1.8) per decade, to a prevalence of 59% (48–71%) by age >79 years [3]. The main radical treatment methods for primary prostate cancer are prostatectomy and radiation therapy. Despite that, recurrence of the PCa is frequent and patients with clinically intermediate or high-risk prostate cancer after initial radical treatment need clinical follow-up. Biochemical recurrence is when PSA levels rise in the blood to a certain threshold after prostate cancer treatment—radical prostatectomy or radiation therapy [4]. The biochemical persistence is defined as a PSA level persistence/recurrence after radical prostatectomy such that the PSA fails to fall to undetectable levels [5].
Serum prostate-specific antigen (PSA) is a biomarker for prostate cancer screening and a reliable marker of PCa recurrence after initial treatment.
Prostate specific membrane antigen (PSMA) is a glycoprotein that is hyper expressed in prostate cancer tissues while its degree of expression correlates with tumor aggressiveness, metastatic disease and disease recurrence. In recent years, PSMA positron emission tomography/computed tomography (PET/CT) has become the gold standard for the staging of primary prostate cancer and restaging biochemical recurrent prostate cancer patients. PSMA labeled radioligands have outperformed conventional imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy, and previous generation radiopharmaceuticals [6][7][8][6,7,8].
PET/CT is a functional and anatomical non-invasive radiological hybrid imaging modality. Several radiopharmaceuticals with different diagnostic sensitivity and specificity are used in the detection and staging of prostate cancer, in the evaluation of treatment efficacy and localization of recurrence [9].
18F-PSMA-1007 is a novel PSMA-based radiopharmaceutical. Fluorine-18 (18F) labelled PSMA radiotracers have several advantages over Gallium-68 (68Ga) labelled radioligands: 18F is a cyclotron produced isotope with a longer half live and a lower positron energy compared to 68Ga, which leads to improved spatial resolution [10]. Additionally, due to non-urinary excretion, studies with 18F-PSMA-1007 have improved detection rates especially in local relapses and pelvic lymph node metastases in proximity to the urinary tract [8][11][8,11].
According to several publications, 18F-PSMA-1007 PET/CT tests are highly valuable for detecting prostate cancer biochemical recurrences. Giesel et al. analyzed 251 patients, and 204 (81.3%) of them had evidence of recurrence on 18F-PSMA-1007 PET/CT examination. The detection rates (DR) were 94.0% (for PSA levels greater than or equal to 2), 90.9% (for PSA levels 1 to less than 2), 74.5% (for PSA levels 0.5 to less than 1), and 61.5% (for PSA levels 0.2 to less than 0.5 ng/mL) [12]. Sprute et al. conducted a study using 18F-PSMA-1007 PET/CT on 96 patients with prostate cancer. In this study 18F-PSMA-1007 PET/CT had a lesion-based sensitivity of 81.7%, a specificity of 99.6%, a positive predictive value (PPV) of 92.4%, and a negative predictive value (NPV) of 98.9% for detecting positive lymph nodes larger than 3 mm [13]. When compared to bone scintigraphy (BS), 18F-PSMA-1007 PET/CT was more effective in detecting small lesions with minimal osteosclerosis or excluded degenerative change although bone scintigraphy is sufficiently sensitive to detect small bone metastasis in most of the recurrent cases [14].
Despite the specificity of 18F-PSMA-1007, physiological uptake of this radiotracer can be seen in the salivary glands, gallbladder, prostate, kidneys, liver, lacrimal glands, spleen and small intestine [15]. However, concentrated foci with localized abnormal radioactivity uptake are considered positive, such as avid uptake in lymph nodes and bones, which can be diagnosed as metastases [16].
2.
18
F-PSMA-1007 Radiotracer Characteristics
PSMA is a type II transmembrane glycoprotein with enzymatic carboxypeptidase activity that is expressed in the cytosol of normal prostatic cells. PSMA is highly overexpressed on the membrane of prostate cancer cells. The intensity of membranous PSMA expression correlates positively with tumor grade, rises under androgen deprivation and in metastatic and castration-resistant cancer, thus rendering it an appropriate target for imaging and treatment [17]. PSMA is an excellent target for several reasons: preferential, marked overexpression by most PCa cells, positive correlation of its expression with tumor grade and disease stage, low presence in the bloodstream by virtue of its transmembrane localization, and internalization and retention within tumor cells after binding to its ligand. Moreover, in personalized medicine there is increased interest in the use of PSMA for therapeutic approaches—in the theranostics setting, that means combining imaging diagnosis with targeted radionuclide therapy [17][18][17,18]. Several PSMA ligands, differing slightly in chemical structure, are commercially available and they may be radiolabeled with different positron-emitting isotopes as Gallium-68 (68Ga), Fluorine-18 (18F) or Copper-64 (64Cu) to obtain PET radiopharmaceuticals used in clinical practice. Labeling of PSMA agents with 18F may offer numerous advantages, including longer half-life and improved image resolution. Due to the lower positron energy, the theoretical achievable resolution of 18F is slightly better in comparison to 68Ga [19][20][21][22][23][24][19,20,21,22,23,24]. Studies with 18F-PSMA-1007 also suggest improved detection rates especially in local relapses and pelvic lymph node metastases in proximity to the urinary tract when compared to 68Ga-PSMA-11 PET/CT [11]. The 18F-PSMA-1007 seems to be more favorable among other 18F-PSMA ligands candidate compounds because it demonstrates high labeling yields, better tumor uptake and non-urinary background clearance [25]. In 18F-PSMA-1007 PET/CT, physiological uptake can be seen in the liver, gallbladder, prostate, kidneys, salivary glands, lacrimal glands, spleen and small intestine [15]. In several studies no drug-related pharmacological effects or physiologic responses were not reported in the patients. All observed parameters (e.g., blood pressure, heart rate and body temperature) remained normal and unchanged during and after the 18F-PSMA-1007 PET/CT examination. Overall, no adverse events due to 18F-PSMA-1007 administration were reported in the included studies. No patient reported subjective symptoms [15][26][15,26].3.
18
F-PSMA-1007 PET/CT Scanning Protocol
All patients described in the studies underwent 18F-PSMA-1007 PET/CT. The time interval between injection and image acquisition varies from 57.7 ± 4.9 min to 120 ± 10 min after injection of 18F-PSMA-1007. The 18F-PSMA-1007 solution was given by intravenous bolus injection [8][14][8,14]. All patients received regular whole-body 18F-PSMA-1007 PET/CT scans (in the most cases from head to the thighs). The popular scanner model was Biograph-mCT PET/CT (Siemens, Erlangen, Germany) [8][12][15][25][27][8,12,15,25,28]. The administered 18F-PSMA-1007 individual dose ranged from 159 ± 31 MBq to 363.93 ± 69.40 MBq [27][28][28,29]. It was confirmed that 18F-PSMA-1007 can be safely administered and results in a mean effective dose of 12.8 ± 0.6 μSv/MBq. Therefore, the total radiation dose is lower than for other PSMA PET agents and in the same range as 18F-DCFPyL [29][30]. In most of the studies all images were interpreted by two physicians—radiologists and/or nuclear medicine physicians in consensus. Image analysis was performed using an appropriate workstation and software. Focal uptake of 18F-PSMA-1007 higher than the surrounding background and not associated with physiologic uptake was considered suggestive of malignancy. Moreover, typical pitfalls in PSMA ligand PET imaging (e.g., uptake in celiac and other ganglia, fractures and degenerative changes) were considered. If at least one PSMA positive lesion suspicious for PCa was described, the PET/CT was counted as positive [8][12][30][8,12,31].4. Local Recurrence
Local recurrence of prostate cancer is defined as focal uptake of 18F-PSMA-1007 radiotracer higher than the surrounding background and not associated with physiologic uptake in PET/CT. It has been reported that the detection rate (DR) of 18F-PSMA-1007 PET/CT in local recurrence is related to PSA serum values. Results of the meta-analysis by Ferrari et al. confirmed that 18F-PSMA-1007 PET/CT demonstrated a good detection rate in biochemical recurrent prostate cancer (81.3%) and higher PSA values were associated with higher DR [31][38]. In addition, in a study by Lengana et al., the detection rate depending on the PSA level was determined. The detection rates for PSA levels 0–<0.5 ng/mL were 31.3% for 0.5–<1 ng/mL—33.3%, for 1–2 ng/mL detection rate were 55.6% and for PSA level > 2 ng/mL detection rate were 72.2%. In this study 7 (29.2%) of the positive patients had been described as negative or equivocal on conventional imaging. In addition, an optimal PSA cut-off level was determined—1.3 ng/mL [32][39]. See Figure 1 for an example of local recurrence in the prostate bed in PET/CT.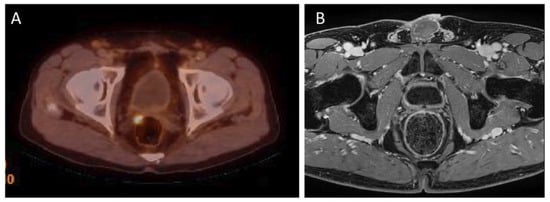
Figure 1. (A)—18F-PSMA-1007 PET/CT examination of a patient (74 years old) after radical prostatectomy eight years before, Gleason Score 7(3 + 4), current PSA level 0.29 ng/mL. 18F-PSMA-1007 focal uptake in right side tissues adjacent to the prostate bed, in the level under seminal vesicle, pararectaly with SUVmax = 6.9 was detected, confirming local recurrence. (B) this lesion cannot be seen on the corresponding MRI examination (Images from Riga Stradins University Radiology research laboratory archive).
5. Lymph Node Metastases
Lymph nodes adjacent to the primary tumor are often the first site of metastases. Additionally, lymph node metastases are commonly detected in prostate cancer recurrence. Detection of lymph node metastasis is one of major prognostic significance for prostate cancer, although lymph node metastases are themselves rarely life threatening [37][38][47,48]. See Figure 2 for example of lymph node metastasis.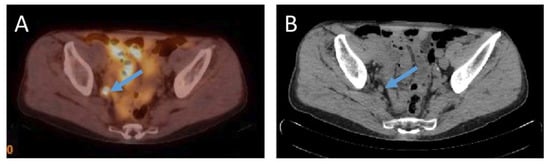
Figure 2. (A)—18F-PSMA-1007 PET/CT examination of a patient (74 years old) after radical prostatectomy ten years before, Gleason Score 6 (3 + 3), current PSA level 4.77 ng/mL. 18F-PSMA-1007 uptake in an 8 mm in size obturatory right side lymph node with SUVmax = 13.7 was detected. (B) an 8 mm in size round shaped obturatory right side lymph node in corresponding computed tomography image in axial plane (Images from Riga Stradins University Radiology research laboratory archive).
6. Bone Metastases
Bone metastases are typical in advanced stages of prostate cancer. Imaging of bone metastases is important for localization and characterization, and for evaluation of their size and number and for follow-up after therapy. Bone metastases formation is triggered by cancer initiating cells in the bone marrow and is facilitated by the release of several growth factors [19]. Figure 3 shows an example of bone metastasis.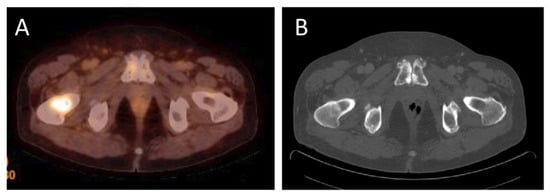
Figure 3. (A)—18F-PSMA-1007 PET/CT examination of a patient (69 years old) after radical prostatectomy seven years before, current PSA level 3.9 ng/mL. 18F-PSMA-1007 uptake in the right femur with SUVmax = 5.7 was detected to approve metastatic activity. (B) in computed tomography mild, local sclerotic lesion in the right femoral neck was inconclusive (Images from Riga Stradins University Radiology research laboratory archive).
