Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Alberto Distefano.
Thyroid eye disease (TED) is an inflammatory disease of orbital tissue characterized by infiltration of lymphocytic cells, orbital fat expansion, and extraocular muscle swelling. The gravity of thyroid eye disease lies in its sight-threatening, debilitating, and disfiguring potential. Despite extensive ongoing research about TED, the disorder remains elusive in its exact pathophysiology, prevention, and ideal treatment.
- thyroid eye disease
- thyroid-associated orbitopathy
- Grave’s disease
- Hashimoto’s thyroiditis
- chronic lymphocytic thyroiditis
- euthyroid eye disease
1. Clinical Evaluation
Ocular and systemic disease present simultaneously in 40% of Thyroid eye disease (TED) patients. Of those who develop TED, 85–90% are hyperthyroid, 5–6% are euthyroid, and 4% are hypothyroid at disease onset [1,2,3][1][2][3]. Typical presenting symptoms include dry eyes, red eyes, double vision, and pain with eye movement. The most common sign is eyelid retraction, followed by exophthalmos and eye motility limitation; additional findings include periorbital swelling and conjunctival injection. In severe cases, patients may present with vision loss secondary to ocular surface compromise and/or optic neuropathy [1].
Several grading scales are available to document TED severity and track inflammatory activity. The European Group on Graves’ Orbitopathy (EUGOGO) protocol and the VISION classification (V: vision, optic neuropathy; I: inflammation, congestion; S: strabismus, motility restriction; A: appearance, exposure) provide clinical grading. The NOSPECS (N: no signs or symptoms; O: only signs; S: soft-tissue involvement; P: proptosis; E: extraocular muscle involvement; C: corneal involvement; S: sight loss due to optic nerve compression) classification and Mourits system are useful for research purposes [1].
1.1. Mild Disease
TED may present with minimal findings that can easily go unrecognized or be misdiagnosed. Patients may report foreign body sensation, photophobia, or tearing, which are indicative of dry eyes secondary to exposure keratopathy (corneal disease) [3]. Examination may reveal mild periorbital swelling, proptosis <3 mm over the normal value for race and gender, eyelid retraction <2 mm, and conjunctival injection (Figure 1) [4,3][3][4].
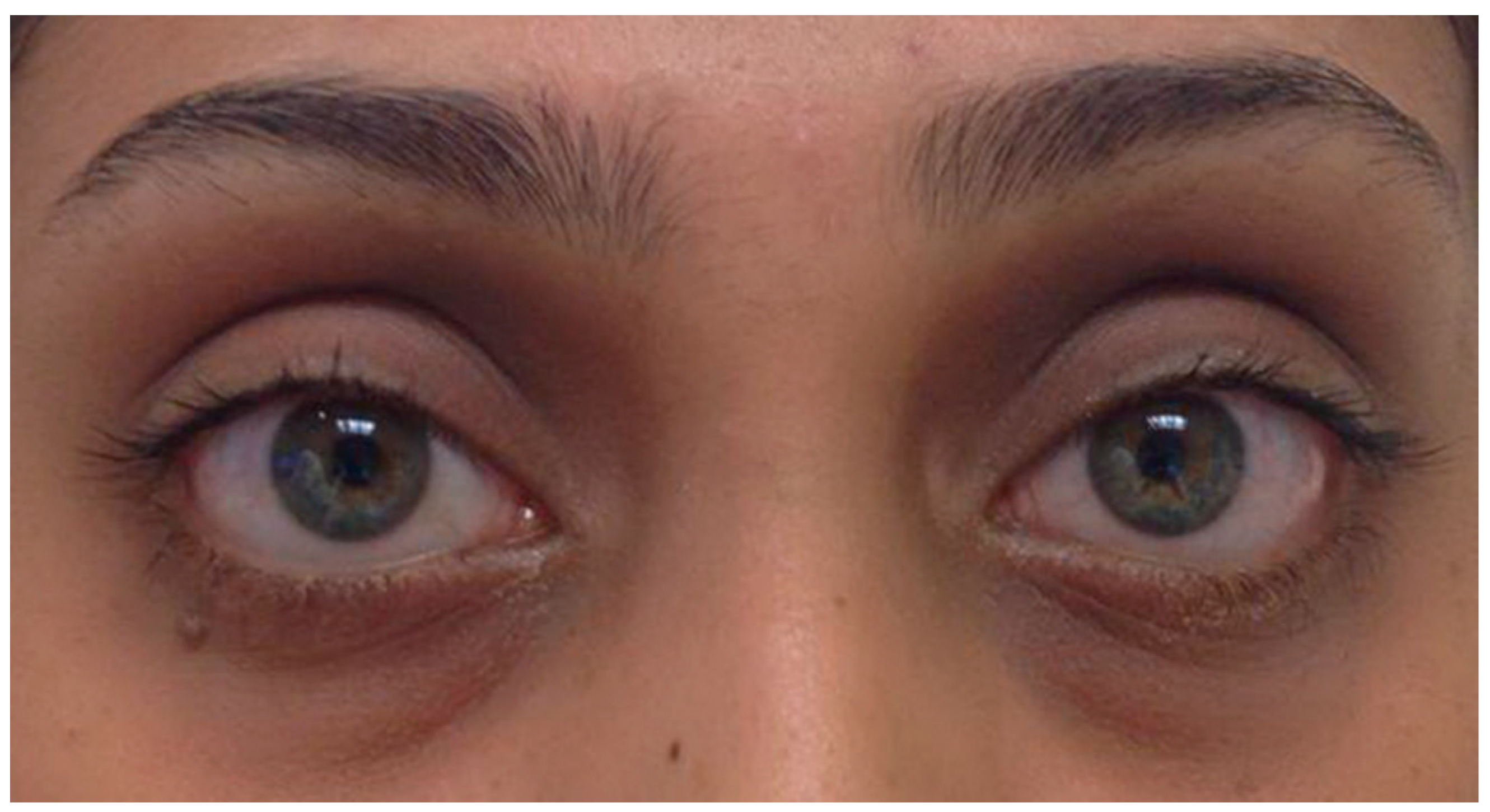
Figure 1. Mild Thyroid Eye Disease. External color photograph showing mild signs of bilateral lower eyelid retraction, lateral upper eyelid flare, periorbital edema, and trace conjunctival injection.
1.2. Moderate Disease
In moderate TED, patients have more apparent signs and symptoms. Orbital inflammation and congestion can lead to significant periorbital edema, conjunctival swelling and injection, corneal disease, pain with eye movements, diplopia, proptosis >2 mm, and eye motility restriction (Figure 2) [4,3][3][4].
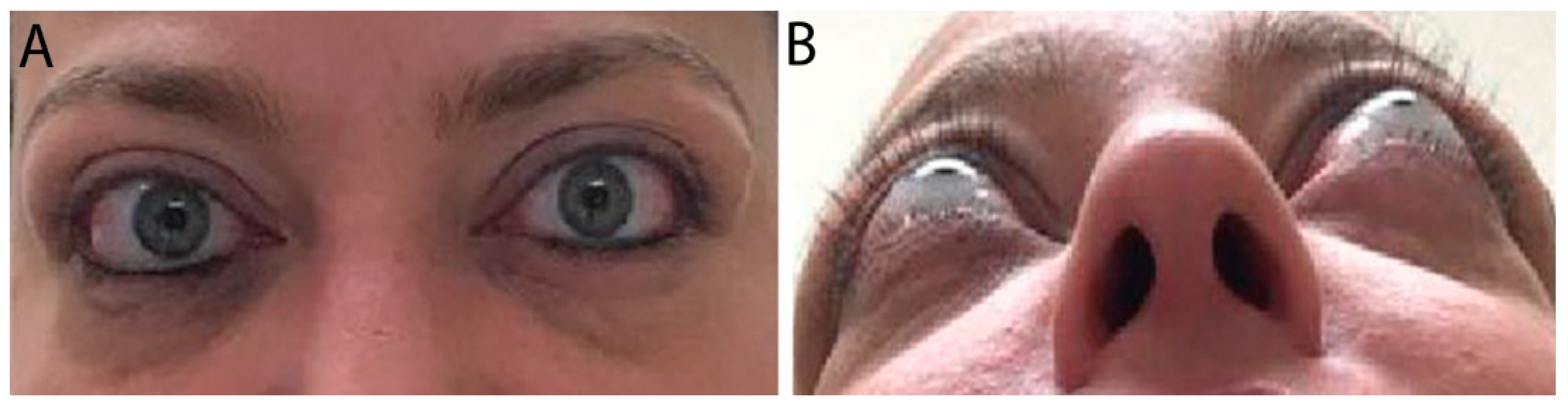
Figure 2. External color photograph of a patient with chronic moderate thyroid eye disease with (A) upper and lower eyelid retraction, lateral flare of the upper eyelids, and moderate periorbital fat protrusion. (B) Proptosis, best seen in “worm’s eye view” (chin-up position).
1.3. Severe Disease
Risk factors for severe TED include advanced age, male gender, and smoking [2]. Patients presenting with optic neuropathy and/or corneal breakdown require urgent referral to ophthalmology as these are definite indications for surgical decompression. Red flag symptoms include persistently blurred or decreased vision, scotomas, and decreased color vision. Examination may reveal lagophthalmos (incomplete eyelid closure), absent Bell’s phenomenon (upward movement of the eye during attempted eye closure which protects the cornea from exposure), relative afferent pupillary defect (if one eye is affected more than the other), corneal opacity, and decreased vision (Figure 3) [3]. Patients with optic neuropathy may not display dramatic proptosis, as orbital soft-tissue volume expansion within an enclosed area is more likely to lead to compression than when there is forward movement of the globe to provide more retrobulbar space [3,5][3][5].
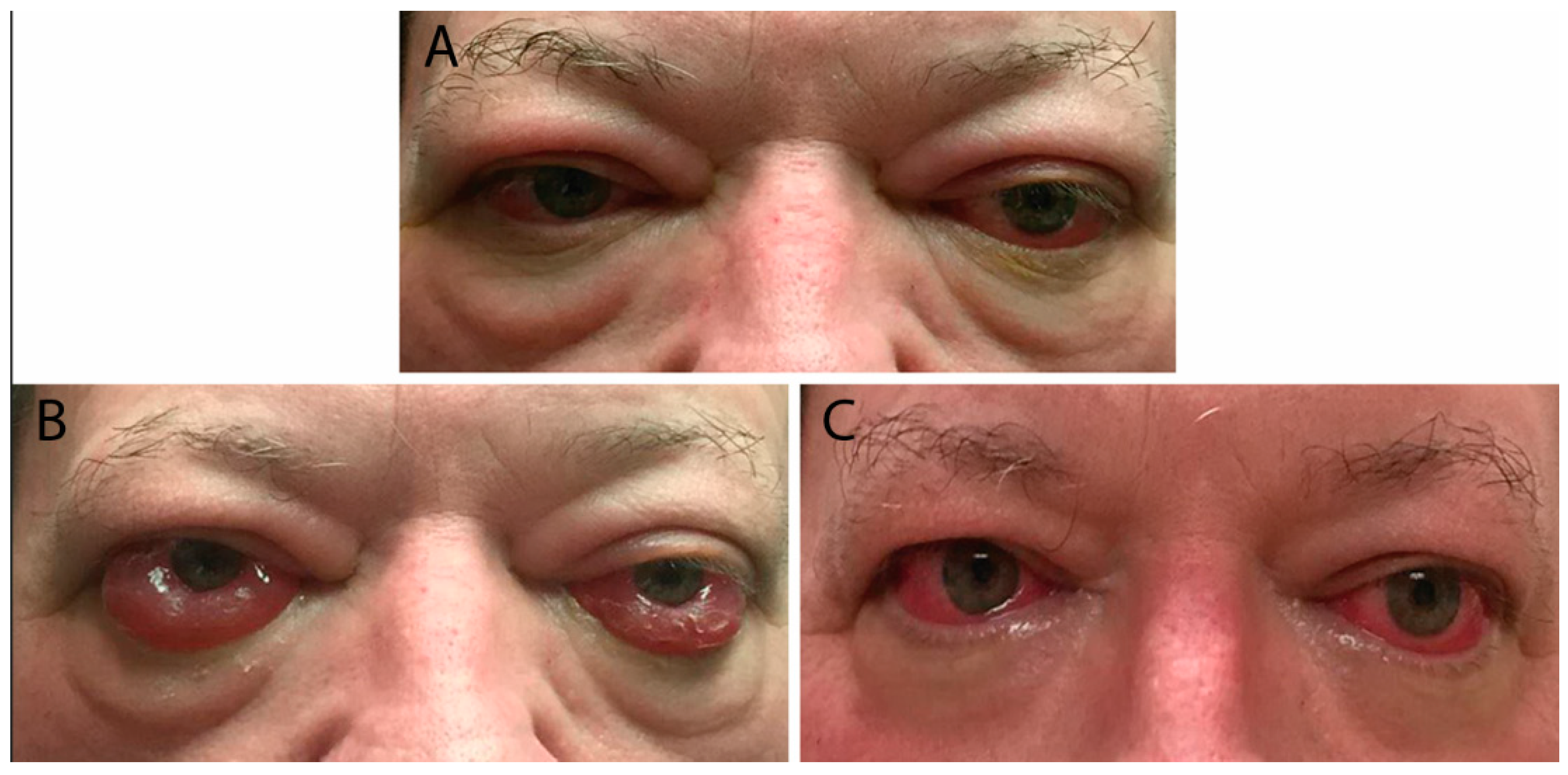
Figure 3. (A) External color photograph of patient with acute moderate thyroid eye disease beginning two months after radioactive iodine therapy. (B) One month later with progression to severe thyroid eye disease. Exam shows increased conjunctival injection and chemosis. Sixty milligrams of oral prednisone with adjunctive radiotherapy was started. (C) Significant improvement in conjunctival and periorbital edema after one month. Steroids were slowly tapered over eight months.
1.4. Natural Course
Although the natural course of TED can vary, disease typically follows an archetype known as Rundle’s curve. TED begins with an active inflammatory phase that rapidly progresses to maximally severe disease before improving to a stable, inactive plateau [1,2,3][1][2][3]. Onset of orbitopathy tends to coincide with development of Graves’ hyperthyroidism, occurring within 18 months of each other in 60–85% of patients [3] [3]. The self-limited disease course is unique compared to other autoimmune disorders and may be ascribed to the absence of lymphoid tissue in the orbit [1]. There may be slow improvement after months to years of inactive, plateaued disease. Nevertheless, most patients with moderate-to-severe TED do not achieve complete recovery back to normal, with proptosis being the least likely manifestation to resolve [3].
2. Diagnosis
2.1. Differential
TED can present in innumerable ways, warranting careful clinical evaluation and targeted ancillary testing based on each individual patient’s constellation of signs and symptoms. The most common cause of both unilateral and bilateral proptosis in adults is TED [6,7][6][7]. When patients present with proptosis, the differential also includes primary and secondary orbital tumors, carotid-cavernous fistula, unilateral myopia, orbital pseudotumor, orbital myositis, and systemic inflammatory disease [3,7][3][7]. Binocular diplopia carries a long list of differential diagnoses, the most relevant of which are myasthenia gravis (characteristically variable diplopia associated with ptosis), orbital tumors, orbital myositis, and orbital pseudotumor in patients with TED. Upper eyelid retraction can develop in midbrain neurological disease, and lower eyelid retraction can occur along with decreased ocular motility in chronic progressive extranuclear ophthalmoplegia [3]. Restrictive limitations in extraocular motility are associated with orbital fracture, Brown syndrome, Duane syndrome, and congenital fibrosis of the extraocular muscles [8]. When TED is suspected, complete work up encompasses multiple investigatory modalities. Diagnosis is based on ophthalmic signs and symptoms on physical examination, presence of thyroid autoimmunity on laboratory testing, and exclusion of an alternative diagnosis [4].
2.2. Laboratory Testing
Serologic work up should assess thyroid function and thyroid autoimmunity. Thyroid testing includes thyroid-stimulating hormone (TSH) and free T4; if both are normal but there is a strong suspicion for TED, free T3 can be considered. Antibody testing must look for antithyroid peroxidase (TPO), antithyroid-stimulating immunoglobulins (TSI), TSHR-stimulating antibodies (TSAb), and thyroid-binding inhibitory immunoglobulins (TBIIs) [1,4][1][4]. TSI levels correlate to TED inflammation and can serve as a marker for disease activity and prognosis. Free T3 and TPO levels have also been correlated with active inflammation [4].
2.3. Imaging
Orbital imaging is a helpful component of TED evaluation, as 90% of patients with Graves’ disease have abnormalities in imaging studies [9]. Methods to visualize and monitor disease activity in TED include orbital ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), somatostatin receptor scintigraphy with radio-labeled somatostatin analog (Octreotide, octreoscan), and optical coherence tomography (OCT) [7].
2.3.1. Orbital Ultrasound
Ultrasound is a cost-effective screening modality that can detect extraocular muscle enlargement, though is less sensitive than CT and MRI. There are two types: the A-scan can identify enlargement greater than the 95th percentile; and the B-scan visualizes TED signs such as posterior scalloped orbital fat secondary to muscle enlargement, widening of the echo-free area between orbital fat and bone, and optic nerve sheath “doubling” in optic neuropathy. Advantages of orbital ultrasound include no patient exposure to radiation, short investigation time, and accessibility in ophthalmology clinics. Nevertheless, orbital ultrasound is rarely used to detect and monitor TED given the availability and improved accuracy of CT and MRI [7].
2.3.2. Computed Tomography
CT provides superior bone resolution and is essential to orbital surgeons when planning decompression surgery [1,7,9][1][7][9]. Additionally, it is readily available with short study duration, accurately assesses volume of orbital tissues, and provides precise imaging of the orbital apex and sinuses. Disadvantages include patient radiation exposure and inability to monitor disease activity. Radiographic signs suggestive of TED include extraocular muscle enlargement sparing tendon insertions, slight bowing of the medial orbital wall (“Coca-Cola sign”), optic nerve compression by enlarged extraocular muscles, fat prolapse with optic nerve compression, and absence of orbital masses, vascular enlargement, and sinus involvement (Figure 4). The inferior rectus is affected most commonly, and rarely, the lacrimal gland may be enlarged [7]. Orbital CT is required for preoperative assessment for orbital decompression surgery [9] [9].
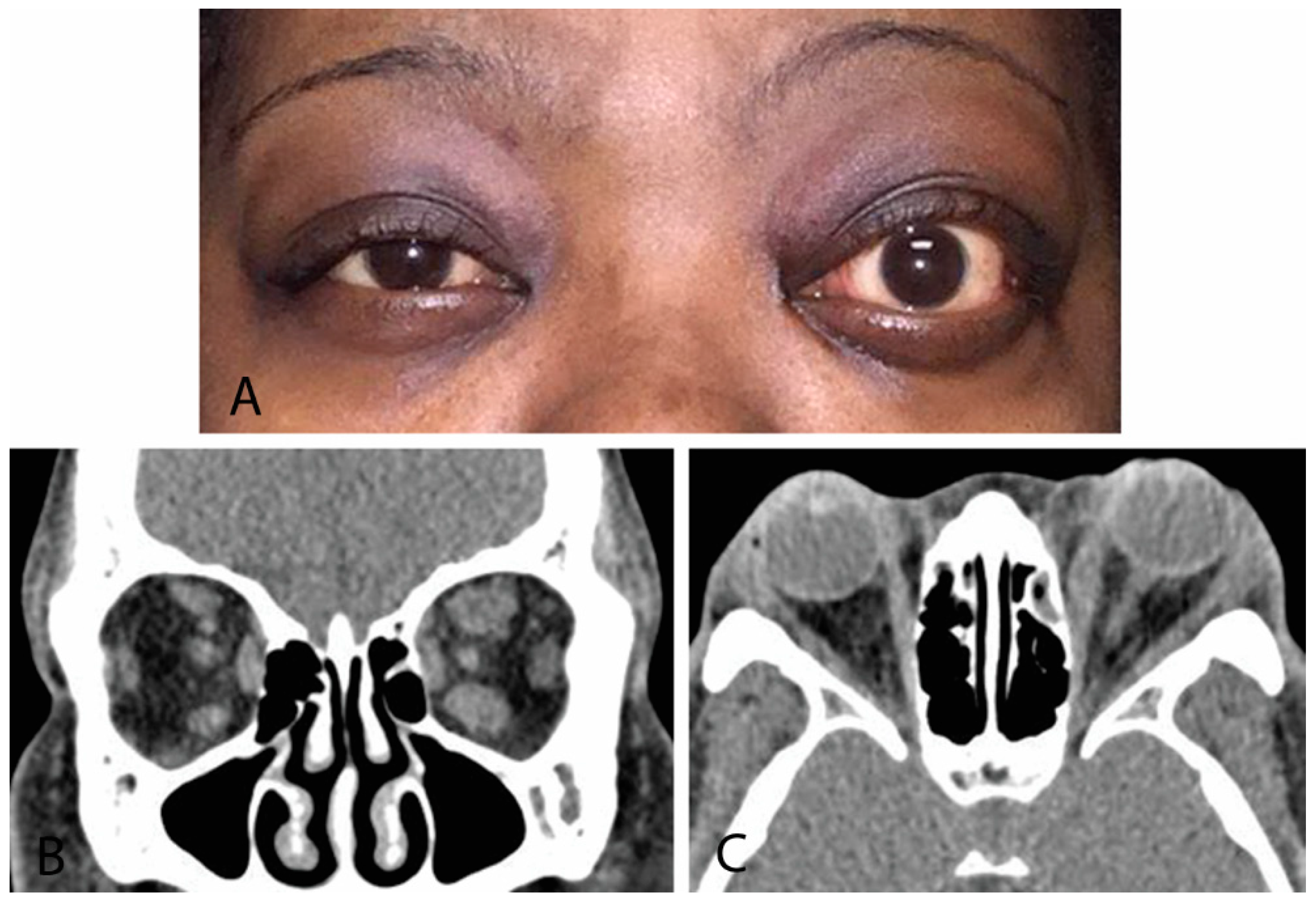
Figure 4. (A) External color photograph of chronic moderate thyroid eye disease with asymmetric proptosis on left more than right. (B) Coronal CT of orbits with thickening of recti muscles on left more than right. (C) Sagittal cut of same CT scan.
2.3.3. Magnetic Resonance Imaging
MRI with gadolinium contrast and fat suppression is the modality of choice for identifying and monitoring active disease [1,7][1][7]. It provides high-resolution images that enable orbital soft-tissue differentiation and the ability to detect changes in water content (interstitial edema) within extraocular muscles and orbital fat expansion without radiation exposure (Figure 5). MRI should be used to assess whether vision loss in TED is secondary to optic nerve compression or a noncompressive etiology and to differentiate inflammatory from fibrotic tissue [7,9][7][9]. Serial orbital MRI investigations can be used to monitor response to treatment and activity of orbital disease [9]. Barriers to MRI include relatively high cost, greater length of study time, and potential claustrophobia. It is less helpful than CT for bony structure evaluation [7].
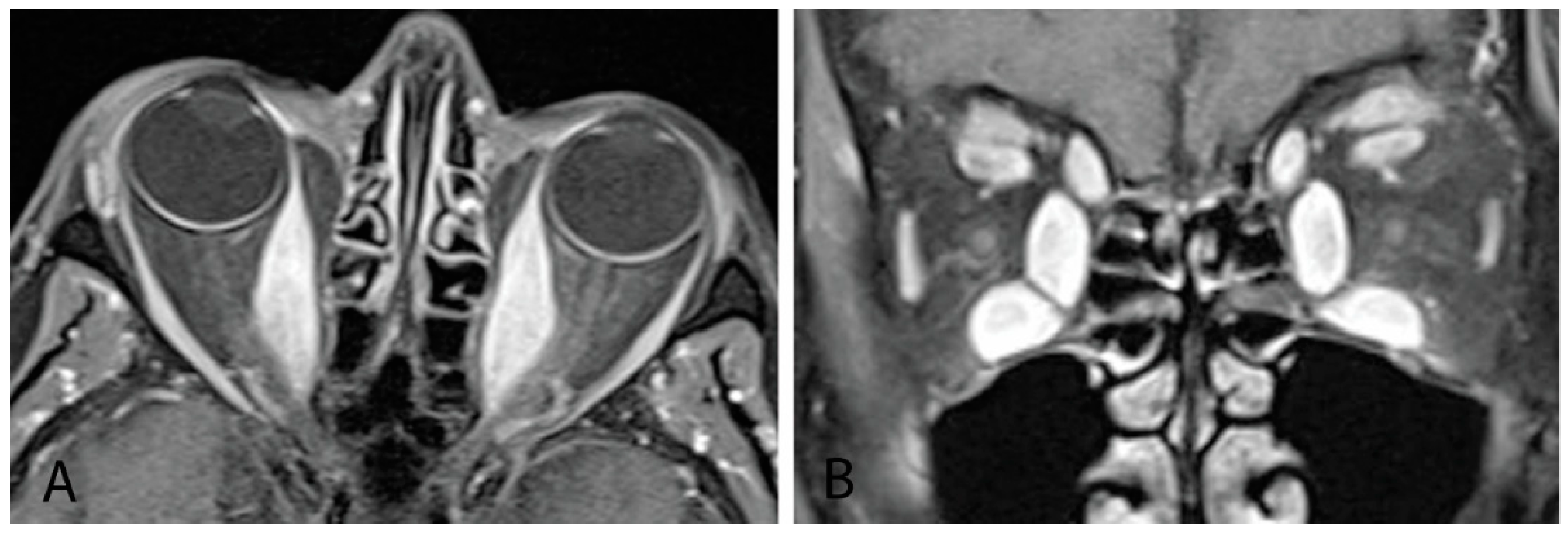
Figure 5. (A) MRI orbits with gadolinium with sagittal cut showing thickening of bilateral medial recti muscles. (B) Coronal cut from same patient showing thickening of all bilateral inferior, medial, and superior recti muscles, as well as levator palpebrae superioris and superior oblique muscles.
2.3.4. Octreoscan
Octreoscan, also known as somatostatin receptor scintigraphy, uses octreotide, a radio-labeled somatostatin analog, to detect inflammation through accumulation associated with lymphocytes, myoblasts, fibroblasts, endothelial cells, or localized blood pooling from inflammation-induced venous stasis. It is highly sensitive for detecting inflammation of orbital tissue, and it can be used to predict response to immunosuppressive therapy and monitor disease activity in TED. However, it poses significant disadvantages, including whole-body radiation exposure, lack of morphologic assessment, and significant cost. Octreoscan is mainly reserved for cases in which disease activity is unclear from clinical evaluation and MRI is not available [7].
2.3.5. Optical Coherence Tomography (OCT)
OCT is a noninvasive imaging technique routinely used in ophthalmology, particularly in glaucoma and neuro-ophthalmic settings. Recently, changes in the retinal nerve fiber and ganglion cell layers have been studied in TED. Subclinical thinning has been noted, which can be important for disease monitoring and treatment [10]. Optical coherence tomography angiography (OCT-A) has also been used to study peripapillary and macular vessel density changes, which can further provide subclinical information on disease progression [11]. Lastly, subfoveal choroidal thickness has been correlated with thyroid eye disease activity and is currently under further review for its potential role in disease prognostication [12]. It is important to note that the above OCT changes are not specific to thyroid eye disease and may be confounded by other ocular diseases.
2.4. Biopsy
Most cases of TED in patients with unknown thyroid dysfunction can be diagnosed without surgical biopsy. However, suspicion of malignancy serves as a potential indication for extraocular muscle or orbital soft-tissue biopsy [3]. The main histologic features of TED include normal extraocular muscle fibers separated by abnormally wide spaces of connective tissue and hydrophilic extracellular matrix materials, T lymphocytes, and macrophages. There may be focal accumulations of B lymphocytes and natural killer cells. After chronic compression, muscle fibers display a fibrotic, atrophic appearance [5].
3. Additional Considerations
3.1. Pediatric Thyroid Eye Disease
3.1.1. Epidemiology
Although the risk of developing TED in children is comparable to that of adults, disease severity is characteristically mild and regresses after restoring euthyroidism [4,13][4][13]. Pediatric TED typically presents in Graves’ disease but may develop in Hashimoto’s thyroiditis. The overall incidence is 0.79–6.5 per 100,000 children, occurring at a rate of 1.7–3.5 per 100,000 per year. TED in children is more common among girls than boys and occurs more frequently in the adolescent population (11–18 years old; 68.2%) than in children younger than 11 years old (31.8%). The latter trend may be related to increased smoking among adolescents. Even passive smoking appears to impose risk, as evidenced by a higher incidence of TED in children younger than 11 years old in countries where tobacco use is higher [4].
3.1.2. Clinical Considerations
Pediatric TED usually manifests with signs and symptoms similar to those of adult TED but is milder and tends to regress after systemic treatment. The most common symptoms in children are pain, foreign body sensation, photophobia, and excessive tearing. Diplopia is sometimes reported. The most common physical examination findings include upper eyelid lag in downgaze (Graefe sign), upper eyelid retraction, and proptosis. With puberty, rates of more serious consequences, such as extraocular muscle restriction and ocular surface compromise secondary to exposure, occur [4]. Studies that have investigated TED disease activity after RAI, drug therapy, or subtotal thyroidectomy in children found improvement in 90%, 73%, and 75%, respectively [13]. More severe complications, such as eye motility restriction, tend to improve more often than proptosis, which may be attributed to the normal increase in exophthalmometry (measurement of eye protrusion) with age [4].
Long-term sequelae of pediatric TED include an association with refractive changes and a potential for permanent poor vision. TED increases the risk of developing myopia and astigmatism in children. Additionally, patients who are already near-sighted experience higher rates of myopic progression [4]. Amblyopia can develop if the visual pathway does not fully develop during childhood. Causes in TED include sensory deprivation from ocular surface compromise, uncorrected changes in refractive error, and restrictive strabismus.
3.1.3. Management
Clinicians can use the same physical examination, laboratory markers, and disease classification systems to evaluate TED activity and severity in children [4].
3.2. Pregnancy
While it is well established that the systemic manifestations of Graves’ hyperthyroidism improve during pregnancy and rebound in the postpartum period, there remains a dearth of rigorous evaluation of TED activity during pregnancy and in the postpartum period [6,14][6][14]. If TED is linked to systemic autoimmunity, the natural course of TED should reflect the same improvement and subsequent rebound activity. However, conclusions from one retrospective study suggest this is not the case. TED activity varied considerably in seven patients with Graves’ hyperthyroidism, who had ophthalmopathy and/or upper eyelid retraction, and twelve Hashimoto’s thyroiditis patients, who manifested with only upper eyelid retraction. Approximately 70% of all patients experienced improvement or no change in TED severity while 30% developed increased severity during pregnancy. The authors conclude systemic thyroid autoimmunity and TED are affected differently by pregnancy, though this is limited by small study sample numbers, assessment of only mild-to-moderate TED, and lack of subgroup analysis based on confounding factors such as active or passive smoking and genetic factors [14]. One case reported onset and worsening of Graves’ TED during pregnancy despite treatment initiation. On postpartum day one, the patient experienced complete resolution of eyelid retraction, pain with eye movements, and diplopia. The authors hypothesize TED may worsen during pregnancy secondary to generalized hypervolemia and abruptly improve after significantly decreasing body fluid volume with delivery [15]. More rigorous investigation is warranted to fully explore the effect of pregnancy on TED activity to aid in patient counseling and clinical management.
References
- Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves' or-bitopathy. N Engl J Med. 2011;364(20):1920-31.McAlinden, C. An overview of thyroid eye disease. Eye Vis. 2014, 1, 9.
- Wall JR, Lahooti H, Hibbert EJ, Champion B. Relationship between Clinical and Immunological Features of Thyroid Au-toimmunity and Ophthalmopathy during Pregnancy. J Thyroid Res. 2015;2015:698470.Stan, M.N. Natural History, Risk Factors, and Management of Patients with Mild GO. In Graves’ Disease—A Comprehensive Guide for Clinicians; Bahn, R.S., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 241–255.
- Abbouda A, Trimboli P, Bruscolini A. A mild Grave's ophthalmopathy during pregnancy. Semin Ophthalmol. 2014;29(1):8-10.Perros, P.; Dickinson, A.J.; Kendall-Taylor, P. Clinical Presentation and Natural History of Graves’ Ophthalmopathy. In Thyroid Eye Disease; Bahn, R.S., Ed.; Springer Science+Business Media: New York, NY, USA, 2001; pp. 119–136.
- Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the Eu-ropean group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008;18(3):333-46.Szczapa-Jagustyn, J.; Gotz-Wieckowska, A.; Kociecki, J. An update on thyroid-associated ophthalmopathy in children and adolescents. J. Pediatr. Endocrinol. Metab. 2016, 29, 1115–1122.
- Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (Lond). 2007;21(9):1135-45.Yamada, M.; Li, A.W.; Wall, J.R. Thyroid-associated ophthalmopathy: Clinical features, pathogenesis, and management. Crit. Rev. Clin. Lab. Sci. 2000, 37, 523–549.
- Graves' Disease--A Comprehensive Guide for Clinicians. Bahn RS, editor. New York: Springer Science+Business Media; 2015. 167-78 p.Stagnaro-Green, A. Graves’ Disease and Pregnancy. In Graves’ Disease—A Comprehensive Guide for Clinicians; Bahn, R.S., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 167–178.
- Thyroid Eye Disease. Bahn RS, editor. New York: Springer Science+Business Media; 2001. 137-57 p.Kahaly, G.J.; Muller-Forell, W.; Forster, G.J.; Pitz, S.; Rosier, H.P.; Mann, W.J. Imaging in Graves’ Ophthalmopathy. In Thyroid Eye Disease; Bahn, R.S., Ed.; Springer Science+Business Media: New York, NY, USA, 2001; pp. 137–162.
- Merino P, de Liano PG, Ruiz Y, Franco G. Atypical restrictive strabismus secondary to an anomalous orbital structure: differential diagnosis. Strabismus. 2012;20(4):162-5.Merino, P.; de Liano, P.G.; Ruiz, Y.; Franco, G. Atypical restrictive strabismus secondary to an anomalous orbital structure: Differential diagnosis. Strabismus 2012, 20, 162–165.
- Muller-Forell W, Kahaly GJ. Neuroimaging of Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):259-71.Muller-Forell, W.; Kahaly, G.J. Neuroimaging of Graves’ orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 259–271.
- Graves' Disease--A Comprehensive Guide for Clinicians. Bahn RS, editor. New York: Springer Science+Business Media; 2015. 155 p.Guo, J.; Li, X.; Ma, R.; Gan, L.; Qian, J. The changes of retinal nerve fibre layer and ganglion cell layer with different severity of thyroid eye disease. Eye 2022, 36, 129–134.
- Wall JR, Lahooti H, Hibbert EJ, Champion B. Relationship between Clinical and Immunological Features of Thyroid Au-toimmunity and Ophthalmopathy during Pregnancy. J Thyroid Res. 2015;2015:698470.Jamshidian Tehrani, M.; Mahdizad, Z.; Kasaei, A.; Fard, M.A. Early macular and peripapillary vasculature dropout in active thyroid eye disease. Graefes. Arch. Clin. Exp. Ophthalmol. 2019, 257, 2533–2540.
- Abbouda A, Trimboli P, Bruscolini A. A mild Grave's ophthalmopathy during pregnancy. Semin Ophthalmol. 2014;29(1):8-10.Kurt, M.M.; Akpolat, C.; Evliyaoglu, F.; Yilmaz, M.; Ordulu, F. Evaluation of Retinal Neurodegeneration and Choroidal Thickness in Patients with Inactive Graves’ Ophthalmopathy. Klin. Monbl. Augenheilkd. 2021, 238, 797–802.
- Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the Eu-ropean group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008;18(3):333-46.Rivkees, S.A. Graves’ Disease in Childhood. In Graves’ Disease—A Comprehensive Guide for Clinicians; Bahn, R.S., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 147–166.
- Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (Lond). 2007;21(9):1135-45.Wall, J.R.; Lahooti, H.; Hibbert, E.J.; Champion, B. Relationship between Clinical and Immunological Features of Thyroid Autoimmunity and Ophthalmopathy during Pregnancy. J. Thyroid Res. 2015, 2015, 698470.
- Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, et al. Selenium and the course of mild Graves' or-bitopathy. N Engl J Med. 2011;364(20):1920-31.Abbouda, A.; Trimboli, P.; Bruscolini, A. A mild Grave’s ophthalmopathy during pregnancy. Semin Ophthalmol. 2014, 29, 8–10.
More
