Gene immunization comprises mRNA and DNA vaccines, which stand out due to their simple design, maintenance, and high efficacy. Several studies indicate promising results in preclinical and clinical trials regarding immunization against ebola, human immunodeficiency virus (HIV), influenza, and human papillomavirus (HPV). The efficiency of nucleic acid vaccines has been highlighted in the fight against COVID-19 with unprecedented approval of their use in humans.
- vaccines
- nucleic acids
- synthetic genes
1. Introduction
Throughout history, we have experienced how several disease outbreaks have caused health risks, many of them with pandemic potential, culminating in the deaths of millions of people worldwide. The emergence of new diseases accompanied by population growth and globalization indicates the need to obtain new tools capable of reducing the transmission of infectious agents and the risk of future pandemics [1,2][1][2]. In this context, vaccines represent a valuable measure for maintaining global health, offering protection, and contributing to the control and combat of several pathogens that threaten human and veterinary health [3]. More than two centuries after the creation of the first vaccine, the field of vaccinology has promoted the improvement of classic immunization techniques. These approaches include use of attenuated or inactivated pathogens, or even toxoids, and the creation and application of new strategies, such as live vectors and nucleic acids [4].
As seen in recent epidemic outbreaks, future pandemics are likely to require the continued development of new models and approaches for designing nucleic acid vaccines. Particularly with viral infections, there is a demand for rapid production and updating of vaccine platforms. These improvements are essential not only for diseases not controlled through vaccination, but also in the context of the appearance of mutations that lead to the emergence of variants or the establishment of new serotypes. In addition, it is also essential to invest in the vaccine targets’ presentation, which can be whole genes or constructs based on epitopes predicted in silico, besides the development of adjuvants and immunomodulators [6,7][5][6]. Therefore, this research field is continuously expanding, especially regarding third-generation vaccines.
2. New Technologies: Gene-bBased Vaccines
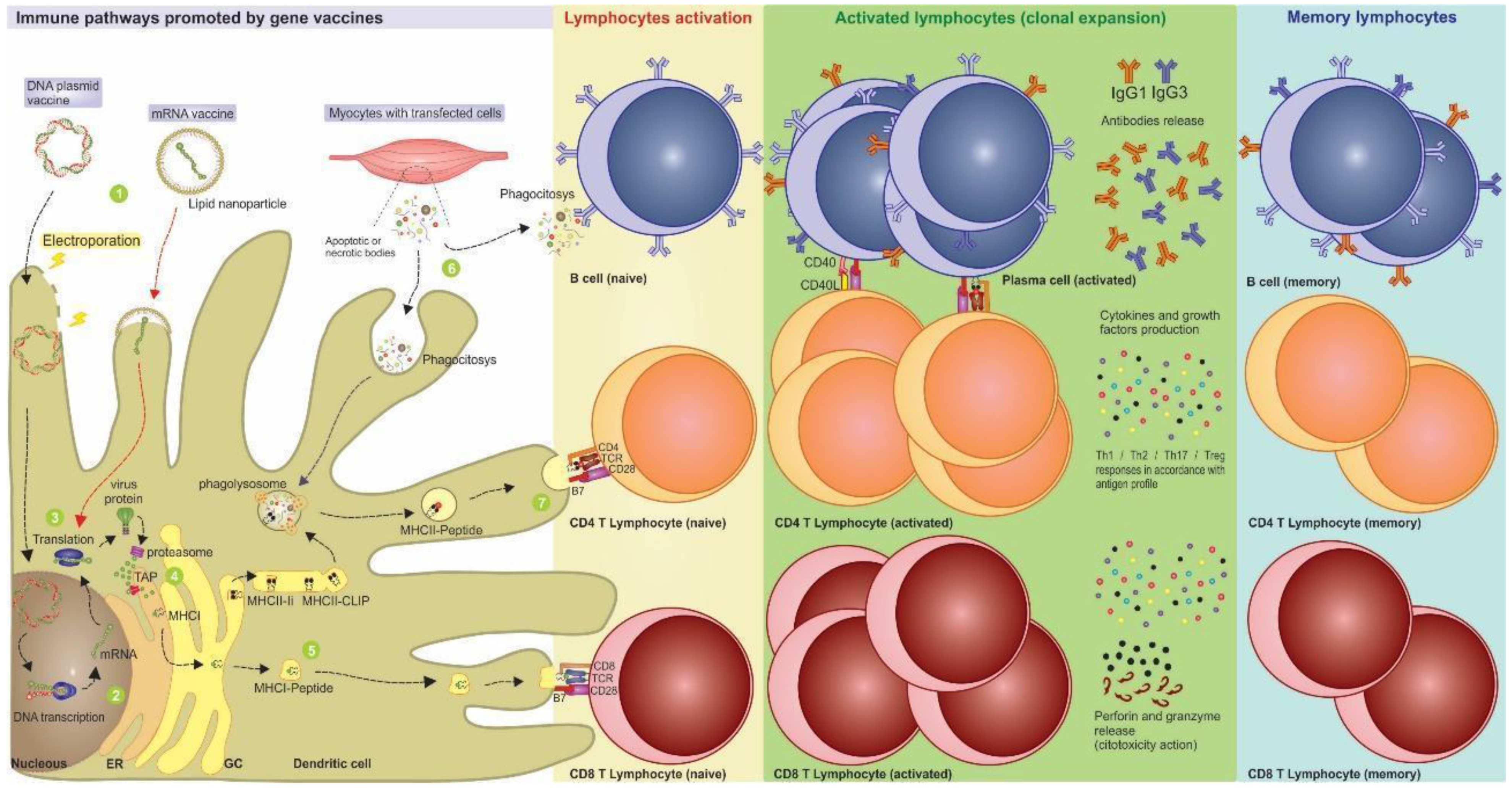
3. Nucleic Acid Vaccines Allow Better Immune Response Directing
References
- Domínguez-Andrés, J.; van Crevel, R.; Divangahi, M.; Netea, M.G. Designing the Next Generation of Vaccines: Relevance for Future Pandemics. mBio 2020, 11, e02616–e02620.
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544.
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11.
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, 231–241.
- Rappuoli, R.; De Gregorio, E.; Del Giudice, G.; Phogat, S.; Pecetta, S.; Pizza, M.; Hanon, E. Vaccinology in the Post−COVID-19 Era. Proc. Natl. Acad. Sci. USA 2021, 118, e2020368118.
- González-Romo, F.; Picazo, J.J. El desarrollo de nuevas vacunas. Enferm. Infecc. Y Microbiol. Clínica 2015, 33, 557–568.
- Carter, C.; Houser, K.V.; Yamshchikov, G.V.; Bellamy, A.R.; May, J.; Enama, M.E.; Sarwar, U.; Larkin, B.; Bailer, R.T.; Koup, R.; et al. Safety and Immunogenicity of Investigational Seasonal Influenza Hemagglutinin DNA Vaccine Followed by Trivalent Inactivated Vaccine Administered Intradermally or Intramuscularly in Healthy Adults: An Open-Label Randomized Phase 1 Clinical Trial. PLoS ONE 2019, 14, e0222178.
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of MRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854.
- Tombácz, I.; Weissman, D.; Pardi, N. Vaccination with Messenger RNA: A Promising Alternative to DNA Vaccination. In DNA Vaccines; Sousa, Â., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2197, pp. 13–31. ISBN 978-1-07-160871-5.
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of MRNA-Based Vaccines. Pharmaceutics 2020, 12, 102.
- Sheets, R.L.; Stein, J.; Manetz, T.S.; Duffy, C.; Nason, M.; Andrews, C.; Kong, W.-P.; Nabel, G.J.; Gomez, P.L. Biodistribution of DNA Plasmid Vaccines against HIV-1, Ebola, Severe Acute Respiratory Syndrome, or West Nile Virus Is Similar, without Integration, despite Differing Plasmid Backbones or Gene Inserts. Toxicol. Sci. 2006, 91, 610–619.
- Wang, J.-C.E.; Livingstone, A.M. Cutting Edge: CD4+ T Cell Help Can Be Essential for Primary CD8 + T Cell Responses In Vivo. J. Immunol. 2003, 171, 6339–6343.
- Lee, S.-H.; Danishmalik, S.N.; Sin, J.-I. DNA Vaccines, Electroporation and Their Applications in Cancer Treatment. Hum. Vaccines Immunother. 2015, 11, 1889–1900.
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. MRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931.
- Rittig, S.M.; Haentschel, M.; Weimer, K.J.; Heine, A.; Muller, M.R.; Brugger, W.; Horger, M.S.; Maksimovic, O.; Stenzl, A.; Hoerr, I.; et al. Intradermal Vaccinations With RNA Coding for TAA Generate CD8+ and CD4+ Immune Responses and Induce Clinical Benefit in Vaccinated Patients. Mol. Ther. 2011, 19, 990–999.
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. MRNA Vaccine–Induced Neoantigen-Specific T Cell Immunity in Patients with Gastrointestinal Cancer. J. Clin. Investig. 2020, 130, 5976–5988.
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet 2015, 386, 2078–2088.
- Kim, T.J.; Jin, H.-T.; Hur, S.-Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.-W.; Kim, S.; Woo, J.-W.; Park, K.S.; et al. Clearance of Persistent HPV Infection and Cervical Lesion by Therapeutic DNA Vaccine in CIN3 Patients. Nat. Commun. 2014, 5, 5317.
- Houseley, J.; Tollervey, D. The Many Pathways of RNA Degradation. Cell 2009, 136, 763–776.
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, Safety, and Immunogenicity of the DNA SARS-CoV-2 Vaccine (ZyCoV-D): The Interim Efficacy Results of a Phase 3, Randomised, Double-Blind, Placebo-Controlled Study in India. Lancet 2022, 399, 1313–1321.
- van de Wall, S.; Ljungberg, K.; Ip, P.P.; Boerma, A.; Knudsen, M.L.; Nijman, H.W.; Liljeström, P.; Daemen, T. Potent Therapeutic Efficacy of an Alphavirus Replicon DNA Vaccine Expressing Human Papilloma Virus E6 and E7 Antigens. OncoImmunology 2018, 7, e1487913.
- Ljungberg, K.; Liljeström, P. Self-Replicating Alphavirus RNA Vaccines. Expert Rev. Vaccines 2015, 14, 177–194.
- Le, T.P.; Coonan, K.M.; Hedstrom, R.C.; Charoenvit, Y.; Sedegah, M.; Epstein, J.E.; Kumar, S.; Wang, R.; Doolan, D.L.; Maguire, J.D.; et al. Safety, Tolerability and Humoral Immune Responses after Intramuscular Administration of a Malaria DNA Vaccine to Healthy Adult Volunteers. Vaccine 2000, 18, 1893–1901.
- Palucka, K.; Banchereau, J. Cancer Immunotherapy via Dendritic Cells. Nat. Rev. Cancer 2012, 12, 265–277.
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA Nanoparticles Generate Protective Immunity against Lethal Ebola, H1N1 Influenza, and Toxoplasma Gondii Challenges with a Single Dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142.
- Stitz, L.; Vogel, A.; Schnee, M.; Voss, D.; Rauch, S.; Mutzke, T.; Ketterer, T.; Kramps, T.; Petsch, B. A Thermostable Messenger RNA Based Vaccine against Rabies. PLoS Negl. Trop. Dis. 2017, 11, e0006108.
- Liu A Comparison of Plasmid DNA and MRNA as Vaccine Technologies. Vaccines 2019, 7, 37.
- Hollister, K.; Chen, Y.; Wang, S.; Wu, H.; Mondal, A.; Clegg, N.; Lu, S.; Dent, A. The Role of Follicular Helper T Cells and the Germinal Center in HIV-1 Gp120 DNA Prime and Gp120 Protein Boost Vaccination. Hum. Vaccines Immunother. 2014, 10, 1985–1992.
- Holdsworth, S.R.; Kitching, A.R.; Tipping, P.G. Th1 and Th2 T Helper Cell Subsets Affect Patterns of Injury and Outcomes in Glomerulonephritis. Kidney Int. 1999, 55, 1198–1216.
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.-Y.; Mitzner, W.; et al. Lack of Antibody Affinity Maturation Due to Poor Toll-like Receptor Stimulation Leads to Enhanced Respiratory Syncytial Virus Disease. Nat. Med. 2009, 15, 34–41.
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-Dependent Enhancement and SARS-CoV-2 Vaccines and Therapies. Nat. Microbiol. 2020, 5, 1185–1191.
