You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Anna Cieślińska.
Bovine beta-casein has been a subject of increasing scientific interest because its genetic A1 variant during gastrointestinal digestion releases opioid-like peptide β-casomorphin-7 (β-CM-7). Since β-CM-7 is involved in the dysregulation of many physiological processes, there is a growing discussion of whether the consumption of the β-casein A1 variant has an influence on human health. The clinical studies on humans showed a negative effect of variant A1 on serum glutathione level, digestive well-being, cognitive performance score in children, and mood score in women.
- bovine
- β-casein
- β-casomorphin-7
- A2 milk
1. Introduction
Milk accompanies humans from childhood, first as food from their mother, and then as a product used in the daily diet. It should be noted that humans are almost the only such case in nature who ingest milk in adulthood and who consume the milk of other species. From an evolutionary point of view, this phenomenon is relatively new, as historical data suggest that it has existed for about three thousand years. An increase in consumer interest in milk and dairy products has recently been observed [1]. One aspect of this interest is a discussion about the potential influence of the β-casein A1 variant on human health. The hypothesis assuming that the A1 variant of β-casein may have a negative effect on the health of milk consumers was formulated at the beginning of this century. According to this hypothesis, the digestion of cows’ milk with the A1 variant in the gastrointestinal tract may give rise to the opioid peptide β-casomorphin-7, which could be a risk factor in the development of heart disease, insulin-dependent diabetes, or neurological diseases. Unfortunately, there are many conflicting reports on this subject in the scientific literature. The numerous studies and reports that have appeared in recent years do not provide a definitive answer of whether milk containing the A1 variant of β-casein has a negative effect on the human body, and whether there are grounds for avoiding the A1 allele [2,3,4][2][3][4].
The significance and possible negative effects of the β-casein variant A1 on human health have been widely discussed and have aroused interest in the institutions in charge of food safety. The first such report was prepared for the Food Safety Authority of New Zealand [5]. This document concluded that the effects of β-casein A1 consumption on human health are important and require further investigation. Although the report of the European Food Safety Authority (EFSA 2009) “Scientific Report—Review of the potential health impact of β-casomorphins and related peptides” has not supported the hypothesis of the causal relationship between β-casomorphin-7 exposure and the etiology of human diseases, it also does not resolve the credibility hypothesis of the negative impact of the A1 β-casein variant, arguing that there is insufficient evidence and suggests further research in this field [6].
The hypothesis that a high consumption of A1 β-casein increases the risk of diabetes mellitus type 1 (DM-1), ischemic heart disease (IHD), sudden infant death syndrome (SIDS), schizophrenia, and autism spectrum disorder (ASD) is very intriguing and interesting for basic as well as application studies [2,3,4][2][3][4].
2. β-Casein Gene
2.1. Structure and Genetic Variants
β-casein (β-CN, 25–35% of milk proteins) is one of four caseins that together constitute nearly 80% of cow’s milk proteins [4,7,8][4][7][8]. Casein genes have been mapped in cattle on chromosome 6 (BTA6) in the region q31–33 and in the order: CSN1S1 (αs1-casein), CSN2 (β-casein), CSN1S2 (αs2-casein), CSN3 (κ-casein), starting from the 5’ end [9,10,11,12][9][10][11][12]. Casein cluster is represented by a DNA fragment of about 250 kb [9,11,13][9][11][13] and its expression is coordinated by multihormonal factors. This region is very conservative in many mammalian species, both in terms of construction and organization [13]. The first complete sequence of the bovine β-casein gene (CSN2, 8.5 kb) was published by Bonsing et al. [14] in 1998. Nowadays, it is known that this gene consists of nine exons and eight introns, has a total length of 10,338 bp (GenBank: M55158.1), and is classified as the most polymorphic gene of all bovine caseins, with most mutations located in exon 7. The polymorphism of β-casein was first discovered by Aschaffenburg in 1961 [15]. Up until now, 15 genetic variants of β-casein coding regions have been reported and named according to the order of discovery as A1, A2 [16[16][17],17], A3 [17], B, C [18[18][19],19], D [15], E [20], F [21], G [22], H1 [23], H2 [24], I [25], J, K, L [26]. For the next two reported variants of bovine β-CN, A4 and B2, nucleotide substitutions have not been recognized yet. An additional A5 variant with nucleotide substitution found in the intron of the β-casein gene had no implication on the protein structure. All identified and confirmed changes in the amino acid sequence of the β-casein variants are shown in Figure 1. Since the primary gene product (224 amino acids; GenBank: AAA30431.1) also contains a signal peptide that is removed post-transcriptionally, the final protein molecule is composed of 209 amino acid residues [27,28][27][28]. Basically, all the β-casein variants differed in 1–3 amino acid substitutions at different positions, but they could generally be classified as the β-casein A2 type (10 variants) or A1 type (5 variants) depending on the Pro or His presence (respectively) at position 67 of the protein sequence. The cause of it is a single nucleotide polymorphism (SNP) at codon 67 of the β-casein gene in exon 7. It is accepted that SNP67 is the effect of the natural mutation with the change of cytosine (A2 allele: CCT, proline) into adenine (A1 allele: CAT, histidine) [29,30][29][30].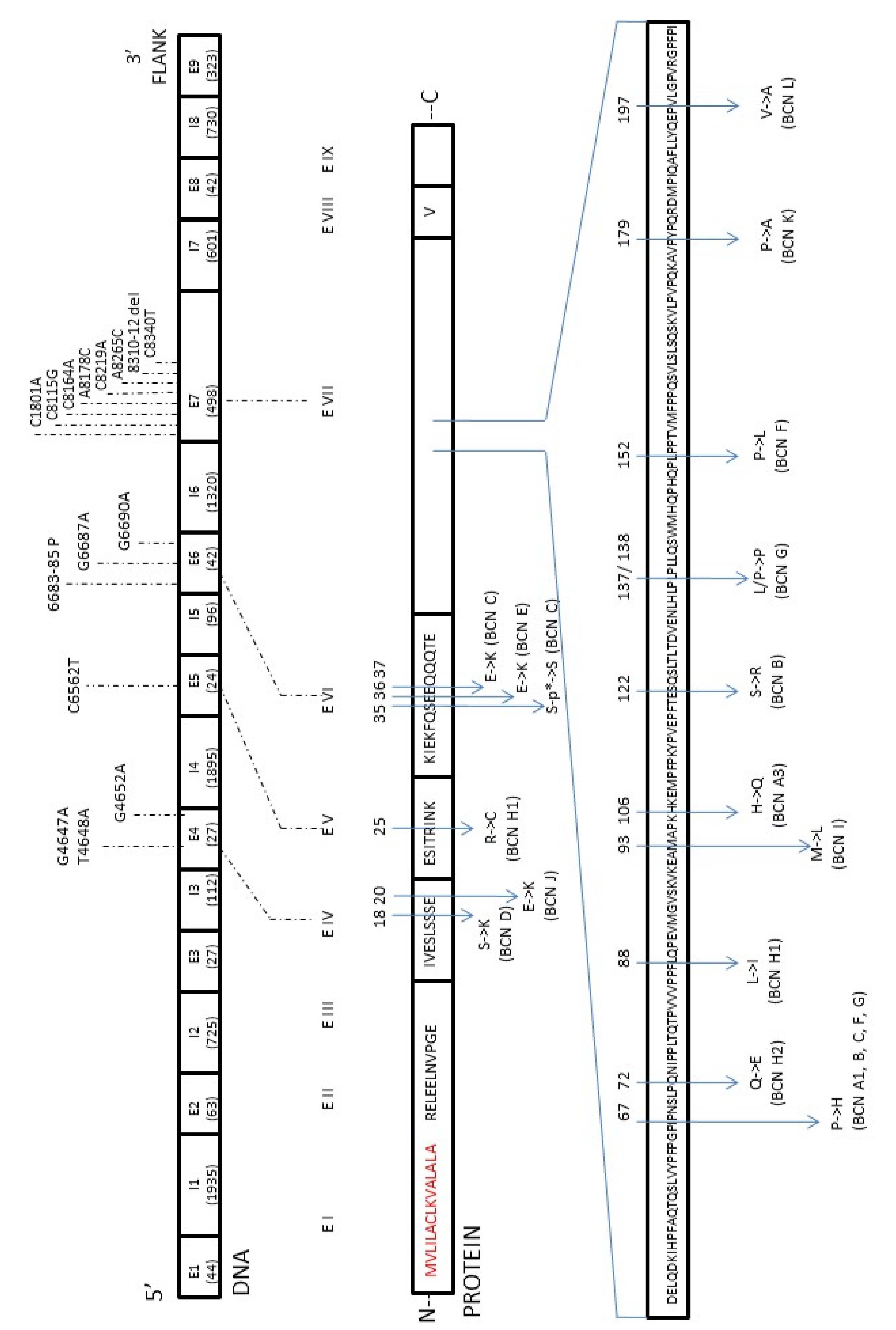
Figure 1.
The map of mutations found in the β-casein (CSN2) gene in relation to the amino acid changes with the β-casein protein (β-CN).
2.2. Variants and Their Frequency in Dairy Cattle Breeds
Many early data on the presence and prevalence of β-CN variants were based on a starch gel electrophoresis, and the method that allowed for the differentiation of only A, B, and C variants [18,19][18][19]. Later, Seibert et al. in 1985 [31] and Caroli et al. in 2016 [32] proposed the isoelectric focusing electrophoresis (IEF) method for the detection of the β-casein A1, A2, A3, B, and C variants in bovine milk. At the same time, chromatographic methods (RP-HPLC) and mass spectrometry (MS) have been proposed for the identification of variant F, the β-casein with the electroneutral substitution of amino acid with a different hydrophobic index [21]. Generally, genetic methods such as PCR-RFLP, Real-Time PCR, or sequencing are used to identify polymorphism in the β-casein gene. The most common variants of β-casein in dairy cattle breeds are A1 and A2, while B is less common, and A3 and C are considered rare [33]. Other β-CN types are very rare or identified exclusively in humped cattle (Zebu) or African cattle (Ankole) [15,20,34,35,36][15][20][34][35][36] [15,20,34,35,36][15][20][34][35][36]. Only seven of β-CN variants (A1, A2, A3, B, C, I, and E) have been detected in European cattle breeds. Although reported as the second type, the A2 variant is considered as the oldest one from which the others originated via mutation [37]. The presence of this variant is dominant in African and Asian cattle, where the average A1 and A2 frequency was found at the level of 0.16 and 0.82, respectively [15,20,34,35,38][15][20][34][35][38]. Studies conducted over the last few decades within European, American, and Australian dairy cattle populations indicate that the average prevalence of the A1 allele in these regions is 0.35, while the A2 allele is 0.61. The distribution of frequency clearly depends on the breed of cattle. Variant A1 is dominant in Ayrshire and Red Denmark cattle (between 0.51 and 0.72), while its low frequency is found in Guernsey, Jersey, Brown Swiss, and Brown Italian cows (between 0.04 and 0.14). In the world’s most common breed of dairy cattle, Holstein-Friesian (HF), the frequency of the A1 variant ranges from 0.25 to 0.51. The occurrence of the CSN2 A1 allele in various dairy breeds and countries is shown in Figure 2 (based on [22,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60][22][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60]).
Figure 2.
Frequency of the A1 β-casein gene variant in various breeds and countries.
3. Beta-Casein Protein
The biological function of the αs1-CN, αs2-CN, and β-CN is to form micelles, which are macromolecular structures responsible for the transfer of calcium to the newborn. The fourth casein (κ-CN) is the stabilizing factor of the micelles and could play an important protective function against Helicobacter pylori infections in infants [11,61][11][61]. β-casein is the most hydrophobic casein multilaterally bounded in casein micelles in an aqueous solution. As it lacks Cys, β-casein has a flexible and open conformation with a little tertiary structure. The C-terminal region of β-casein is hydrophobic, while the N-terminal domain rich in phosphate groups is highly negatively charged and polar [62]. Phosphoserine residues in the polar part of the molecule interacts electrostatically with colloidal calcium phosphate (CCP) to form nanoclusters. The nonpolar part of the molecule enhances micellar stability by forming hydrophobic bonds with other caseins [62,63,64][62][63][64]. A study by Raynes et al. showed structural differences between bovine A1 and A2 β-casein [65]. As already mentioned, β-casein participates in the formation of the casein micelles, forms oligomeric micelles itself, and functions as a molecular chaperone, preventing the aggregation of a wide range of proteins that also include other caseins. Differences in micelle assembly and chaperone activity may explain differences in the functionality of A1 and A2 milk. The A2 β-casein variant forms smaller micelles than the A1 β-casein. The monomer-micelle form equilibrium of the A2 β-casein is shifted toward the monomer, where the shift comes from the structural differences between the two β-casein variants associated with the adoption of the greater polyproline-II helix in the A2 β-casein, which may lead to enhanced chaperone activity of the A2 β-casein in comparison to the A1 β-casein [65]. To the researchers' best knowledge, the first study investigating the differences in the protein composition of casein micelles, milk whey, and fat globule membrane (MFGM) in three milk variants (A1A1, A2A2, and A1A2) was presented by Wang et al. (2020), who used the proteomic method with a label-free approach to analyze this aspect [66]. They found different contents of the protein cargo not only in casein micelles, but also in the whey and MFGM fractions. The overall analysis of these three fractions showed that several proteins were significantly associated with each of the milk variants including ceruloplasmin, protein S100-A9, and cathelicidin-2 in A1A1 milk, lactoferrin, protein S100-A8, CD5L, and protein S100-A12 in A2A2 milk, and selenoprotein P, β-glucuronidase, and osteopontin in A1A2 milk. However, the genetic rationale for these findings corresponding to the β-casein variants, its biological meaning, and physiological implications for consumer health remains unclear.4. β-Casein Digestion
β-casein is a relatively slowly digestible protein that can be completely degraded, or due to the limited proteolysis, can release bioactive peptides with antioxidant, ACE-inhibitory, or opioid activity in a way that is dependent on the genetic polymorphism [67,68,69,70][67][68][69][70]. Especially interesting here are morphine-like peptides (β-casomorphins). They are suspected to play an important role in the response to stress, pain, regulation of food intake, or perform other pathobiological functions as they are able to bind to the opioid μ-receptors (MORs) found principally in the central nervous system, immunological system, and the gastrointestinal tract [71,72,73,74,75][71][72][73][74][75]. Thus, as milk and dairy products are often the main component of the diet, their consumption may predispose hypersensitive individuals to adverse health effects. Peptides can be released from the parent structure in a few ways: hydrolysis by digestive enzymes in the digestive tract, as a consequence of microbiome activity, or as an effect of technological food processing [76,77,78,79,80,81,82,83,84,85][76][77][78][79][80][81][82][83][84][85].5. β-Casomorphins
β-casomorphins (βCMs) are a group of peptides with a chain length of 4–11 amino acids, all starting with the tyrosine residue critical to their opioid activity (Figure 3) [70]. The first isolated, and the most often identified later on, was the β-casomorphin-7 (BCM-7) heptapeptide, the sequence of which corresponds to the fragment 60–66 of the parent protein [68]. It was shown that in simulated gastrointestinal conditions in vitro, β-CM-7 is yielded by the successive gastrointestinal proteolytic digestion of β-casein A1 and B (but not A2) by pepsin, pancreatic elastase, and leucine aminopeptidase [82,84,86][82][84][86]. The cause of this difference is due to single nucleotide polymorphism of the β-casein gene (SNP67) and proline substitution by histidine in A1 of the β-casein molecule. This amino acid substitution results in the conformational difference in the expressed protein secondary structure, which may exert an influence on the physical properties of the respective casein micelles [87,88][87][88]. Additionally, the peptide bond between proline and isoleucine in the A2 variant has higher enzymatic resistance than that between histidine and isoleucine in the A1 variant. Therefore, the A1 β-casein is more readily hydrolyzed, resulting in the release of β-CM-7 [84,89][84][89]. The release of β-CM-7 during simulated gastrointestinal digestion (SGID) of A1A1 and A1A2 milk β-casein was confirmed in vitro [52]. However, it should be noted that Cieślińska et al. [53] and Duarte-Vazquez et al. [90] further showed that small amounts of β-casomorphin-7 could also be produced from β-casein A2. The release of β-casomorphin-7 from both the A1 and A2 milk β-caseins was recently confirmed by Lambers et al. [91], but not by Haq et al. [86], who did not find β-CMs in the hydrolyzed milk A2. The presence of β-casomorphin-7 was also identified in vivo, in the jejunum of healthy humans who ingested bovine milk or casein. Although the authors did not specify the parental protein variant, they estimated that the amount of β-casomorphin-7 was sufficient to elicit its biological action [92,93,94][92][93][94].
Figure 3.
The amino acid composition of the β-casomorphins formed in cow’s milk (based on Ramabadran and Bansinath [95]).
6. β-Casomorphin-7 in Milk and Milk Products
β-Casomorphins and their precursors have been identified in milk and various dairy products. A quantitative examination of the β-CM-7 in the fresh and hydrolyzed (by digestive enzymes) bovine milk revealed that in hydrolyzed A1 milk, there was a 4-fold higher level of β-CM-7 than in A2 milk, whereas in the non-hydrolyzed milk, traces of β-CM-7 were found [52,89][52][89]. Small amounts of β-CM-7 after digestion of the A2 milk β-casein were also detected by Duarte-Vazquez et al. [90] and Lambers et al. [91]. Other results were obtained by Haq et al. [86], who found a 3.2 times higher level of β-CM-7 released from the A1A1 variant after enzymatic digestion in comparison to the A1A2 variant of β-casein, and no β-casomorphin-7 after the digestion of the A2A2 variant of β-casein. It should be noted here that Lambers et al. also found that higher amounts of this peptide were liberated from the raw milk proteins than from heat-processed milk [91]. β-Casein-derived opioid peptides have been identified in fermented milk products and different types of cheeses. An example of fermented milk drinks in which β-casomorphin-7 has been identified are natural yogurt and kefir [104,105][104][105]. The content of peptides was rather low in the examined products, but as suggested by Nguyen et al., factors such as the time of fermentation, time, and conditions of product storage could strongly influence the opioid peptide concentration [106]. Precursors of β-CMs or β-CM-9 and β-CM-10 were also found in Gouda, Swiss, Blue, Limburger, and Brie cheeses, but not in mature Cheddar cheese, perhaps due to degradation during the ripening process [107,108,109][107][108][109]. Other researchers have reported the presence of β-casomorphin-7 in Gorgonzola, Gouda, Fontina, and Cheddar [104], Edamski, Gouda, Kasztelan, Rokpol and Brie, Kaszkawał, and Camping and Brie cheese [110,111][110][111]. Many of these findings were qualitative, however, based on the available data, it seems that short-ripening soft cheeses (mold-cheeses, French type) contain more β-CM-7 than the Dutch-type semi-hard cheeses that are riper for longer. Finally, several reports also showed the presence of β-CM-like and morphiceptin-like activities or exactly β-casomorphin-5 and -7 in infant formulas [104,112,113][104][112][113]. Working in this area, Duarte-Vazquez et al. developed an infant formula based on β-casein A2 milk where the concentration of β-CM7 was significantly lower than in other tested infant formulas including a formula based on A1 β-casein milk [90].7. β-Casein Variants A1/A2 and Human Health
As presented above, it is thought that the β-casein variant A1 yields the bioactive peptide β-casomorphin-7, which is thought to play a role in the higher incidence of some human diseases. At the end of the 1990s, some reports suggested that casein variant A1 consumption is a risk factor of type 1 (insulin-dependent) diabetes mellitus [114] and ischemic heart disease in humans [115]. Additionally, a relation of β-casomorphin to sudden infant death syndrome (SIDS) [2,87,88,116,117,118,119][2][87][88][116][117][118][119] and autism [120,121][120][121] has been suggested. Another potential impact of milk proteins on human health is its hypothetical correlation to milk allergy and atopic dermatitis (AD) [122,123,124,125][122][123][124][125]. In contrast, Zoghbi et al. claim that dairy products containing β-casomorphin-7 may improve intestinal protection and could have dietary and health applications [126]. β-CM-7 is known to influence the endocrine, nervous, and immune systems by activating μ-opioid receptors, which leads to different effects such as analgesia, sedation, reduced blood pressure, nausea, decreasing respiration, and bowel motility [114]. The known influence of β-CM-7 on human body systems is presented in Figure 4.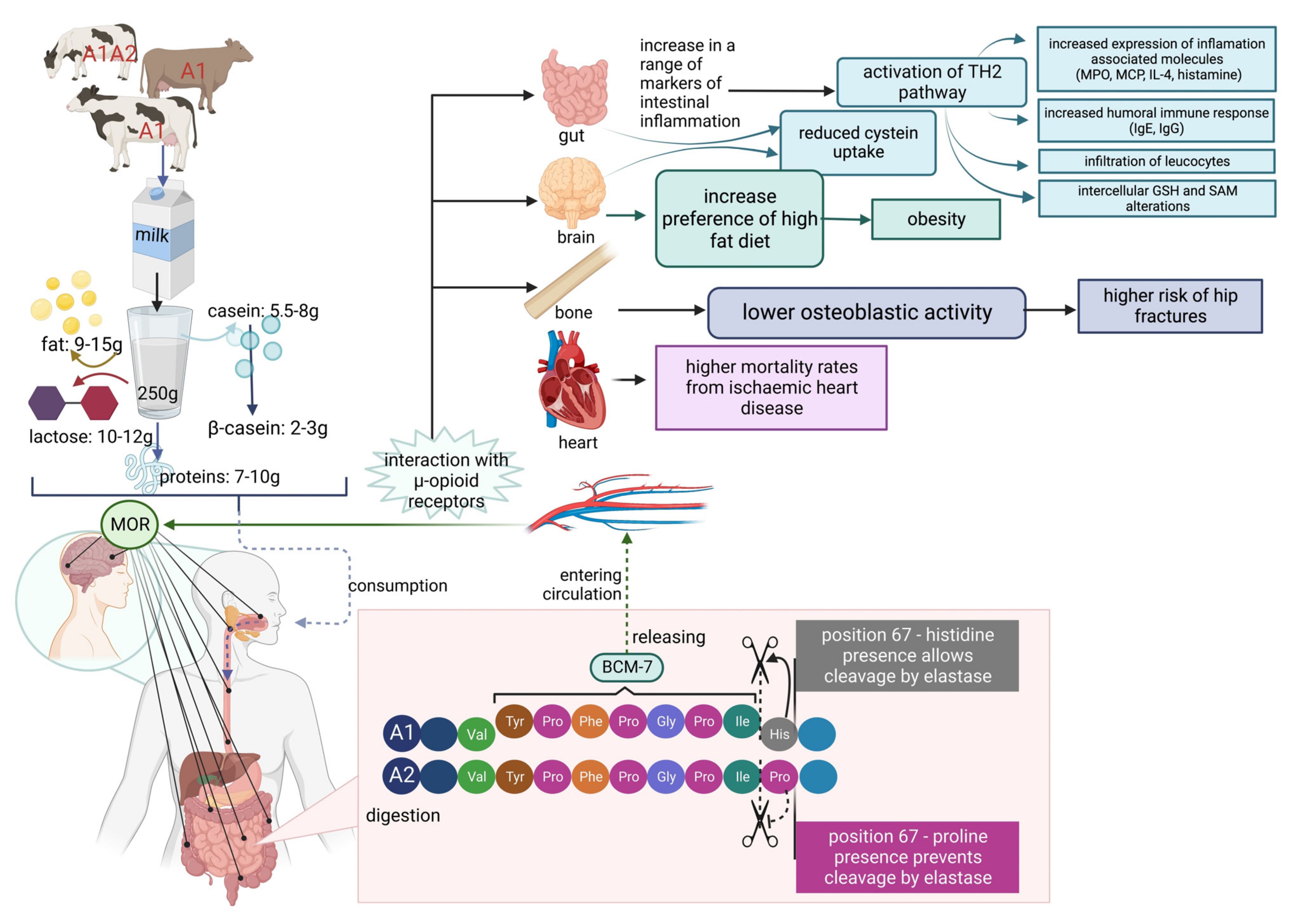
Figure 4.
β-Casomorphin-7 influence on human body systems.
8. β-Casein Variants A1/A2 in Dairy Cattle Breeding
If the risks associated with variant A1 β-casein consumption are confirmed, consumers may wish to reduce or remove this kind of milk from their diet. The farmers should take appropriate steps to allow for a systematic reduction in the number of cows and bulls with the A1 allele of β-casein and consequently reduce the spread of this undesirable allele in a dairy cattle population. Genetic polymorphism related to the differences in animal breeding value can be considered in the selection process. Research by Oleński et al. [183,184][127][128] showed that the A1 allele is associated with lower levels of milk yield traits, and the A2 variant increased the breeding values for the milk yield and milk protein content. Norwegian researchers [184][128] have suggested increasing the frequency of the allele A2 β-casein in the Norwegian cattle population due to its very positive effect on milk traits. Similar conclusions were proposed by Heck et al. [49]. The work of Gustavsson et al. [56] suggests that a higher frequency of β-casein A1A2 could have positive effects on the processing of cheese. Additional benefits of the A2 variant have also been spotted and economically estimated by Morris et al. [185][129], who indicated that the A2A2 milk, because of the better characteristics, had a higher daily yield of milk (about 2.1% higher than the value of A1A1 and A1A2 together). Furthermore, Kearney et al. [186][130] calculated that A2A2 cows produce a higher profit for milk than A1A2 or A1A1 cows.
References
- Daniloski, D.; McCarthy, N.A.; Markoska, T.; Auldist, M.J.; Vasiljevic, T. Conformational and Physicochemical Characteristics of Bovine Skim Milk Obtained from Cows with Different Genetic Variants of β-Casein. Food Hydrocoll. 2022, 124, 107186.
- Kaminski, S.; Cieslinska, A.; Kostyra, E. Polymorphism of Bovine Beta-Casein and Its Potential Effect on Human Health. J. Appl. Genet. 2007, 48, 189–198.
- Sodhi, M.; Mukesh, M.; Kataria, R.S.; Mishra, B.P.; Joshii, B.K. Milk Proteins and Human Health: A1/A2 Milk Hypothesis. Indian J. Endocrinol. Metab. 2012, 16, 856.
- Thiruvengadam, M.; Venkidasamy, B.; Thirupathi, P.; Chung, I.-M.; Subramanian, U. β-Casomorphin: A Complete Health Perspective. Food Chem. 2021, 337, 127765.
- Swinburn, B. Beta Casein A 1 and A 2 in Milk and Human Health; Report to New Zealand Food Safety Authority; School of Health Sciences: Melbourne, Australia, 2004.
- Review of the Potential Health Impact of β-Casomorphins and Related Peptides | EFSA. Available online: https://www.efsa.europa.eu/pl/efsajournal/pub/rn-231 (accessed on 2 October 2022).
- Miller, M.J.; Witherly, S.A.; Clark, D.A. Casein: A Milk Protein with Diverse Biologic Consequences. Proc. Soc. Exp. Biol. Med. 1990, 195, 143–159.
- Ostersen, S.; Foldager, J.; Hermansen, J.E. Effects of Stage of Lactation, Milk Protein Genotype and Body Condition at Calving on Protein Composition and Renneting Properties of Bovine Milk. J. Dairy Res. 1997, 64, 207–219.
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Invited Review: Milk Protein Polymorphisms in Cattle: Effect on Animal Breeding and Human Nutrition. J. Dairy Sci. 2009, 92, 5335–5352.
- Cieslak, J.; Wodas, L.; Borowska, A.; Pawlak, P.; Czyzak-Runowska, G.; Wojtowski, J.; Puppel, K.; Kuczynska, B.; Mackowski, M. 5’-Flanking Variants of Equine Casein Genes (CSN1S1, CSN1S2, CSN2, CSN3) and Their Relationship with Gene Expression and Milk Composition. J. Appl. Genet. 2019, 60, 71–78.
- Threadgill, D.W.; Womack, J.E. Genomic Analysis of the Major Bovine Milk Protein Genes. Nucleic Acids Res. 1990, 18, 6935–6942.
- Ferretti, L.; Leone, P.; Sgaramella, V. Long Range Restriction Analysis of the Bovine Casein Genes. Nucleic Acids Res. 1990, 18, 6829.
- Rijnkels, M.; Elnitski, L.; Miller, W.; Rosen, J.M. Multispecies Comparative Analysis of a Mammalian-Specific Genomic Domain Encoding Secretory Proteins. Genomics 2003, 82, 417–432.
- Bonsing, J.; Ring, J.M.; Stewart, A.F.; Mackinlay, A.G. Complete Nucleotide Sequence of the Bovine Beta-Casein Gene. Aust. J. Biol. Sci. 1988, 41, 527–537.
- Aschaffenburg, R.; Sen, A.; Thompson, M.P. Genetic Variants of Casein in Indian and African Zebu Cattle. Comp. Biochem. Physiol. 1968, 25, 177–184.
- Peterson, R.F.; Kopfler, F.C. Detection of New Types of β-Casein by Polyacrylamide Gel Electrophoresis at Acid PH: A Proposed Nomenclature. Biochem. Biophys. Res. Commun. 1966, 22, 388–392.
- Kiddy, C.A.; Peterson, R.F.; Kopfler, F.C. Genetic control of the variants of β-casein A. J. Dairy Sci. 1966, 49, 742.
- Aschaffenburg, R. Imherited Casein Variants in Cow’s Milk. Nature 1961, 192, 431–432.
- Aschaffenburg, R. Inherited Casein Variants in Cow’s Milk: II. Breed Differences in the Occurrence of β-Casein Variants. J. Dairy Res. 1963, 30, 251–258.
- Voglino, G.F. A New β-Casein Variant in Piedmont Cattle. Anim. Blood Groups Biochem. Genet. 1972, 3, 61–62.
- Visser, S.; Slangen, C.J.; Lagerwerf, F.M.; Van Dongen, W.D.; Haverkamp, J. Identification of a New Genetic Variant of Bovine Beta-Casein Using Reversed-Phase High-Performance Liquid Chromatography and Mass Spectrometric Analysis. J. Chromatogr. A 1995, 711, 141–150.
- Dong, C.; Ng-Kwai-Hang, K.F. Characterization of a Non-Electrophoretic Genetic Variant of β-Casein by Peptide Mapping and Mass Spectrometric Analysis. Int. Dairy J. 1998, 8, 967–972.
- Han, S.K.; Shin, Y.C.; Byun, H.D. Biochemical, Molecular and Physiological Characterization of a New β-Casein Variant Detected in Korean Cattle. Anim. Genet. 2000, 31, 49–51.
- Senocq, D.; Mollé, D.; Pochet, S.; Léonil, J.; Dupont, D.; Levieux, D. A New Bovine β-Casein Genetic Variant Characterized by a Met93→Leu93 Substitution in the Sequence A2. Lait 2002, 82, 171–180.
- Jann, O.; Ceriotti, G.; Caroli, A.; Erhardt, G. A New Variant in Exon VII of Bovine β-Casein Gene (CSN2) and Its Distribution among European Cattle Breeds. J. Anim. Breed. Genet. 2002, 119, 65–68.
- Gallinat, J.L.; Qanbari, S.; Drögemüller, C.; Pimentel, E.C.G.; Thaller, G.; Tetens, J. DNA-Based Identification of Novel Bovine Casein Gene Variants. J. Dairy Sci. 2013, 96, 699–709.
- Swaisgood, H.E. Review and Update of Casein Chemistry1, 2. J. Dairy Sci. 1993, 76, 3054–3061.
- Truswell, A.S. The A2 Milk Case: A Critical Review. Eur. J. Clin. Nutr. 2005, 59, 623–631.
- Roginski, H.; Fuquay, J.W.; Fox, P.F. Encyclopedia of Dairy Sciences; Academic Press: Cambridge, MA, USA, 2003; Volume 1–4.
- Groves, M.L. Some Minor Components of Casein and Other Phosphoproteins in Milk. A Review. J. Dairy Sci. 1969, 52, 1155–1165.
- Seibert, B.; Erhardt, G.; Senft, B. Procedure for Simultaneous Phenotyping of Genetic Variants in Cow’s Milk by Isoelectric Focusing. Anim. Blood Groups Biochem. Genet. 1985, 16, 183–191.
- Caroli, A.M.; Savino, S.; Bulgari, O.; Monti, E. Detecting β-Casein Variation in Bovine Milk. Molecules 2016, 21, 141.
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk--Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674.
- Grosclaude, F.; Mahé, M.-F.; Mercier, J.-C. Comparaison Du Polymorphisme Génétique Des Lactoprotéines Du Zébu et Des Bovins. Ann. Genet. Sel. Anim. 1974, 6, 305–329.
- Ganguly, I.; Gaur, G.K.; Singh, U.; Kumar, S.; Kumar, S.; Mann, S. Beta-Casein (CSN2) Polymorphism in Ongole (Indian Zebu) and Frieswal (HF × Sahiwal Crossbred) Cattle. Indian J. Biotechnol. 2013, 12, 195–198.
- Rahman, S.; Islam, A.; Alam, M.; Hossain, M.; Alim, M.A.; Salimmullah, M.; Alam, J. In Analysis of Beta-Casein Gene Variants of Milk in Cattle. In Proceedings of the 3rd International Exhibition on Dairy Aqua and Pet, Dhaka, Bangladesh, 18–20 February 2016; pp. 45–50.
- Jiménez-Montenegro, L.; Alfonso, L.; Mendizabal, J.A.; Urrutia, O. Worldwide Research Trends on Milk Containing Only A2 β-Casein: A Bibliometric Study. Animals 2022, 12, 1909.
- Rahman, M.M.; Hosen, M.B.; Faruk, M.O.; Hasan, M.M.; Kabir, Y.; Howlader, M.Z.H. Association of Vitamin D and Vitamin D Binding Protein (DBP) Gene Polymorphism with Susceptibility of Type 2 Diabetes Mellitus in Bangladesh. Gene 2017, 636, 42–47.
- Bech, A.-M.; Kristiansen, K.R. Milk Protein Polymorphism in Danish Dairy Cattle and the Influence of Genetic Variants on Milk Yield. J. Dairy Res. 1990, 57, 53–62.
- Van Eenennaam, A.; Medrano, J.F. Milk Protein Polymorphisms in California Dairy Cattle. J. Dairy Sci. 1991, 74, 1730–1742.
- Swaisgood, H.E. Chemistry of the Caseins. Adv. Dairy Chem. 1992, 1, 63.
- Baranyi, M.; Boesze, Z.; Buchberger, J.; Krause, I. Genetic Polymorphism of Milk Proteins in Hungarian Cattle Breeds and PCR Amplification of Beta-Lactoglobulin EXON 5 to Identify Genetic Variant J by RFLP; International Dairy Federation: Brussels, Belgium, 1997.
- Lien, S.; Rogne, S. Bovine Casein Haplotypes: Number, Frequencies and Applicability as Genetic Markers. Anim. Genet. 1993, 24, 373–376.
- Ikonene, T.; Ojala, M.; Ruottinen, O. Effects of Beta- and Kappa-Casein Genotypes on First Lactation Milk Production Traits in Finnish Ayrshire Cows; International Dairy Federation: Brussels, Belgium, 1997.
- Curik, I.; Havranek, J.; Samarzija, D. Milk Protein Polymorphism and Genetic Structure of Croatian Simmental Cattle; International Dairy Federation: Brussels, Belgium, 1997.
- Ehrmann, S.; Bartenschlager, H.; Geldermann, H. Quantification of Gene Effects on Single Milk Proteins in Selected Groups of Dairy Cows. J. Anim. Breed. Genet. 1997, 114, 121–132.
- Winkelman, A.M.; Wickham, B.W. Associations between Milk Protein Genetic Variants and Production Traits in New Zealand Dairy Cattle; International Dairy Federation: Brussels, Belgium, 1997.
- Boettcher, P.J.; Caroli, A.; Stella, A.; Chessa, S.; Budelli, E.; Canavesi, F.; Ghiroldi, S.; Pagnacco, G. Effects of Casein Haplotypes on Milk Production Traits in Italian Holstein and Brown Swiss Cattle. J. Dairy Sci. 2004, 87, 4311–4317.
- Kamiński, S.; Ruść, A.; Cieślińska, A. A Note on Frequency of A1 and A2 Variants of Bovine Beta-Casein Locus in Polish Holstein Bulls. J. Anim. Feed Sci. 2006, 15, 195–198.
- Heck, J.M.L.; Schennink, A.; van Valenberg, H.J.F.; Bovenhuis, H.; Visker, M.H.P.W.; van Arendonk, J.A.M.; van Hooijdonk, A.C.M. Effects of Milk Protein Variants on the Protein Composition of Bovine Milk. J. Dairy Sci. 2009, 92, 1192–1202.
- Visker, M.H.P.W.; Dibbits, B.W.; Kinders, S.M.; van Valenberg, H.J.F.; van Arendonk, J.A.M.; Bovenhuis, H. Association of Bovine β-Casein Protein Variant I with Milk Production and Milk Protein Composition. Anim. Genet. 2011, 42, 212–218.
- Molee, A.; Boonek, L.; Rungsakinnin, N. The Effect of Beta and Kappa Casein Genes on Milk Yield and Milk Composition in Different Percentages of Holstein in Crossbred Dairy Cattle. Anim. Sci. J. 2011, 82, 512–516.
- Cieślińska, A.; Kostyra, E.; Kostyra, H.; Oleński, K.; Fiedorowicz, E.; Kamiński, S. Milk from Cows of Different β-Casein Genotypes as a Source of β-Casomorphin-7. Int. J. Food Sci. Nutr. 2012, 63, 426–430.
- Dinc, H.; Ozkan, E.; Koban, E.; Togan, I. Beta-Casein A1/A2, Kappa-Casein and Beta-Lactoglobulin Polymorphisms in Turkish Cattle Breeds. Arch. Anim. Breed. 2013, 56, 650–657.
- Mir, S.N.; Ullah, O.; Sheikh, R. Genetic Polymorphism of Milk Protein Variants and Their Association Studies with Milk Yield in Sahiwal Cattle. Afr. J. Biotechnol. 2014, 13, 555–565.
- Gustavsson, F.; Buitenhuis, A.J.; Johansson, M.; Bertelsen, H.P.; Glantz, M.; Poulsen, N.A.; Lindmark Månsson, H.; Stålhammar, H.; Larsen, L.B.; Bendixen, C.; et al. Effects of Breed and Casein Genetic Variants on Protein Profile in Milk from Swedish Red, Danish Holstein, and Danish Jersey Cows. J. Dairy Sci. 2014, 97, 3866–3877.
- Gholami, M.; Hafezian, S.H.; Rahimi, G.; Farhadi, A.; Rahimi, Z.; Kahrizi, D.; Kiani, S.; Karim, H.; Vaziri, S.; Muhammadi, S.; et al. Allele Specific-PCR and Melting Curve Analysis Showed Relatively High Frequency of β-Casein Gene A1 Allele in Iranian Holstein, Simmental and Native Cows. Cell. Mol. Biol. (Noisy-Le-Grand) 2016, 62, 138–143.
- Dai, R.; Fang, Y.; Zhao, W.; Liu, S.; Ding, J.; Xu, K.; Yang, L.; He, C.; Ding, F.; Meng, H. Identification of Alleles and Genotypes of Beta-Casein with DNA Sequencing Analysis in Chinese Holstein Cow. J. Dairy Res. 2016, 83, 312–316.
- Rangel, A.H.N.; Zaros, L.G.; Lima, T.C.; Borba, L.H.F.; Novaes, L.P.; Mota, L.F.M.; Silva, M.S. Polymorphism in the Beta Casein Gene and Analysis of Milk Characteristicsin Gir and Guzerá Dairy Cattle. Genet. Mol. Res. 2017, 16, gmr16029592.
- Massella, E.; Piva, S.; Giacometti, F.; Liuzzo, G.; Zambrini, A.V.; Serraino, A. Evaluation of Bovine Beta Casein Polymorphism in Two Dairy Farms Located in Northern Italy. Ital. J. Food Saf. 2017, 6, 6904.
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine Milk Proteins: Chemistry, Structure and Nutritional Significance. Int. Dairy J. 2010, 20, 609–629.
- Cheng, H.; Dong, H.; Wusigale; Liang, L. A Comparison of β-Casein Complexes and Micelles as Vehicles for Trans-/Cis-Resveratrol. Food Chem. 2020, 330, 127209.
- Duerasch, A.; Herrmann, P.; Hogh, K.; Henle, T. Study on β-Casein Depleted Casein Micelles: Micellar Stability, Enzymatic Cross-Linking, and Suitability as Nanocarriers. J. Agric. Food Chem. 2020, 68, 13940–13949.
- Li, M.; Fokkink, R.; Ni, Y.; Kleijn, J.M. Bovine Beta-Casein Micelles as Delivery Systems for Hydrophobic Flavonoids. Food Hydrocoll. 2019, 96, 653–662.
- Raynes, J.K.; Day, L.; Augustin, M.A.; Carver, J.A. Structural Differences between Bovine A1 and A2 β-Casein Alter Micelle Self-Assembly and Influence Molecular Chaperone Activity. J. Dairy Sci. 2015, 98, 2172–2182.
- Wang, X.; Yu, Z.; Zhao, X.; Han, R.; Huang, D.; Yang, Y.; Cheng, G. Comparative Proteomic Characterization of Bovine Milk Containing β-Casein Variants A1A1 and A2A2, and Their Heterozygote A1A2. J. Sci. Food Agric. 2021, 101, 718–725.
- Petrat-Melin, B.; Andersen, P.; Rasmussen, J.T.; Poulsen, N.A.; Larsen, L.B.; Young, J.F. In Vitro Digestion of Purified β-Casein Variants A(1), A(2), B, and I: Effects on Antioxidant and Angiotensin-Converting Enzyme Inhibitory Capacity. J. Dairy Sci. 2015, 98, 15–26.
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel Opioid Peptides Derived from Casein (Beta-Casomorphins). I. Isolation from Bovine Casein Peptone. Hoppe Seylers Z. Physiol. Chem. 1979, 360, 1211–1216.
- Chang, K.J.; Su, Y.F.; Brent, D.A.; Chang, J.K. Isolation of a Specific Mu-Opiate Receptor Peptide, Morphiceptin, from an Enzymatic Digest of Milk Proteins. J. Biol. Chem. 1985, 260, 9706–9712.
- Kostyra, E.; Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Krawczuk, S.; Kostyra, H. Opioid Peptides Derived from Milk Proteins. Pol. J. Food Nutr. Sci. 2004, 13, 25–35.
- Meisel, H.; FitzGerald, R.J. Opioid Peptides Encrypted in Intact Milk Protein Sequences. Br. J. Nutr. 2000, 84 (Suppl. 1), S27–S31.
- Nylund, G.; Pettersson, A.; Bengtsson, C.; Khorram-Manesh, A.; Nordgren, S.; Delbro, D.S. Functional Expression of Mu-Opioid Receptors in the Human Colon Cancer Cell Line, HT-29, and Their Localization in Human Colon. Dig. Dis. Sci. 2008, 53, 461–466.
- Bidlack, J.M. Detection and Function of Opioid Receptors on Cells from the Immune System. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723.
- Mansour, A.; Fox, C.A.; Akil, H.; Watson, S.J. Opioid-Receptor MRNA Expression in the Rat CNS: Anatomical and Functional Implications. Trends Neurosci. 1995, 18, 22–29.
- Teschemacher, H. Opioid Receptor Ligands Derived from Food Proteins. Curr. Pharm. Des. 2003, 9, 1331–1344.
- Phelan, M.; Aherne, A.; FitzGerald, R.J.; O’Brien, N.M. Casein-Derived Bioactive Peptides: Biological Effects, Industrial Uses, Safety Aspects and Regulatory Status. Int. Dairy J. 2009, 19, 643–654.
- Schmelzer, C.E.H.; Schöps, R.; Reynell, L.; Ulbrich-Hofmann, R.; Neubert, R.H.H.; Raith, K. Peptic Digestion of β-Casein: Time Course and Fate of Possible Bioactive Peptides. J. Chromatogr. A 2007, 1166, 108–115.
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960.
- Johansson, M.; Åkerstedt, M.; Li, S.; Zamaratskaia, G.; Sternesjö Lundh, Å. Casein Breakdown in Bovine Milk by a Field Strain of Staphylococcus Aureus. J. Food Prot. 2013, 76, 1638–1642.
- Noni, I.D. Release of β-Casomorphins 5 and 7 during Simulated Gastro-Intestinal Digestion of Bovine β-Casein Variants and Milk-Based Infant Formulas. Food Chem. 2008, 110, 897–903.
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of Processing on Bioactive Proteins and Peptides. Trends Food Sci. Technol. 1998, 9, 307–319.
- Hartwig, A. Einfluß Genetischer Varianten Bovinen β-Caseins auf Entstehung und Aktivität Biologisch Aktiver Peptide. Ph.D. Dissertation, University of Giessen, Giessen, Germany, 1997.
- Meisel, H. Biochemical Properties of Regulatory Peptides Derived from Mil Proteins. Pept. Sci. 1997, 43, 119–128.
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic Release of Neocasomorphin and Beta-Casomorphin from Bovine Beta-Casein. Peptides 1999, 20, 957–962.
- Korhonen, H.; Pihlanto, A. Food-Derived Bioactive Peptides—Opportunities for Designing Future Foods. Curr. Pharm. Des. 2003, 9, 1297–1308.
- Ul Haq, M.R.; Kapila, R.; Kapila, S. Release of β-Casomorphin-7/5 during Simulated Gastrointestinal Digestion of Milk β-Casein Variants from Indian Crossbred Cattle (Karan Fries). Food Chem. 2015, 168, 70–79.
- Elliott, R.B.; Harris, D.P.; Hill, J.P.; Bibby, N.J.; Wasmuth, H.E. Type I (Insulin-Dependent) Diabetes Mellitus and Cow Milk: Casein Variant Consumption. Diabetologia 1999, 42, 292–296.
- McLachlan, C.N. Beta-Casein A1, Ischaemic Heart Disease Mortality, and Other Illnesses. Med. Hypotheses 2001, 56, 262–272.
- Cieślińska, A.; Kamiński, S.; Kostyra, E.; Sienkiewicz-Szłapka, E. Beta-Casomorphin 7 in Raw and Hydrolyzed Milk Derived from Cows of Alternative β-Casein Genotypes. Milchwiss. Milk Sci. Int. 2007, 62, 125–127.
- Duarte-Vázquez, M.Á.; García-Ugalde, C.; Villegas-Gutiérrez, L.M.; García-Almendárez, B.E.; Rosado, J.L. Production of Cow’s Milk Free from Beta-Casein A1 and Its Application in the Manufacturing of Specialized Foods for Early Infant Nutrition. Foods 2017, 6, 50.
- Lambers, T.T.; Broeren, S.; Heck, J.; Bragt, M.; Huppertz, T. Processing Affects Beta-Casomorphin Peptide Formation during Simulated Gastrointestinal Digestion in Both A1 and A2 Milk. Int. Dairy J. 2021, 121, 105099.
- Svedberg, J.; de Haas, J.; Leimenstoll, G.; Paul, F.; Teschemacher, H. Demonstration of Beta-Casomorphin Immunoreactive Materials in in Vitro Digests of Bovine Milk and in Small Intestine Contents after Bovine Milk Ingestion in Adult Humans. Peptides 1985, 6, 825–830.
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tomé, D.; Leonil, J. Sequential Release of Milk Protein-Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323.
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive Peptides from Milk Proteins. J. Nutr. 2004, 134, 980S–988S.
- Ramabadran, K. Pharmacology of β-Casomorphins, Opioid Peptides Derived from Milk Protein. Asia Pac. J. Pharm. 1989, 4, 45–58.
- Hafeez, Z.; Cakir-Kiefer, C.; Girardet, J.-M.; Jardin, J.; Perrin, C.; Dary, A.; Miclo, L. Hydrolysis of Milk-Derived Bioactive Peptides by Cell-Associated Extracellular Peptidases of Streptococcus Thermophilus. Appl. Microbiol. Biotechnol. 2013, 97, 9787–9799.
- Asledottir, T.; Picariello, G.; Mamone, G.; Ferranti, P.; Røseth, A.; Devold, T.G.; Vegarud, G.E. Degradation of β-Casomorphin-7 through in Vitro Gastrointestinal and Jejunal Brush Border Membrane Digestion. J. Dairy Sci. 2019, 102, 8622–8629.
- Engel, M.; Hoffmann, T.; Wagner, L.; Wermann, M.; Heiser, U.; Kiefersauer, R.; Huber, R.; Bode, W.; Demuth, H.-U.; Brandstetter, H. The Crystal Structure of Dipeptidyl Peptidase IV (CD26) Reveals Its Functional Regulation and Enzymatic Mechanism. Proc. Natl. Acad. Sci. USA 2003, 100, 5063–5068.
- Lambeir, A.-M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-Peptidase IV from Bench to Bedside: An Update on Structural Properties, Functions, and Clinical Aspects of the Enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294.
- Kreil, G.; Umbach, M.; Brantl, V.; Teschemacher, H. Studies on the Enzymatic Degradation of Beta-Casomorphins. Life Sci. 1983, 33 (Suppl. 1), 137–140.
- Osborne, S.; Chen, W.; Addepalli, R.; Colgrave, M.; Singh, T.; Tran, C.; Day, L. In Vitro Transport and Satiety of a Beta-Lactoglobulin Dipeptide and Beta-Casomorphin-7 and Its Metabolites. Food Funct. 2014, 5, 2706–2718.
- Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Bielikowicz, K. Transport of Bovine Milk-Derived Opioid Peptides across a Caco-2 Monolayer. Int. Dairy J. 2009, 19, 252–257.
- Jarmołowska, B.; Teodorowicz, M.; Fiedorowicz, E.; Sienkiewicz-Szłapka, E.; Matysiewicz, M.; Kostyra, E. Glucose and Calcium Ions May Modulate the Efficiency of Bovine β-Casomorphin-7 Permeability through a Monolayer of Caco-2 Cells. Peptides 2013, 49, 59–67.
- De Noni, I.; Cattaneo, S. Occurrence of β-Casomorphins 5 and 7 in Commercial Dairy Products and in Their Digests Following in Vitro Simulated Gastro-Intestinal Digestion. Food Chem. 2010, 119, 560–566.
- Jarmołowska, B.; Krawczuk, S. The Influence of Storage on Contents of Selected Antagonist and Agonist Opioid Peptides in Fermented Milk Drinks. Milchwissenschaft 2012, 67, 130–133.
- Nguyen, D.D.; Solah, V.A.; Johnson, S.K.; Charrois, J.W.A.; Busetti, F. Isotope Dilution Liquid Chromatography–Tandem Mass Spectrometry for Simultaneous Identification and Quantification of Beta-Casomorphin 5 and Beta-Casomorphin 7 in Yoghurt. Food Chem. 2014, 146, 345–352.
- Muehlenkamp, M.R.; Warthesen, J.J. Beta-Casomorphins: Analysis in Cheese and Susceptibility to Proteolytic Enzymes from Lactococcus Lactis Ssp. Cremoris. J. Dairy Sci. 1996, 79, 20–26.
- Saito, T. Antihypertensive Peptides Derived from Bovine Casein and Whey Proteins. Adv. Exp. Med. Biol. 2008, 606, 295–317.
- Toelstede, S.; Hofmann, T. Quantitative Studies and Taste Re-Engineering Experiments toward the Decoding of the Nonvolatile Sensometabolome of Gouda Cheese. J. Agric. Food. Chem. 2008, 56, 5299–5307.
- Jarmołowska, B.; Kostyra, E.; Krawczuk, S.; Kostyra, H. β-Casomorphin-7 Isolated from Brie Cheese. J. Sci. Food Agric. 1999, 79, 1788–1792.
- Contents of Agonistic and Antagonistic Opioid Peptides in Different Cheese Varieties. Available online: https://nauka-polska.pl/#/profile/publication?id=2118297&_k=z8mnca (accessed on 2 November 2022).
- Jarmołowska, B.; Bielikowicz, K.; Iwan, M.; Sidor, K.; Kostyra, E.; Kaczmarski, M. Serum Activity of Dipeptidyl Peptidase IV (DPPIV; EC 3.4.14.5) in Breast-Fed Infants with Symptoms of Allergy. Peptides 2007, 28, 678–682.
- Sturner, R.; Chang, K. Opioid Peptide Content in Infant Formulas. Pediatr. Res 1988, 23, 4–10.
- Küllenberg de Gaudry, D.; Lohner, S.; Schmucker, C.; Kapp, P.; Motschall, E.; Hörrlein, S.; Röger, C.; Meerpohl, J.J. Milk A1 β-Casein and Health-Related Outcomes in Humans: A Systematic Review. Nutr. Rev. 2019, 77, 278–306.
- Kumar, S.; Singh, R.V.; Kumar, A.; Yadav, J.S. Analysis of Beta-Casein Gene (CSN2) Polymorphism in Tharparkar and Frieswal Cattle. Indian J. Anim. Res. 2019, 54, 1–5.
- Thorsdottir, I.; Birgisdottir, B.E.; Johannsdottir, I.M.; Harris, D.P.; Hill, J.; Steingrimsdottir, L.; Thorsson, A.V. Different Beta-Casein Fractions in Icelandic versus Scandinavian Cow’s Milk May Influence Diabetogenicity of Cow’s Milk in Infancy and Explain Low Incidence of Insulin-Dependent Diabetes Mellitus in Iceland. Pediatrics 2000, 106, 719–724.
- Laugesen, M.; Elliott, R. Ischaemic Heart Disease, Type 1 Diabetes, and Cow Milk A1 Beta-Casein. N. Z. Med. J. 2003, 116, U295.
- Sun, Z.; Zhang, Z.; Wang, X.; Cade, R.; Elmir, Z.; Fregly, M. Relation of β-Casomorphin to Apnea in Sudden Infant Death Syndrome. Peptides 2003, 24, 937–943.
- Tailford, K.A.; Berry, C.L.; Thomas, A.C.; Campbell, J.H. A Casein Variant in Cow’s Milk Is Atherogenic. Atherosclerosis 2003, 170, 13–19.
- Kost, N.V.; Sokolov, O.Y.; Kurasova, O.B.; Dmitriev, A.D.; Tarakanova, J.N.; Gabaeva, M.V.; Zolotarev, Y.A.; Dadayan, A.K.; Grachev, S.A.; Korneeva, E.V.; et al. β-Casomorphins-7 in Infants on Different Type of Feeding and Different Levels of Psychomotor Development. Peptides 2009, 30, 1854–1860.
- Reichelt, K.L.; Tveiten Bioengineer, D.; Knivsberg, A.-M.; Brønstad, G. Peptides’ Role in Autism with Emphasis on Exorphins. Microb. Ecol. Health Dis. 2012, 23, 18958.
- Kaczmarski, M.; Wasilewska, J.; Lasota, M. Hypersensitivity to Hydrolyzed Cow’s Milk Protein Formula in Infants and Young Children with Atopic Eczema/Dermatitis Syndrome with Cow’s Milk Protein Allergy. Rocz. Akad. Med. Bialymst. 2005, 50, 274–278.
- Arvola, T.; Moilanen, E.; Vuento, R.; Isolauri, E. Weaning to Hypoallergenic Formula Improves Gut Barrier Function in Breast-Fed Infants with Atopic Eczema. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 92–96.
- Chatchatee, P.; Järvinen, K.-M.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE- and IgG-Binding Epitopes on As1-Casein: Differences in Patients with Persistent and Transient Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2001, 107, 379–383.
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent Bioactive Peptides in Milk Proteins: Proteolytic Activation and Significance in Dairy Processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239.
- Zoghbi, S.; Trompette, A.; Claustre, J.; Homsi, M.E.; Garzón, J.; Jourdan, G.; Scoazec, J.-Y.; Plaisancié, P. β-Casomorphin-7 Regulates the Secretion and Expression of Gastrointestinal Mucins through a μ-Opioid Pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1105–G1113.
- Olenski, K.; Kamiński, S.; Szyda, J.; Cieslinska, A. Polymorphism of the Beta-Casein Gene and Its Associations with Breeding Value for Production Traits of Holstein–Friesian Bulls. Livest. Sci. 2010, 131, 137–140.
- Nilsen, H.; Olsen, H.G.; Hayes, B.; Sehested, E.; Svendsen, M.; Nome, T.; Meuwissen, T.; Lien, S. Casein Haplotypes and Their Association with Milk Production Traits in Norwegian Red Cattle. Genet. Sel. Evol. 2009, 41, 24.
- Morris, C.A.; Hickey, S.M.; Cullen, N.G.; Prosser, C.G.; Anderson, R.M.; Tate, M.L. Associations between Β-casein Genotype and Milk Yield and Composition in Grazing Dairy Cows. N. Z. J. Agric. Res. 2005, 48, 441–450.
- Kearney, J.F.; Amer, P.R.; Villanueva, B. Cumulative Discounted Expressions of Sire Genotypes for the Complex Vertebral Malformation and β-Casein Loci in Commercial Dairy Herds. J. Dairy Sci. 2005, 88, 4426–4433.
More
