Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Ruixia Chu.
The booming electric vehicle industry continues to place higher requirements on power batteries related to economic-cost, power density and safety. The positive electrode materials play an important role in the energy storage performance of the battery. The nickel-rich NCM (LiNixCoyMnzO2 with x + y + z = 1) materials have received increasing attention due to their high energy density, which can satisfy the demand of commercial-grade power batteries. Prominently, single-crystal nickel-rich electrodes with s unique micron-scale single-crystal structure possess excellent electrochemical and mechanical performance, even when tested at high rates, high cut-off voltages and high temperatures.
- single-crystal
- nickel-rich NCM materials
- cathode
1. Introduction
Since the economic crisis in 2008, the global energy crisis and environmental pressures are becoming increasingly serious. In order to improve industrial competitiveness, and maintain sustainable economic and social development, the major automobile producing countries (e.g., the United States, Germany, Japan) have adopted the development of electric vehicles as a major strategy [1]. At present, the electric vehicle industry is one of the strategic emerging industries pursued by many countries [2,3][2][3]. After decades of development, ever-increasing requirements for energy storage devices have been identified, such as, higher energy density, better safety and longer service life, etc. [4,5][4][5]. However, realization of these goals mainly depends on the cathode material in the power batteries [6,7,8][6][7][8].
Ternary layered transition metal oxide, LiNixCoyMnzO2 (NCM, x + y + z = 1), was first proposed by J. R. Dahn’s group in 2001 [9]. According to the report, Li[NixCo1−2xMnx]O2 with x = 1/4 or 3/8 could be prepared by the ‘‘mixed hydroxide’’ method which combines a two-step calcination progress. The obtained samples have a layered α-NaFeO2-type structure, and deliver a stable capacity above 150 mAh g−1 at the current density of 40 mA g−1 in the voltage range of 2.5–4.4 V vs. Li. In particular, the capacity retention behavior of Li[Ni3/8Co1/4Mn1/4]O2 was close to that of LiCoO2 in the same potential window (2.5–4.2 V) and the thermal stability was better. As mentioned above, NCM is based on the hexagonal crystal system of the α-NaFeO2-type layered structure, which belongs to the R m space group and can be regarded as a solid solution of three compounds: LiCoO2, LiNiO2 and LiMnO2. The layered structure and compositional phase diagram of an NCM cathode are shown as Figure 1. the NCM cathode combines the advantages of LiCoO2, LiNiO2 and LiMnO2, exhibiting high operating voltage, large energy density and relatively good cycling performance [10,11,12,13,14,15,16][10][11][12][13][14][15][16]. In 2021, the Ministry of Industry and Information Technology of the People’s Republic of China officially released the “Lithium-ion Battery Industry Specification Conditions (2021)”, which states that the energy density of ternary materials-based batteries should not be lower than 210 Wh kg−1, the energy density of battery packs should not less than 150 Wh kg−1, and the specific capacity of ternary materials cathode should not less than 165 Ah kg−1. There has been some research investigating how to improve the stability, safety, and meanwhile reduce the cost of NCM material while ensuring its high energy density [15,17,18][15][17][18].
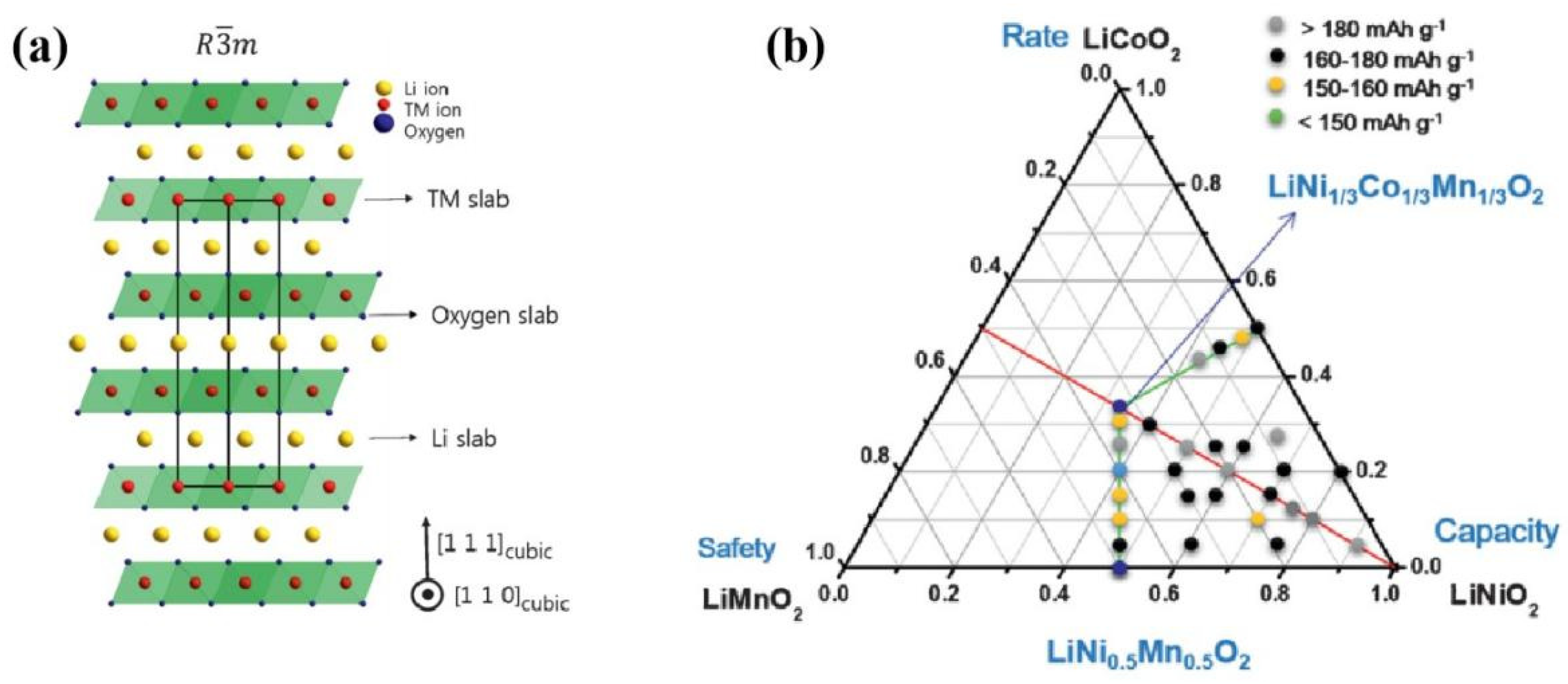
Figure 1. Illustration of the ordered layer structure and phase diagram of NCM materials: (a) the hexagonal crystal system layered structure; (b) NCM composition phase diagram of several typical Ni-Co-Mn ratios, reproduced from Ref. [15], Copyright 2008, The Royal Society of Chemistry.
It is well known that nickel, cobalt and manganese in NCM materials have obvious synergistic effects. Cobalt can stabilize the layered structure, improve electric conductivity, and thus promote the cycle and rate capability for NCM. However, excessive cobalt content leads to more serious economical and environmental problems due to its cost and toxic nature. Nickel can improve the volume energy density of the NCM, while ternary materials with high nickel content lead to cation mixing and cause various problems including capacity loss, structure deterioration and poor thermal stability. Manganese can reduce the costs and improve the safety and structural stability of the NCM. However, a higher manganese content leads to reduced electrochemical activity in the charging/discharging process, and lower specific capacity of the NCM [4,19,20,21,22,23,24,25,26,27,28,29][4][19][20][21][22][23][24][25][26][27][28][29]. For these reasons, numerous scholars have improved the electrochemical properties of NCM by adjusting the elemental ratios of Ni-Co-Mn in order to obtain ternary materials with better performance in all aspects. The relevant NCM materials that have been studied include LiNi1/3Co1/3Mn1/3O2 (NCM111) [30[30][31][32][33][34][35],31,32,33,34,35], LiNi0.4Co0.2Mn0.4O2 (NCM424) [30[30][31][32][33][34],31,32,33,34], LiNi0.5Co0.2Mn0.3O2 (NCM523) [36,37,38,39,40,41][36][37][38][39][40][41] and other NCM materials with non-stoichiometry ratios of Ni-Co-Mn elements [42[42][43][44][45][46][47][48][49][50][51][52],43,44,45,46,47,48,49,50,51,52], etc. In particular, the nickel-rich NCM materials LiNi0.6Co0.2Mn0.2O2 (NCM622) [52[52][53][54][55][56][57][58][59][60][61],53,54,55,56,57,58,59,60,61], LiNi0.8Co0.1Mn0.1O2 (NCM811) [7,62,63,64,65,66,67,68,69,70,71][7][62][63][64][65][66][67][68][69][70][71] and other NCM with more than 90% nickel content [69,72,73,74,75[69][72][73][74][75][76][77][78],76,77,78], even the cobalt-free ternary materials [79,80,81,82,83,84,85,86[79][80][81][82][83][84][85][86][87][88][89][90][91],87,88,89,90,91], have become increasingly popular active cathode materials because of the higher potential vs. Li, higher energy densities, less toxicity, and lower priced raw materials, which can better meet the needs of electric vehicles. Nevertheless, there still are many remaining challenges in the commercialization of NCM cathodes with high-nickel content (Ni ≥ 60%) including performance deterioration and potential safety concerns. The poor lithium storage and safety of nickel-rich NCM cathodes mainly originate from the following aspects [14,15,16,28,92,93,94,95,96,97,98,99][14][15][16][28][92][93][94][95][96][97][98][99]. (i) During the cycling, heavy Ni2+ and Li+ cation mixing and more vacancies accompanied by oxygen release occur at the stage of the deep delithiation, resulting in irreversible phases transformation from the original layered structure into the spinel-like phase or inactive rock-salt phase, thus leading to poor kinetics, structural stability, thermal stability and cycling performance. (ii) Predomination of the highly oxidized Ni4+ ions at the end of charge process leads to a list of issues including the dissolution of transition metal ions, electrolyte decomposition, undesired side interfacial reactions, and the formation of cathode solid electrolyte interfaces (cSEI), which result in low coulombic efficiencies and rapid capacity reduction. (iii) During the lithiation and delithiation process, large lattice volumetric change brings about stress accumulation inside nickel-rich NCM materials, which results in secondary particle microcracks along the grain boundaries, and thus induces further structural degradation and sustained capacity loss.
To address or alleviate the above issues and improve the electrochemical performance of nickel-rich NCM materials, some feasible strategies have been proposed. First, ionic doping is an effective method to stabilize the structure of a nickel-rich NCM electrode. The purpose of doping is to make the dopant ions enter the lattice, replace some of the ions in the raw lattice, stabilize the raw material structure and improve the cycling stability during the charging and discharging process [100,101,102][100][101][102]. In particular, about ten years ago, John B. Goodenough, and Arumugam Manthiram had conducted an in-depth study into the effects of element doping, cation ordering and lithium content on the conductivity and electrochemical characteristics of high-voltage spinel transition metal oxide cathode materials [26,27][26][27]. The relationship between the conductivity, phase transformation mechanisms and the content of Mn3+, and the degree of cation ordering were investigated. It was found that Mn3+ content and ordering of spinel were not critical to the phase transformation behavior but benefited the high rate capability. Studies of ionic doping in nickel-rich NCM materials include a multitude of dopants such as Mg [102[102][103],103], Al [4,102[4][102][104][105],104,105], Zr [106,107,108][106][107][108], Ti [44[44][109][110][111],109,110,111], Nb [112[112][113],113], Mo [114[114][115],115], Cr [116], F [117[117][118][119][120],118,119,120], B [76[76][121][122],121,122], etc. Proper doping can also boost the cycling performance of the conductivity and lithium ion migration rate of the NCM electrode [15,123][15][123]. In addition, the design of the concentration-gradient structured materials can effectively improve the rate and cycling performance [124,125][124][125]. Second, surface coating is considered an effective method to improve the capacity retention, rate capability, and thermal stability of an NCM cathode. With the surface coating, the cathode and electrolyte are mechanically separated, and the dissolution of transition metal ions and the interfacial side reaction between cathodes and liquid electrolytes are effectively suppressed, improving the cycling performance of the NCM materials. The surface coating can also reduce the collapse of the material structure during long charging and discharging processes, which is beneficial to the cycling and thermal stability of the NCM materials [15,16,28,92,93,94,95,126,127][15][16][28][92][93][94][95][126][127]. Various coating materials applied on the NCM cathode can be divided into the following categories, such as oxides (SiO2 [128[128][129][130],129,130], Al2O3 [131[131][132][133][134][135],132,133,134,135], ZrO2 [136[136][137][138][139][140][141],137,138,139,140,141], TiO2 [138[138][142][143][144][145][146],142,143,144,145,146], etc.), phosphates (AlPO4 [147,148][147][148], FePO4 [149[149][150],150], Ni3(PO4)2 [151], CaHPO4 [152], Mn3(PO4)2 [153[153][154],154], ZrP2O7 [155[155][156],156], etc.), Li-containing compounds (Li3PO4 [157,158,159][157][158][159], Li2ZrO3 [160,161,162,163][160][161][162][163], Li2TiO3 [160[160][164][165],164,165], Li1.5Al0.5Ge1.5(PO4)3 [166], etc.), electron conducting coatings (graphene or reduced graphene oxide (rGO) [167[167][168][169][170][171],168,169,170,171], permeable poly (3,4-ethylenedioxythiophene) (PEDOT) [172[172][173],173], polyaniline (PANI) [174[174][175],175], poly-pyrrole (PPy) [176[176][177],177], etc.), etc. Combining a concentration gradient and surface coating, the NCM materials with a core-shell structure represent the advantages of the high capacity and high thermal and mechanical stability [178,179][178][179]. Third, electrolyte optimization is another effective strategy toward improved performances. As mentioned earlier in the text, the cSEI film reconstruction occurs easily between the solid cathode and organic liquid electrolytes during the cycling, degrading the electrochemical properties of NCM cathodes, especially at high voltages. Different electrolyte additives have been considered to adapt to the high operating voltage of NMC, restrain undesired side reactions and stabilize the surface structure [18,30,46,68,126,180][18][30][46][68][126][180]. With the addition of LiBOB dopamine [181], metal-organic framework (MOF) [182], acetonitrile (AN) [183], vinylene carbonate (VC) [184], fluoroethylene carbonate (FEC) [185] or other tailoring electrolyte additives, the lithium-ion transference number has been effectively increased, the rate capability has been enhanced, and the cycling life has been prolonged [186,187,188][186][187][188]. Typically, the solid-state electrolyte also attracts much attention because of the higher safety and cycling stability compared with combustible organic electrolytes. The solid-state interface engineering strategy is a simple promising method for NCM batteries to meet the ever-increasing requirements of safe power vehicles [189,190,191,192,193,194,195][189][190][191][192][193][194][195].
Nevertheless, the research about NCM materials summarized above, whether on normal ternary materials or nickel-rich, nickel-ultra-rich or cobalt-free materials, mostly focuses on macro-size polycrystalline secondary spheres composed of nano-size primary grains with random orientations. The continuous growth and expansion of deep-rooted cracking along weak internal grain boundaries still cannot be thoroughly eliminated due to the ever-present anisotropic strain among the randomly oriented primary grains during the charge–discharge process. Although the increasing specific surface area improves the lithium ion conductivity, it aggravates the undesired side reactions between the cathode and electrolyte, increasing the degradation of capacity retention and further reducing the cycling stability. In addition, the internal kinetics of polycrystalline materials are more unstable and the stress distribution is more uneven, especially for nanoscale NCM materials with a higher Ni content or operating voltage (≥4.5 V), which makes the materials highly susceptible to structural collapse and capacity decay during prolonged cycling, largely reducing thermal stability and safety [36,67,94,196,197][36][67][94][196][197].
2. Synthesis
At present, various synthesis strategies have been developed, including co-precipitation combined with the multi-calcination method (solid-state method) [36,200,224,225,226,227[36][198][199][200][201][202][203][204][205][206][207][208],228,229,230,231,232,233], the molten salt assistant method [199,211,218,234,235,236,237,238,239,240,241,242,243][209][210][211][212][213][214][215][216][217][218][219][220][221] and the solvothermal method [244,245,246,247][222][223][224][225]. The main synthesis strategies of single-crystal nickel-rich NCM materials are shown in Figure 32.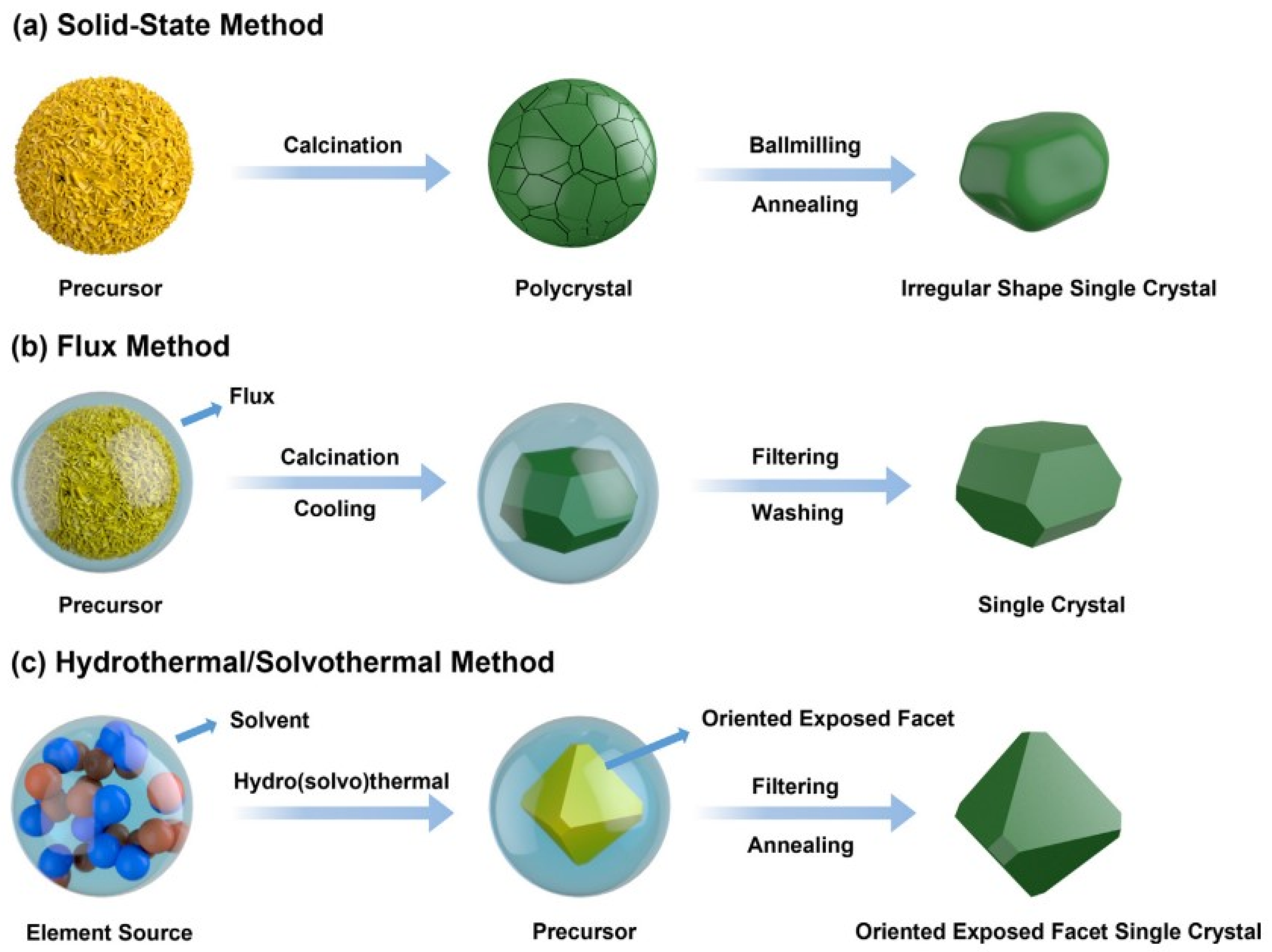
Figure 32. A schematic diagrams of the main synthesis strategies for single-crystal nickel-rich NCM materials, reproduced from Ref. [222]. Copyright 2021, Elsevier.
A schematic diagrams of the main synthesis strategies for single-crystal nickel-rich NCM materials, reproduced from Ref. [226]. Copyright 2021, Elsevier.
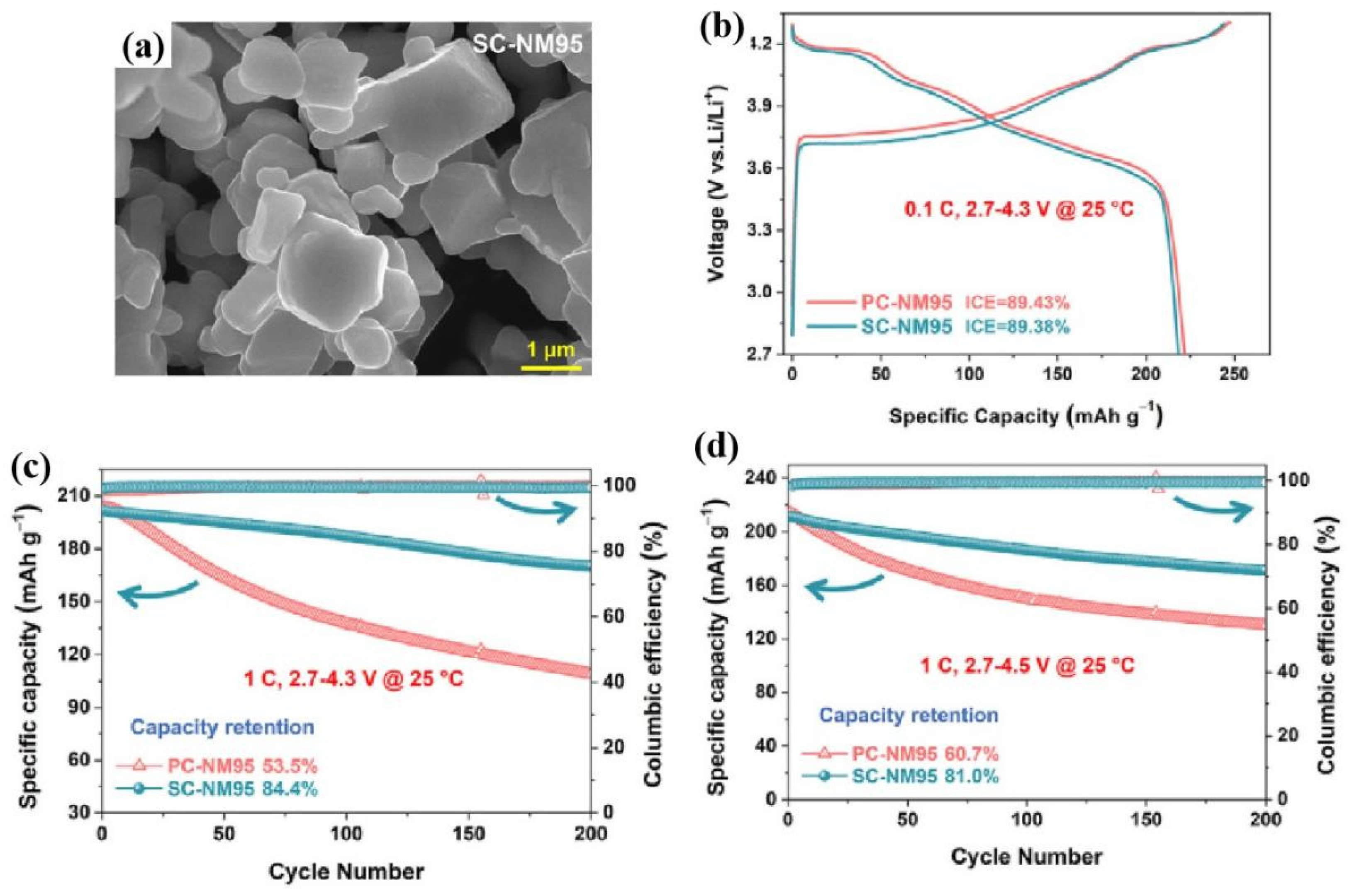
Figure 54. Research content reproduced from Ref. [237][215]: (a) SEM image of fresh SC-NM95 materials; (b) the first discharge/charge curves, and the cycling performance at difficult cut-off voltage of (c) 4.3 V and (d) 4.5 V of comparison between SC-NM95 and PC-NM95 materials, Copyright 2022, Elsevier.
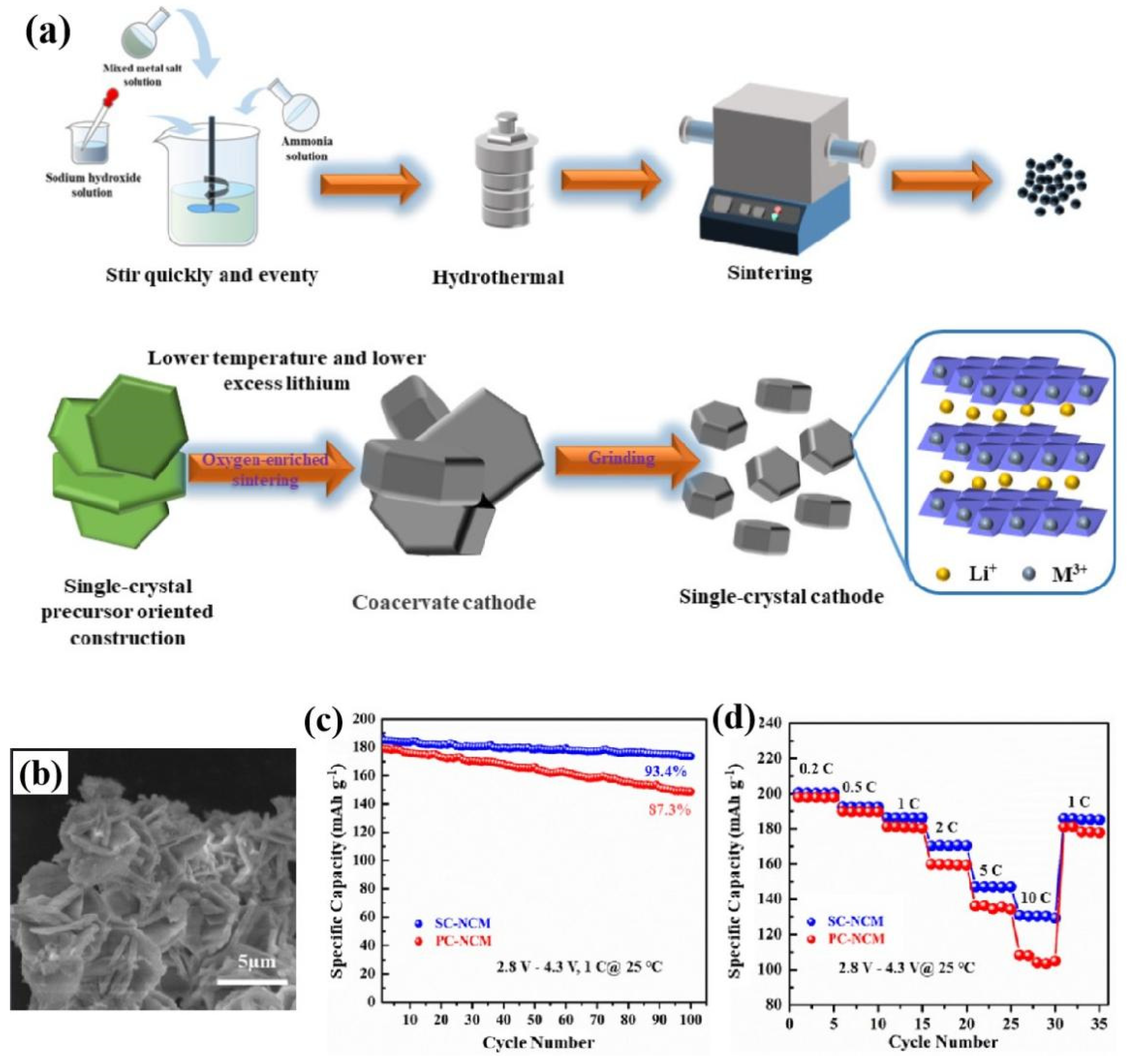
Table 1.
Electrochemical properties of single-crystal nickel-rich NCM materials synthesized via different strategies. (CR = Capacity Retention).
| Material Components | Synthesis Methods | Electrochemical Performance | Ref. |
|---|---|---|---|
| LiNi0.6Co0.1Mn0.3O2 (SC-NCM613) | One-step calcination method | CR of 73.9% after 900 cycles at 1 C, 45 °C, 2.75–4.2 V, pouch full cell | [248][227] |
| LiNi0.6Co0.2Mn0.2O2 (SC-NCM622) | One-step calcination method | CR of 82.6% after 3000 cycles at 1 C, 25 °C, 3.0–4.2 V, pouch full cell | [249][228] |
| Ce-doped Li[Ni0.9Co0.05Mn0.05]O2 (SC-Ce-NCM90) |
One-step calcination method with lower temperature | CR of 80.5% after 100 cycles at 0.5 C, 30 °C, 2.7–4.3 V, half cell | [250][229] |
| Li[Ni0.7Co0.15Mn0.15]O2 (SC-NCM70) | Calcination method | CR of 91% after 100 cycles at 0.5 C, 30 °C, 2.7–4.3 V, half cell | [233][208] |
| LiNi0.91Co0.06Mn0.03O2 (SNCM91) | Molten salt assistant method | initial discharge capacity of 203.8 mAh g−1 at 0.1 C, 3.0–4.3 V, half cell | [242][220] |
| LiNi0.92Co0.06Mn0.02O2 | Molten salt assistant method | CR of 86.3% after 300 cycles at 0.5 C, 25 °C, 2.7–4.2 V, pouch full cell | [235][213] |
| LiNi0.92Co0.06Mn0.01Al0.01O2 (NCMA) | Solid-phase sintering method | 221.4 mAh g−1 at 0.1 C, 3.0–4.3 V, CR of 94.9% after 100 cycles at 45 °C, half cell | [240][218] |
| LiNi0.95Mn0.05O2 (SC-NM95) | Molten salt assistant method | CR of 81% after 200 cycles at 1 C, 25 °C, 2.7–4.5 V, half cell | [237][215] |
| LiNi0.6Co0.2Mn0.2O2 (SC-NCM622) | Molten salt assistant method | 155.1 mAh g−1 at 1 C, CR of 94.3% after 240 cycles at 1 C, 25 °C, 2.8–4.3 V, half cell | [238][216] |
| LiNi0.8Co0.1Mn0.1O2 (SC-NCM811) | Hydrothermal method | 186.2 mAh g−1 at 1 C, CR of 93.4% after 100 cycles at 1 C, 25 °C, 2.8–4.3 V, half cell | [244][222] |
| LiNi0.8Co0.1Mn0.1O2 (SC-NCM811) | Solvothermal method | 226.9 mAh g−1 at 0.1 C, CR of 91.2% after 100 cycles at 1 C, 25 °C, 2.8–4.3 V, half cell | [245][223] |
| LiNi0.6Co0.2Mn0.2O2 (SC-NCM622) | Hydrothermal method | 184.2 mAh g−1 at 0.1 C, CR of 89.6% after 100 cycles at 1 C, 2.8–4.5 V, half cell | [246][224] |
References
- Armand, M.; Tarascon, J.M. Building better batteries: Researchers must find a sustainable way of providing the power our modern lifestyles demand. Nature 2008, 451, 652–657.
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139.
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301.
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732.
- Zaghib, K.; Mauger, A.; Groult, H.; Goodenough, J.B.; Julien, C.M. Advanced electrodes for high power Li-ion batteries. Materials 2013, 6, 1028–1049.
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34.
- Akhilash, M.; Salini, P.S.; John, B.; Mercy, T.D. A journey through layered cathode materials for lithium ion cells—From lithium cobalt oxide to lithium-rich transition metal oxides. J. Alloys Compd. 2021, 869, 159239.
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered LiO2 cathode materials for lithium-ion batteries. Electrochem. Solid-State Lett. 2001, 4, 200–203.
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiCo1/3Ni1/3Mn1/3O2 for Lithium-Ion Batteries. Chem. Lett. 2001, 30, 642–643.
- Hwang, B.J.; Tsai, Y.W.; Carlier, D.; Ceder, G. A Combined computational/experimental study on LiNi1/3Co1/3Mn1/3O2. Chem. Mater. 2003, 15, 3676–3682.
- Radin, M.D.; Hy, S.; Sina, M.; Fang, C.; Liu, H.; Vinckeviciute, J.; Zhang, M.; Whittingham, M.S.; Meng, Y.S.; Van der Ven, A. Narrowing the gap between theoretical and practical capacities in Li-ion layered oxide cathode materials. Adv. Energy Mater. 2017, 7, 1602888.
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603.
- Chakraborty, A.; Kunnikuruvan, S.; Dixit, M.; Major, D.T. Review of computational studies of NCM cathode materials for Li-ion batteries. Isr. J. Chem. 2020, 60, 850–862.
- Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457.
- Jung, C.H.; Shim, H.; Eum, D.; Hong, S.H. Challenges and recent progress in LiNixCoyMn1−x−yO2 (NCM) cathodes for lithium ion batteries. J. Korean Ceram. Soc. 2020, 58, 1–27.
- Li, W.; Dolocan, A.; Oh, P.; Celio, H.; Park, S.; Cho, J.; Manthiram, A. Dynamic behaviour of interphases and its implication on high-energy-density cathode materials in lithium-ion batteries. Nat. Commun. 2017, 8, 14589.
- Maleki Kheimeh Sari, H.; Li, X. Controllable cathode–electrolyte interface of LiO2 for lithium ion batteries: A review. Adv. Energy Mater. 2019, 9, 1901597.
- Lim, B.B.; Yoon, S.J.; Park, K.J.; Yoon, C.S.; Kim, S.J.; Lee, J.J.; Sun, Y.K. Advanced concentration gradient cathode material with two-slope for high-energy and safe lithium batteries. Adv. Funct. Mater. 2015, 25, 4673–4680.
- Koyama, Y.; Arai, H.; Tanaka, I.; Uchimoto, Y.; Ogumi, Z. Defect chemistry in layered LiMO2 (M = Co, Ni, Mn, and Li1/3Mn2/3) by first-principles calculations. Chem. Mater. 2012, 24, 3886–3894.
- Zheng, J.; Liu, T.; Hu, Z.; Wei, Y.; Song, X.; Ren, Y.; Wang, W.; Rao, M.; Lin, Y.; Chen, Z.; et al. Tuning of thermal stability in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2016, 138, 13326–13334.
- Kim, J. Synthesis and electrochemical behavior of LiO2 cathode materials. Solid State Ionics 2003, 164, 43–49.
- Kang, K.; Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 2006, 74, 094105.
- Huang, Z.; Gao, J.; He, X.; Li, J.; Jiang, C. Well-ordered spherical LiNixCo(1−2x)MnxO2 cathode materials synthesized from cobolt concentration-gradient precursors. J. Power Sources 2012, 202, 284–290.
- MacNeil, D.D.; Lu, Z.; Dahn, J.R. Structure and electrochemistry of LiO2 (0 ≤ x ≤ 1/2). J. Electrochem. Soc. 2002, 149, 1332–1336.
- Moorhead, R.Z.; Huq, A.; Goodenough, J.B.; Manthiram, A. Electronic and electrochemical properties of Li1−xMn1.5Ni0.5O4 spinel cathodes as a function of lithium content and cation ordering. Chem. Mater. 2015, 27, 6934–6945.
- Moorhead, R.Z.; Chemelewski, K.R.; Goodenough, J.B.; Manthiram, A. Magnetic measurements as a viable tool to assess the relative degrees of cation ordering and Mn3+ content in doped LiMn1.5Ni0.5O4 spinel cathodes. J. Mater. Chem. A 2013, 1, 10745–10752.
- Yan, J.; Huang, H.; Tong, J.; Li, W.; Liu, X.; Zhang, H.; Huang, H.; Zhou, W. Recent progress on the modification of high nickel content NCM: Coating, doping, and single crystallization. Interdiscip. Mater. 2022, 1, 330–353.
- Wu, F.; Liu, N.; Chen, L.; Su, Y.; Tan, G.; Bao, L.; Zhang, Q.; Lu, Y.; Wang, J.; Chen, S.; et al. Improving the reversibility of the H2-H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy 2019, 59, 50–57.
- Madec, L.; Xia, J.; Petibon, R.; Nelson, K.J.; Sun, J.-P.; Hill, I.G.; Dahn, J.R. Effect of sulfate electrolyte additives on LiNi1/3Mn1/3Co1/3O2/graphite pouch cell lifetime: Correlation between XPS surface studies and electrochemical test results. J. Phys. Chem. C 2014, 118, 29608–29622.
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sources 2012, 207, 134–140.
- Hua, W.; Schwarz, B.; Knapp, M.; Senyshyn, A.; Missiul, A.; Mu, X.; Wang, S.; Kübel, C.; Binder, J.R.; Indris, S.; et al. (De)Lithiation mechanism of hierarchically layered LiNi1/3Co1/3Mn1/3O2 cathodes during high-voltage cycling. J. Electrochem. Soc. 2018, 166, A5025–A5032.
- Zhang, X.; Mauger, A.; Lu, Q.; Groult, H.; Perrigaud, L.; Gendron, F.; Julien, C.M. Synthesis and characterization of LiNi1/3Mn1/3Co1/3O2 by wet-chemical method. Electrochim. Acta 2010, 55, 6440–6449.
- Yan, P.; Zheng, J.; Zhang, J.G.; Wang, C. Atomic resolution structural and chemical imaging revealing the sequential migration of Ni, Co, and Mn upon the battery cycling of layered cathode. Nano Lett. 2017, 17, 3946–3951.
- Wang, Q.; Feng, L.; Sun, J. A multi-component additive to improve the thermal stability of Li(Ni1/3Co1/3Mn1/3)O2-based lithium ion batteries. Energies 2016, 9, 424.
- Li, J.; Cameron, A.R.; Li, H.; Glazier, S.; Xiong, D.; Chatzidakis, M.; Allen, J.; Botton, G.A.; Dahn, J.R. Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J. Electrochem. Soc. 2017, 164, A1534–A1544.
- Berkes, B.B.; Schiele, A.; Sommer, H.; Brezesinski, T.; Janek, J. On the gassing behavior of lithium-ion batteries with NCM523 cathodes. J. Solid State Electrochem. 2016, 20, 2961–2967.
- Zhang, Y.; Wang, Z.; Zhong, Y.; Wu, H.; Li, S.; Cheng, Q.; Guo, P. Coating for improving electrochemical performance of NCM523 cathode for lithium-ion batteries. Ionics 2020, 27, 13–20.
- Li, J.; Huang, J.; Kong, X.; Zeng, J.; Zhao, J. The apparent capacity decay by kinetic degradation of LiNi0.5Co0.2Mn0.3O2 during cycling under the high upper-limit charging potential. J. Power Sources 2021, 496, 229856.
- Jung, S.K.; Gwon, H.; Hong, J.; Park, K.Y.; Seo, D.H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300787.
- Wu, Z.; Ji, S.; Zheng, J.; Hu, Z.; Xiao, S.; Wei, Y.; Zhuo, Z.; Lin, Y.; Yang, W.; Xu, K.; et al. Prelithiation activates Li(Ni0.5Mn0.3Co0.2)O2 for high capacity and excellent cycling stability. Nano Lett. 2015, 15, 5590–5596.
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered LiO2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130.
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767.
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529.
- Hua, W.; Schwarz, B.; Azmi, R.; Müller, M.; Dewi Darma, M.S.; Knapp, M.; Senyshyn, A.; Heere, M.; Missyul, A.; Simonelli, L.; et al. Lithium-ion (de)intercalation mechanism in core-shell layered Li(Ni,Co,Mn)O2 cathode materials. Nano Energy 2020, 78, 105231.
- Cherkashinin, G.; Motzko, M.; Schulz, N.; Späth, T.; Jaegermann, W. Electron spectroscopy study of LiO2/electrolyte interface: Electronic structure, interface composition, and device implications. Chem. Mater. 2015, 27, 2875–2887.
- Ming, J.; Kwak, W.J.; Youn, S.J.; Ming, H.; Hassoun, J.; Sun, Y.-K. Lithiation of an iron oxide-based anode for stable, high-capacity lithium-ion batteries of porous carbon-Fe3O4/LiO2. Energy Technol. 2014, 2, 778–785.
- Azmi, R.; Masoumi, M.; Ehrenberg, H.; Trouillet, V.; Bruns, M. Surface analytical characterization of LiNi0.8-yMnyCo0.2O2(0 ≤ y ≤ 0.4) compounds for lithium-ion battery electrodes. Surf. Interface Anal. 2018, 50, 1132–1137.
- Huang, W.; Lin, C.; Zhang, M.; Li, S.; Chen, Z.; Zhao, W.; Zhu, C.; Zhao, Q.; Chen, H.; Pan, F. Revealing roles of co and Ni in Mn-rich layered cathodes. Adv. Energy Mater. 2021, 11, 2102646.
- Shim, J.H.; Kim, C.Y.; Cho, S.W.; Missiul, A.; Kim, J.-K.; Ahn, Y.J.; Lee, S. Effects of heat-treatment atmosphere on electrochemical performances of Ni-rich mixed-metal oxide (LiNi0.80Co0.15Mn0.05O2) as a cathode material for lithium ion battery. Electrochim. Acta 2014, 138, 15–21.
- Lim, H.; Na, D.; Lee, C.R.; Seo, H.K.; Kwon, O.H.; Kim, J.K.; Seo, I. An integrated device of a lithium-ion battery combined with silicon solar cells. Energies 2021, 14, 6010.
- Zhang, N.; Li, J.; Li, H.; Liu, A.; Huang, Q.; Ma, L.; Li, Y.; Dahn, J.R. Structural, electrochemical, and thermal properties of nickel-rich LiNixMnyCozO2 materials. Chem. Mater. 2018, 30, 8852–8860.
- Zhang, Y.; Cao, H.; Zhang, J.; Xia, B. Synthesis of LiNi0.6Co0.2Mn0.2O2 cathode material by a carbonate co-precipitation method and its electrochemical characterization. Solid State Ionics 2006, 177, 3303–3307.
- Cao, H.; Zhang, Y.; Zhang, J.; Xia, B. Synthesis and electrochemical characteristics of layered LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. Solid State Ionics 2005, 176, 1207–1211.
- Chen, Y.; Zhang, Y.; Chen, B.; Wang, Z.; Lu, C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sources 2014, 256, 20–27.
- Liang, L.; Du, K.; Peng, Z.; Cao, Y.; Duan, J.; Jiang, J.; Hu, G. Co–precipitation synthesis of Ni0.6Co0.2Mn0.2(OH)2 precursor and characterization of LiNi0.6Co0.2Mn0.2O2 cathode material for secondary lithium batteries. Electrochim. Acta 2014, 130, 82–89.
- Yue, P.; Wang, Z.; Li, X.; Xiong, X.; Wang, J.; Wu, X.; Guo, H. The enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials by low temperature fluorine substitution. Electrochim. Acta 2013, 95, 112–118.
- Ju, S.H.; Kang, I.S.; Lee, Y.S.; Shin, W.K.; Kim, S.; Shin, K.; Kim, D.W. Improvement of the cycling performance of LiNi0.6Co0.2Mn0.2O2 cathode active materials by a dual-conductive polymer coating. ACS Appl. Mater. Interfaces 2014, 6, 2546–2552.
- Neudeck, S.; Walther, F.; Bergfeldt, T.; Suchomski, C.; Rohnke, M.; Hartmann, P.; Janek, J.; Brezesinski, T. Molecular surface modification of NCM622 cathode material using organophosphates for improved Li-ion battery full-cells. ACS Appl. Mater. Interfaces 2018, 10, 20487–20498.
- Kim, A.Y.; Strauss, F.; Bartsch, T.; Teo, J.H.; Janek, J.; Brezesinski, T. Effect of surface carbonates on the cyclability of LiNbO3-coated NCM622 in all-solid-state batteries with lithium thiophosphate electrolytes. Sci. Rep. 2021, 11, 1–9.
- Hua, W.; Wang, K.; Knapp, M.; Schwarz, B.; Wang, S.; Liu, H.; Lai, J.; Müller, M.; Schökel, A.; Missyul, A.; et al. Chemical and structural evolution during the synthesis of layered Li(Ni,Co,Mn)O2 oxides. Chem. Mater. 2020, 32, 4984–4997.
- Kim, Y. Lithium nickel cobalt manganese oxide synthesized using alkali chloride flux: Morphology and performance as a cathode material for lithium ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 2329–2333.
- Lim, J.M.; Hwang, T.; Kim, D.; Park, M.S.; Cho, K.; Cho, M. Intrinsic origins of crack generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 2017, 7, 39669.
- Kim, H.R.; Woo, S.G.; Kim, J.H.; Cho, W.; Kim, Y.J. Capacity fading behavior of Ni-rich layered cathode materials in Li-ion full cells. J. Electroanal. Chem. 2016, 782, 168–173.
- Xi, Y.; Liu, Y.; Zhang, D.; Jin, S.; Zhang, R.; Jin, M. Comparative study of the electrochemical performance of LiNi0.5Co0.2Mn0.3O2 and LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium ion batteries. Solid State Ionics 2018, 327, 27–31.
- Huang, Y.; Wang, Z.X.; Li, X.H.; Guo, H.J.; Wang, J.X. Synthesis of Ni0.8Co0.1Mn0.1(OH)2 precursor and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium batteries. Trans. Nonferrous Met. Soc. China 2015, 25, 2253–2259.
- Li, W.; Liu, X.; Xie, Q.; You, Y.; Chi, M.; Manthiram, A. Long-term cyclability of NCM-811 at high voltages in lithium-ion batteries: An in-depth diagnostic study. Chem. Mater. 2020, 32, 7796–7804.
- Song, W.; Harlow, J.; Logan, E.; Hebecker, H.; Coon, M.; Molino, L.; Johnson, M.; Dahn, J.; Metzger, M. A Systematic study of electrolyte additives in single crystal and bimodal LiNi0.8Mn0.1 Co0.1O2/graphite pouch cells. J. Electrochem. Soc. 2021, 168, 090503.
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich LiO2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163.
- Hu, J.P.; Sheng, H.; Deng, Q.; Ma, Q.; Liu, J.; Wu, X.W.; Liu, J.J.; Wu, Y.P. High-rate layered cathode of lithium-ion batteries through regulating three-dimensional agglomerated structure. Energies 2020, 13, 1602.
- Heck, C.A.; von Horstig, M.W.; Huttner, F.; Mayer, J.K.; Haselrieder, W.; Kwade, A. Review—Knowledge-based process design for high quality production of NCM811 cathodes. J. Electrochem. Soc. 2020, 167, 160521.
- Yoon, C.S.; Choi, M.H.; Lim, B.-B.; Lee, E.J.; Sun, Y.K. Review—High-capacity LiO2(x = 0.1, 0.05, 0) cathodes for next-generation Li-ion battery. J. Electrochem. Soc. 2015, 162, A2483–A2489.
- Seong, W.M.; Cho, K.H.; Park, J.W.; Park, H.; Eum, D.; Lee, M.H.; Kim, I.S.; Lim, J.; Kang, K. Controlling residual lithium in high-nickel (>90 %) lithium layered oxides for cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 18662–18669.
- Song, S.H.; Hong, S.; Cho, M.; Yoo, J.G.; Min Jin, H.; Lee, S.H.; Avdeev, M.; Ikeda, K.; Kim, J.; Nam, S.C.; et al. Rational design of Li off-stoichiometric Ni-rich layered cathode materials for Li-ion batteries. Chem. Eng. J. 2022, 448, 137685.
- Jun, D.W.; Yoon, C.S.; Kim, U.H.; Sun, Y.K. High-energy density core–shell structured LiO2 cathode for lithium-ion batteries. Chem. Mater. 2017, 29, 5048–5052.
- Park, K.J.; Jung, H.G.; Kuo, L.Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Improved cycling stability of LiO2 through microstructure modification by boron doping for Li-ion batteries. Adv. Energy Mater. 2018, 8, 1301564.
- Jamil, S.; Yue, L.; Li, C.; Fasehullah, M.; Aizaz Ud Din, M.; Yang, W.; Bao, S.; Xu, M. Significance of gallium doping for high Ni, low Co/Mn layered oxide cathode material. Chem. Eng. J. 2022, 441, 135821.
- Park, N.Y.; Ryu, H.H.; Park, G.T.; Noh, T.C.; Sun, Y.K. Optimized Ni-rich NCMA cathode for electric vehicle batteries. Adv. Energy Mater. 2021, 11, 2003767.
- Zhang, N.; Zaker, N.; Li, H.; Liu, A.; Inglis, J.; Jing, L.; Li, J.; Li, Y.; Botton, G.A.; Dahn, J.R. Cobalt-free nickel-rich positive electrode materials with a core–shell structure. Chem. Mater. 2019, 31, 10150–10160.
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is cobalt needed in Ni-rich positive electrode materials for lithium ion batteries? J. Electrochem. Soc. 2019, 166, A429–A439.
- Rathore, D.; Geng, C.; Zaker, N.; Hamam, I.; Liu, Y.; Xiao, P.; Botton, G.A.; Dahn, J.; Yang, C. Tungsten infused grain boundaries enabling universal performance enhancement of Co-free Ni-rich cathode materials. J. Electrochem. Soc. 2021, 168, 120514.
- Su, L.; Jo, E.; Manthiram, A. Protection of cobalt-free LiNiO2 from degradation with localized saturated electrolytes in lithium-metal batteries. ACS Energy Lett. 2022, 7, 2165–2172.
- Liu, T.; Yu, L.; Liu, J.; Lu, J.; Bi, X.; Dai, A.; Li, M.; Li, M.; Hu, Z.; Ma, L.; et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 2021, 6, 277–286.
- Mu, L.; Yang, Z.; Tao, L.; Waters, C.K.; Xu, Z.; Li, L.; Sainio, S.; Du, Y.; Xin, H.L.; Nordlund, D.; et al. The sensitive surface chemistry of Co-free, Ni-rich layered oxides: Identifying experimental conditions that influence characterization results. J. Mater. Chem. A 2020, 8, 17487–17497.
- Li, N.; Sallis, S.; Papp, J.K.; McCloskey, B.D.; Yang, W.; Tong, W. Correlating the phase evolution and anionic redox in Co-free Ni-rich layered oxide cathodes. Nano Energy 2020, 78, 105365.
- Aishova, A.; Park, G.T.; Yoon, C.S.; Sun, Y.K. Cobalt-free high-capacity Ni-rich layered LiO2 cathode. Adv. Energy Mater. 2019, 10, 1903179.
- Wang, X.; Ding, Y.L.; Deng, Y.P.; Chen, Z. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: Promises and challenges. Adv. Energy Mater. 2020, 10, 1903864.
- Kim, S.; Na, S.; Kim, J.; Jun, T.H.; Oh, M.H.; Min, K.; Park, K. Multifunctional surface modification with Co-free spinel structure on Ni-rich cathode material for improved electrochemical performance. J. Alloys Compd. 2022, 918, 165454.
- Wang, C.; Tan, L.; Yi, H.; Zhao, Z.; Yi, X.; Zhou, Y.; Zheng, J.; Wang, J.; Li, L. Unveiling the impact of residual Li conversion and cation ordering on electrochemical performance of Co-free Ni-rich cathodes. Nano Res. 2022, 15, 9038–9046.
- Xi, Y.; Wang, M.; Xu, L.; Kheimeh Sari, H.M.; Li, W.; Hu, J.; Cao, Y.; Chen, L.; Wang, L.; Pu, X.; et al. A new Co-free Ni-rich LiNi0.8Fe0.1Mn0.1O2 cathode for low-cost Li-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 57341–57349.
- Choi, J.U.; Voronina, N.; Sun, Y.K.; Myung, S.T. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: Yesterday, today, and tomorrow. Adv. Energy Mater. 2020, 10.
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent advances and remaining challenges for lithium ion battery cathodes. J. Electrochem. Soc. 2016, 164, A6220–A6228.
- Liang, L.; Zhang, W.; Zhao, F.; Denis, D.K.; Zaman, F.u.; Hou, L.; Yuan, C. Surface/interface structure degradation of Ni-rich layered oxide cathodes toward lithium-ion batteries: Fundamental mechanisms and remedying strategies. Adv. Mater. Interfaces 2019, 7, 1901749.
- Julien, C.M.; Mauger, A. NCA, NCM811, and the route to Ni-richer lithium-ion batteries. Energies 2020, 13, 6363.
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.J.; Sun, Y.K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2016, 2, 196–223.
- Li, H.; Liu, A.; Zhang, N.; Wang, Y.; Yin, S.; Wu, H.; Dahn, J.R. An unavoidable challenge for Ni-rich positive electrode materials for lithium-ion batteries. Chem. Mater. 2019, 31, 7574–7583.
- Li, W.; Asl, H.Y.; Xie, Q.; Manthiram, A. collapse of LiNi1−x−yCo xMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 2019, 141, 5097–5101.
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Identifying surface degradation, mechanical failure, and thermal instability phenomena of high energy density Ni-rich NCM cathode materials for lithium-ion batteries: A review. RSC Adv. 2022, 12, 5891–5909.
- Ko, D.S.; Park, J.H.; Yu, B.Y.; Ahn, D.; Kim, K.; Han, H.N.; Jeon, W.S.; Jung, C.; Manthiram, A. Degradation of high-nickel-layered oxide cathodes from surface to bulk: A comprehensive structural, chemical, and electrical analysis. Adv. Energy Mater. 2020, 10, 2001035.
- Sun, H.H.; Kim, U.H.; Park, J.H.; Park, S.W.; Seo, D.H.; Heller, A.; Mullins, C.B.; Yoon, C.S.; Sun, Y.K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 1–11.
- Julien, C.; Nazri, G.A.; Rougier, A. Electrochemical performances of layered LiM1-y M’y O2(M = Ni, Co; M’ = Mg, Al, B) oxides in lithium batteries. Solid State Ionics 2000, 135, 121–130.
- Samarasingha, P.B.; Wijayasinghe, A.; Behm, M.; Dissanayake, L.; Lindbergh, G. Development of cathode materials for lithium ion rechargeable batteries based on the system Li(Ni1/3Mn1/3Co(1/3-x)Mx)O2, (M = Mg, Fe, Al and x = 0.00 to 0.33). Solid State Ionics 2014, 268, 226–230.
- Sattar, T.; Lee, S.H.; Sim, S.J.; Jin, B.S.; Kim, H.S. Effect of Mg-doping on the electrochemical performance of LiNi0.84Co0.11Mn0.05O2 cathode for lithium ion batteries. Int. J. Hydrogen Energy 2020, 45, 19567–19576.
- Zhou, F.; Zhao, X.; Lu, Z.; Jiang, J.; Dahn, J.R. The effect of Al substitution on the reactivity of delithiated LiNi1/3Mn1/3Co(1/3−z)AlzO2 with non-aqueous electrolyte. Electrochem. Commun. 2008, 10, 1168–1171.
- Zhang, M.; Wang, C.; Zhang, J.; Li, G.; Gu, L. Preparation and electrochemical characterization of La and Al Co-doped NCM811 cathode materials. ACS Omega 2021, 6, 16465–16471.
- Schipper, F.; Dixit, M.; Kovacheva, D.; Talianker, M.; Haik, O.; Grinblat, J.; Erickson, E.M.; Ghanty, C.; Major, D.T.; Markovsky, B.; et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: Zirconium-doped LiNi0.6Co0.2Mn0.2O2. J. Mater. Chem. A 2016, 4, 16073–16084.
- Han, B.; Xu, S.; Zhao, S.; Lin, G.; Feng, Y.; Chen, L.; Ivey, D.G.; Wang, P.; Wei, W. Enhancing the structural stability of Ni-rich layered oxide cathodes with a preformed Zr-concentrated defective nanolayer. ACS Appl. Mater. Interfaces 2018, 10, 39599–39607.
- Gao, S.; Zhan, X.; Cheng, Y.T. Structural, electrochemical and Li-ion transport properties of Zr-modified LiNi0.8Co0.1Mn0.1O2 positive electrode materials for Li-ion batteries. J. Power Sources 2019, 410–411, 45–52.
- Du, R.; Bi, Y.; Yang, W.; Peng, Z.; Liu, M.; Liu, Y.; Wu, B.; Yang, B.; Ding, F.; Wang, D. Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5V. Ceram. Int. 2015, 41, 7133–7139.
- Sun, H.; Cao, Z.; Wang, T.; Lin, R.; Li, Y.; Liu, X.; Zhang, L.; Lin, F.; Huang, Y.; Luo, W. Enabling high rate performance of Ni-rich layered oxide cathode by uniform titanium doping. Mater. Today Energy 2019, 13, 145–151.
- Zhang, D.; Liu, Y.; Wu, L.; Feng, L.; Jin, S.; Zhang, R.; Jin, M. Effect of Ti ion doping on electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material. Electrochim. Acta 2019, 328, 135086.
- Jia, X.; Yan, M.; Zhou, Z.; Chen, X.; Yao, C.; Li, D.; Chen, D.; Chen, Y. Nd-doped LiNi0.5Co0.2Mn0.3O2 as a cathode material for better rate capability in high voltage cycling of Li-ion batteries. Electrochim. Acta 2017, 254, 50–58.
- Liu, S.; Chen, X.; Zhao, J.; Su, J.; Zhang, C.; Huang, T.; Wu, J.; Yu, A. Uncovering the role of Nb modification in improving the structure stability and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode charged at higher voltage of 4.5 V. J. Power Sources 2018, 374, 149–157.
- Su, Y.; Yang, Y.; Chen, L.; Lu, Y.; Bao, L.; Chen, G.; Yang, Z.; Zhang, Q.; Wang, J.; Chen, R.; et al. Improving the cycling stability of Ni-rich cathode materials by fabricating surface rock salt phase. Electrochim. Acta 2018, 292, 217–226.
- Breuer, O.; Chakraborty, A.; Liu, J.; Kravchuk, T.; Burstein, L.; Grinblat, J.; Kauffman, Y.; Gladkih, A.; Nayak, P.; Tsubery, M.; et al. Understanding the role of minor molybdenum doping in LiNi0.5Co0.2Mn0.3O2 electrodes: From structural and surface analyses and theoretical modeling to practical electrochemical cells. ACS Appl. Mater. Interfaces 2018, 10, 29608–29621.
- Li, L.J.; Wang, Z.X.; Liu, Q.C.; Ye, C.; Chen, Z.Y.; Gong, L. Effects of chromium on the structural, surface chemistry and electrochemical of layered LiNi0.8−xCo0.1Mn0.1CrxO2. Electrochim. Acta 2012, 77, 89–96.
- Yue, P.; Wang, Z.; Wang, J.; Guo, H.; Xiong, X.; Li, X. Effect of fluorine on the electrochemical performance of spherical LiNi0.8Co0.1Mn0.1O2 cathode materials via a low temperature method. Powder Technol. 2013, 237, 623–626.
- Binder, J.O.; Culver, S.P.; Pinedo, R.; Weber, D.A.; Friedrich, M.S.; Gries, K.I.; Volz, K.; Zeier, W.G.; Janek, J. Investigation of fluorine and nitrogen as anionic dopants in nickel-rich cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 44452–44462.
- Li, C.; Kan, W.H.; Xie, H.; Jiang, Y.; Zhao, Z.; Zhu, C.; Xia, Y.; Zhang, J.; Xu, K.; Mu, D.; et al. Inducing favorable cation antisite by doping halogen in Ni-rich layered cathode with ultrahigh stability. Adv. Sci. News 2019, 6, 1801406.
- Kim, H.; Kim, S.B.; Park, D.H.; Park, K.W. Fluorine-doped LiNi0.8Mn0.1Co0.1O2 cathode for high-performance lithium-ion batteries. Energies 2020, 13, 4808.
- Lee, S.H.; Jin, B.S.; Kim, H.S. Superior performances of B-doped LiNi0.84Co0.10Mn0.06O2 cathode for advanced LIBs. Sci. Rep. 2019, 9, 1–7.
- Roitzheim, C.; Kuo, L.Y.; Sohn, Y.J.; Finsterbusch, M.; Möller, S.; Sebold, D.; Valencia, H.; Meledina, M.; Mayer, J.; Breuer, U.; et al. Boron in Ni-rich NCM811 cathode material: Impact on atomic and microscale properties. ACS Appl. Energy Mater. 2021, 5, 524–538.
- Weigel, T.; Schipper, F.; Erickson, E.M.; Susai, F.A.; Markovsky, B.; Aurbach, D. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 2019, 4, 508–516.
- Zhang, Y.; Li, H.; Liu, J.; Zhang, J.; Cheng, F.; Chen, J. LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries. J. Mater. Chem. A 2019, 7, 20958–20964.
- Liu, T.; Yu, L.; Lu, J.; Zhou, T.; Huang, X.; Cai, Z.; Dai, A.; Gim, J.; Ren, Y.; Xiao, X.; et al. Rational design of mechanically robust Ni-rich cathode materials via concentration gradient strategy. Nat. Commun. 2021, 12, 1–10.
- Zhang, H.; Xu, J.; Zhang, J. Surface-coated LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode materials by Al2O3, ZrO2, and Li2O-2B2O3 thin-layers for improving the performance of lithium ion batteries. Front. Mater. 2019, 6, 309.
- Karayaylali, P.; Tatara, R.; Zhang, Y.; Chan, K.L.; Yu, Y.; Giordano, L.; Maglia, F.; Jung, R.; Lund, I.; Shao, H.Y. Editors’ Choice—Coating-dependent electrode-electrolyte interface for Ni-rich positive electrodes in Li-ion batteries. J. Electrochem. Soc. 2019, 166, A1022–A1030.
- Cho, W.; Kim, S.M.; Song, J.H.; Yim, T.; Woo, S.G.; Lee, K.W.; Kim, J.S.; Kim, Y.J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50.
- Liang, L.; Hu, G.; Jiang, F.; Cao, Y. Electrochemical behaviours of SiO2-coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J. Alloys Compd. 2016, 657, 570–581.
- Dou, L.; Hu, P.; Shang, C.; Wang, H.; Xiao, D.; Ahuja, U.; Aifantis, K.; Zhang, Z.; Huang, Z. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 with SiO2 surface coating Via homogeneous precipitation. ChemElectroChem 2021, 8, 4321–4327.
- Liu, W.; Gao, F.; Zang, Y.; Qu, J.; Xu, J.; Ji, S.; Huo, Y.; Qiu, J. Boosting cycle stability of NCM811 cathode material via 2D Mg-Al-LDO nanosheet coating for lithium-ion battery. J. Alloys Compd. 2021, 867, 159079.
- Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges. Coatings 2019, 9, 92.
- Feng, Y.; Xu, H.; Wang, B.; Wang, S.; Ai, L.; Li, S. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials by Al2O3 coating. J. Electrochem. Energy Convers. Storage 2021, 18, 1–14.
- Neudeck, S.; Strauss, F.; Garcia, G.; Wolf, H.; Janek, J.; Hartmann, P.; Brezesinski, T. Room temperature, liquid-phase Al2O3 surface coating approach for Ni-rich layered oxide cathode material. Chem. Commun. (Camb.) 2019, 55, 2174–2177.
- Liu, W.; Li, X.; Xiong, D.; Hao, Y.; Li, J.; Kou, H.; Yan, B.; Li, D.; Lu, S.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120.
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv. Energy Mater. 2018, 8, 1701682.
- Woo, S.G.; Han, J.H.; Kim, K.J.; Kim, J.H.; Yu, J.S.; Kim, Y.J. Surface modification by sulfated zirconia on high-capacity nickel-based cathode materials for Li-ion batteries. Electrochim. Acta 2015, 153, 115–121.
- Herzog, M.J.; Esken, D.; Janek, J. Improved Cycling Performance of High-Nickel NMC by dry powder coating with nanostructured fumed Al2O3,TiO2,and ZrO2: A comparison. Batter. Supercaps 2021, 4, 1003–1017.
- Tubtimkuna, S.; Phattharasupakun, N.; Bunyanidhi, P.; Sawangphruk, M. Diffusion of zirconium (iv) ions from coated thick zirconium oxide shell to the bulk structure of Ni-rich NMC811 cathode leading to high-performance 18650 cylindrical Li-ion batteries. Adv. Mater. Technol. 2022.
- Li, W.; Li, Y.; Yang, L.; Zhu, J.; Guo, J.; Yang, J.C. Enhanced the electrochemical performances of LiNi0.7Co0.15Mn0.15O2 cathodes by the hybrid ZrO2-Li2ZrO3 layer. Ionics 2020, 27, 479–489.
- Jo, S.J.; Hwang, D.Y.; Lee, S.H. Use of zirconium dual-modification on the LiNi0.8Co0.1Mn0.1O2 cathode for improved electrochemical performances of lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 3693–3700.
- Kim, H.; Jang, J.; Byun, D.; Kim, H.S.; Choi, W. Selective TiO2 nanolayer coating by polydopamine modification for highly stable Ni-rich layered oxides. ChemSusChem 2019, 12, 5253–5264.
- Hwang, D.-Y.; Sim, S.-J.; Jin, B.-S.; Kim, H.-S.; Lee, S.-H. Suppressed microcracking and F penetration of Ni-rich layered cathode via the combined effects of titanium dioxide doping and coating. ACS Appl. Energy Mater. 2021, 4, 1743–1751.
- Moryson, Y.; Walther, F.; Sann, J.; Mogwitz, B.; Ahmed, S.; Burkhardt, S.; Chen, L.; Klar, P.J.; Volz, K.; Fearn, S.; et al. Analyzing nanometer-thin cathode particle coatings for lithium-ion batteries—The example of TiO2 on NCM622. ACS Appl. Energy Mater. 2021, 4, 7168–7181.
- Xi, X.; Fan, Y.; Liu, Y.; Chen, Z.; Zou, J.; Zhu, S. Enhanced cyclic stability of NCM-622 cathode by Ti3+ doped TiO2 coating. J. Alloys Compd. 2021, 872, 159664.
- Razmjoo Khollari, M.A.; Azar, M.K.; Esmaeili, M.; Malekpour, N.; Hosseini-Hosseinabad, S.M.; Moakhar, R.S.; Dolati, A.; Ramakrishna, S. Electrochemical performance and elevated temperature properties of the TiO2-coated LiO2 cathode material for high-safety li-ion batteries. ACS Appl. Energy Mater. 2021, 4, 5304–5315.
- Peng, Z.; Li, T.; Zhang, Z.; Du, K.; Hu, G.; Cao, Y. Surface architecture decoration on enhancing properties of LiNi0.8Co0.1Mn0.1O2 with building bi-phase Li3PO4 and AlPO4 by Al(H2PO4)3 treatment. Electrochim. Acta 2020, 338, 135870.
- Wu, Y.; Ming, H.; Li, M.; Zhang, J.; Wahyudi, W.; Xie, L.; He, X.; Wang, J.; Wu, Y.; Ming, J. New organic complex for lithium layered oxide modification: Ultrathin coating, high-voltage, and safety performances. ACS Energy Lett. 2019, 4, 656–665.
- Zha, G.; Luo, Y.; Hu, N.; Ouyang, C.; Hou, H. Surface modification of the LiNi0.8Co0.1Mn0.1O2 cathode material by coating with FePO4 with a yolk-shell structure for improved electrochemical performance. ACS Appl. Mater. Interfaces 2020, 12, 36046–36053.
- Min, K.; Park, K.; Park, S.Y.; Seo, S.W.; Choi, B.; Cho, E. Improved electrochemical properties of LiNi0.91Co0.06Mn0.03O2 cathode material via Li-reactive coating with metal phosphates. Sci. Rep. 2017, 7, 1–10.
- Lee, D.J.; Scrosati, B.; Sun, Y.K. Ni3(PO4)2-coated LiO2 lithium battery electrode with improved cycling performance at 55 °C. J. Power Sources 2011, 196, 7742–7746.
- Sattar, T.; Lee, S.H.; Sim, S.J.; Jin, B.S.; Kim, H.S. Understanding the combined effect of Ca doping and phosphate coating on Ni-rich LiNi0.91Co0.06Mn0.03O2 cathode material for Li-ion batteries. Electrochim. Acta 2021, 399, 139417.
- Xu, L.; Zhou, F.; Liu, B.; Zhou, H.; Zhang, Q.; Kong, J.; Wang, Q. Progress in preparation and modification of LiNi0.6Mn0.2Co0.2O2 cathode material for high energy density li-ion batteries. Int. J. Electrochem. 2018, 2018, 1–12.
- Cho, W.; Kim, S.M.; Lee, K.W.; Song, J.H.; Jo, Y.N.; Yim, T.; Kim, H.; Kim, J.S.; Kim, Y.J. Investigation of new manganese orthophosphate Mn3(PO4)2 coating for nickel-rich LiNi0.6Co0.2Mn0.2O2 cathode and improvement of its thermal properties. Electrochim. Acta 2016, 198, 77–83.
- Akella, S.H.; Taragin, S.; Wang, Y.; Aviv, H.; Kozen, A.C.; Zysler, M.; Wang, L.; Sharon, D.; Lee, S.B.; Noked, M. Improvement of the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 via atomic layer deposition of lithium-rich zirconium phosphate coatings. ACS Appl. Mater. Interfaces 2021, 13, 61733–61741.
- Hu, G.; Zhang, Z.; Li, T.; Gan, Z.; Du, K.; Peng, Z.; Xia, J.; Tao, Y.; Cao, Y. In Situ Surface Modification for Improving the Electrochemical Performance of Ni-Rich Cathode Materials by Using ZrP2O7. ChemSusChem 2020, 13, 1603–1612.
- Zhao, Z.; Wen, Z.; Liu, X.; Yang, H.; Chen, S.; Li, C.; Lv, H.; Wu, F.; Wu, B.; Mu, D. Tuning a compatible interface with LLZTO integrated on cathode material for improving NCM811/LLZTO solid-state battery. Chem. Eng. J. 2021, 405, 127031.
- Jo, C.H.; Cho, D.H.; Noh, H.J.; Yashiro, H.; Sun, Y.K.; Myung, S.T. An effective method to reduce residual lithium compounds on Ni-rich LiO2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res. 2014, 8, 1464–1479.
- Fan, Q.; Yang, S.; Liu, J.; Liu, H.; Lin, K.; Liu, R.; Hong, C.; Liu, L.; Chen, Y.; An, K.; et al. Mixed-conducting interlayer boosting the electrochemical performance of Ni-rich layered oxide cathode materials for lithium ion batteries. J. Power Sources 2019, 421, 91–99.
- Li, J.; Liu, Y.; Yao, W.; Rao, X.; Zhong, S.; Qian, L. Li2TiO3 and Li2ZrO3 co-modification LiNi0.8Co0.1Mn0.1O2 cathode material with improved high-voltage cycling performance for lithium-ion batteries. Solid State Ionics 2020, 349, 115292.
- Song, B.; Li, W.; Oh, S.M.; Manthiram, A. Long-life nickel-rich layered oxide cathodes with a uniform Li2ZrO3 surface coating for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 9718–9725.
- Zhao, C.; Liu, Z.Q.; Weng, W.; Wu, M.; Yan, X.Y.; Yang, J.; Lu, H.M.; Yao, X.Y. Stabilized cathode/sulfide solid electrolyte interface via Li2ZrO3 coating for all-solid-state batteries. Rare Met. 2022, 41, 3639–3645.
- Yang, J.; Ren, K.; Hong, M.; Wang, T.; Wang, D.; Wang, T.; Wang, H. New insight into lattice variations of Ni-rich NMC811 cathode induced by Li2ZrO3 coating. Mater. Technol. 2021, 37, 1926–1935.
- Yang, G.; Pan, K.; Lai, F.; Wang, Z.; Chu, Y.; Yang, S.; Han, J.; Wang, H.; Zhang, X.; Li, Q. Integrated co-modification of PO43−polyanion doping and Li2TiO3 coating for Ni-rich layered LiNi0.6Co0.2Mn0.2O2 cathode material of Lithium-Ion batteries. Chem. Eng. J. 2021, 421, 129964.
- Wang, J.; Yu, Y.; Li, B.; Fu, T.; Xie, D.; Cai, J.; Zhao, J. Improving the electrochemical properties of LiNi(0.5)Co(0.2)Mn(0.3)O2 at 4.6 V cutoff potential by surface coating with Li2TiO3 for lithium-ion batteries. Phys. Chem. Chem. Phys. 2015, 17, 32033–32043.
- Liu, M.; Zhou, J.; Cheng, T.; Feng, Z.; Qin, Y.; Guo, B. Dual design of the surface via an ion conductor coating and in situ electrochemical diffusion enabling a long life for a Ni-rich cathode. ACS Appl. Energy Mater. 2022, 5, 9181–9188.
- Jan, S.S.; Nurgul, S.; Shi, X.; Xia, H.; Pang, H. Improvement of electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by graphene nanosheets modification. Electrochim. Acta 2014, 149, 86–93.
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1561.
- Luu, N.S.; Lim, J.-M.; Torres-Castanedo, C.G.; Park, K.-Y.; Moazzen, E.; He, K.; Meza, P.E.; Li, W.; Downing, J.R.; Hu, X.; et al. Elucidating and mitigating high-voltage interfacial chemomechanical degradation of nickel-rich lithium-ion battery cathodes via conformal graphene coating. ACS Appl. Energy Mater. 2021, 4, 11069–11079.
- Shim, J.H.; Kim, Y.M.; Park, M.; Kim, J.; Lee, S. Reduced graphene oxide-wrapped nickel-rich cathode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 18720–18729.
- Ning, R.; Yuan, K.; Zhang, K.; Shen, C.; Xie, K. A scalable snowballing strategy to construct uniform rGO-wrapped LiNi0.8Co0.1Mn0.1O2 with enhanced processability and electrochemical performance. Appl. Surf. Sci. 2021, 542, 148663.
- Xu, G.L.; Liu, Q.; Lau, K.K.S.; Liu, Y.; Liu, X.; Gao, H.; Zhou, X.; Zhuang, M.; Ren, Y.; Li, J.; et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 2019, 4, 484–494.
- Sun, Z.; Li, Z.; Gao, L.; Zhao, X.; Han, D.; Gan, S.; Guo, S.; Niu, L. Grafting benzenediazonium tetrafluoroborate onto LiNixCoyMnzO2 materials achieves subzero-temperature high-capacity lithium-ion storage via a diazonium soft-chemistry method. Adv. Energy Mater. 2018, 9, 1802946.
- Diao, H.; Jia, M.; Zhao, N.; Guo, X. LiNi0.6Co0.2Mn0.2O2 cathodes coated with dual-conductive polymers for high-rate and long-life solid-state lithium batteries. ACS Appl. Mater. Interfaces 2022, 14, 24929–24937.
- Gan, Q.; Qin, N.; Zhu, Y.; Huang, Z.; Zhang, F.; Gu, S.; Xie, J.; Zhang, K.; Lu, L.; Lu, Z. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-rich LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 12594–12604.
- Fan, Q.; Lin, K.; Shi, Z.; Guan, S.; Chen, J.; Feng, S.; Liu, L. Constructing high conductive composite coating with tin and polypyrrole to improve the performance of LiNi0.8Co0.1Mn0.1O2 at high cutoff voltage of 4.5 V. ACS Appl. Energy Mater. 2021, 4, 10012–10024.
- Yu, H.; Zhu, H.; Yang, Z.; Liu, M.; Jiang, H.; Li, C. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-life for Ni-rich layered cathodes. Chem. Eng. J. 2021, 412, 128625.
- Sun, Y.K.; Myung, S.T.; Park, B.C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324.
- Kim, U.H.; Ryu, H.H.; Kim, J.H.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Microstructure-controlled Ni-rich cathode material by microscale compositional partition for next-generation electric vehicles. Adv. Energy Mater. 2019, 9, 1803902.
- Ryu, H.M.; Kim, M.Y.; Jung, H.Y.; Lim, J.S.; Kim, Y.A.; Kim, H.S. Fabrication and electrochemical behavior of thin composite solid electrolyte for all-solid lithium batteries. Ionics 2020, 26, 2863–2874.
- Cheng, F.; Zhang, X.; Qiu, Y.; Zhang, J.; Liu, Y.; Wei, P.; Ou, M.; Sun, S.; Xu, Y.; Li, Q.; et al. Tailoring electrolyte to enable high-rate and super-stable Ni-rich NCM cathode materials for Li-ion batteries. Nano Energy 2021, 88, 106301.
- Shen, L.; Wu, H.B.; Liu, F.; Shen, J.; Mo, R.; Chen, G.; Tan, G.; Chen, J.; Kong, X.; Lu, X.; et al. Particulate anion sorbents as electrolyte additives for lithium batteries. Adv. Funct. Mater. 2020, 30, 2003055.
- Shi, X.; Zheng, T.; Xiong, J.; Zhu, B.; Cheng, Y.J.; Xia, Y. Stable electrode/electrolyte interface for high-voltage NCM523 cathode constructed by synergistic positive and passive approaches. ACS Appl. Mater. Interfaces 2021, 13, 57107–57117.
- Pham, H.Q.; Thi Tran, Y.H.; Han, J.; Song, S.W. Roles of nonflammable organic liquid electrolyte in stabilizing the interface of the LiNi0.8Co0.1Mn0.1O2 Cathode at 4.5 V and improving the battery performance. J. Phys. Chem. C 2019, 124, 175–185.
- Lee, Y.; Lee, T.K.; Kim, S.; Lee, J.; Ahn, Y.; Kim, K.; Ma, H.; Park, G.; Lee, S.M.; Kwak, S.K.; et al. Fluorine-incorporated interface enhances cycling stability of lithium metal batteries with Ni-rich NCM cathodes. Nano Energy 2020, 67, 104309.
- Wu, Q.; Mao, S.; Wang, Z.; Tong, Y.; Lu, Y. Improving LiNixCoyMn1−x−yO2 cathode electrolyte interface under high voltage in lithium ion batteries. Nano Sel. 2020, 1, 111–134.
- An, F.; Zhao, H.; Zhou, W.; Ma, Y.; Li, P. S-containing and Si-containing compounds as highly effective electrolyte additives for SiOx -based anodes/NCM 811 cathodes in lithium ion cells. Sci. Rep. 2019, 9, 1–16.
- Yan, P.; Zheng, J.; Liu, J.; Wang, B.; Cheng, X.; Zhang, Y.; Sun, X.; Wang, C.; Zhang, J.G. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600–605.
- Xu, S.; Sun, Z.; Sun, C.; Li, F.; Chen, K.; Zhang, Z.; Hou, G.; Cheng, H.M.; Li, F. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv. Funct. Mater. 2020, 30, 2007172.
- Kitsche, D.; Tang, Y.; Ma, Y.; Goonetilleke, D.; Sann, J.; Walther, F.; Bianchini, M.; Janek, J.; Brezesinski, T. High performance all-solid-state batteries with a Ni-rich NCM cathode coated by atomic layer deposition and lithium thiophosphate solid electrolyte. ACS Appl. Energy Mater. 2021, 4, 7338–7345.
- Walther, F.; Strauss, F.; Wu, X.; Mogwitz, B.; Hertle, J.; Sann, J.; Rohnke, M.; Brezesinski, T.; Janek, J. The working principle of a Li2CO3/LiNbO3 coating on NCM for thiophosphate-based all-solid-state batteries. Chem. Mater. 2021, 33, 2110–2125.
- Zhao, C.Z.; Zhao, Q.; Liu, X.; Zheng, J.; Stalin, S.; Zhang, Q.; Archer, L.A. Rechargeable lithium metal batteries with an in-built solid-state polymer electrolyte and a high voltage/loading Ni-rich layered cathode. Adv. Mater. 2020, 32, e1905629.
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 1–8.
- Negi, R.S.; Yusim, Y.; Pan, R.; Ahmed, S.; Volz, K.; Takata, R.; Schmidt, F.; Henss, A.; Elm, M.T. A Dry-Processed Al2O3/LiAlO2 coating for stabilizing the cathode/electrolyte interface in high-Ni NCM-based all-solid-state batteries. Adv. Mater. Interfaces 2021, 9, 2101428.
- Yersak, T.A.; Hao, F.; Kang, C.; Salvador, J.R.; Zhang, Q.; Malabet, H.J.G.; Cai, M. Consolidation of composite cathodes with NCM and sulfide solid-state electrolytes by hot pressing for all-solid-state Li metal batteries. J. Solid State Electrochem. 2022, 26, 709–718.
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452.
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059.
- Li, J.; Li, H.; Stone, W.; Weber, R.; Hy, S.; Dahn, J.R. Synthesis of single crystal LiNi0.5Mn0.3Co0.2O2 for lithium ion batteries. J. Electrochem. Soc. 2017, 164, A3529–A3537.
- Yang, C. Superior cycle stability of single crystal nickel-rich layered oxides with micron-scale grain size as cathode material for lithium ion batteries. Int. J. Electrochem. Sci. 2020, 15, 5031–5041.
- Kim, M.; Zhu, J.; Li, L.; Wang, C.; Chen, G. Understanding reactivities of Ni-rich LiO2 single-crystal cathode materials. ACS Appl. Energy Mater. 2020, 3, 12238–12245.
- Pang, P.; Tan, X.; Wang, Z.; Cai, Z.; Nan, J.; Xing, Z.; Li, H. Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim. Acta 2021, 365, 137380.
- Guo, Q.; Huang, J.; Liang, Z.; Potapenko, H.; Zhou, M.; Tang, X.; Zhong, S. The use of a single-crystal nickel-rich layered NCM cathode for excellent cycle performance of lithium-ion batteries. New J. Chem. 2021, 45, 3652–3659.
- Tian, R.Z.; Wang, Z.X.; Wang, X.Q.; Zhang, H.Z.; Ma, Y.; Song, D.W.; Shi, X.X.; Zhang, L.Q. Preparation and electrochemical investigation of single-crystal LiNi0.6Co0.2Mn0.2O2 for high-performance lithium-ion batteries. New J. Chem. 2022, 46, 4877–4883.
- Zhao, Y.; Liu, L.; Cheng, J.; Yang, Z.; Dong, P.; Meng, Q.; Zhang, Y.; Li, Y. Toward high stability single crystal material by structural regulation with high and low temperature mixing sinter. Ceram. Int. 2022.
- Zhu, H.; Tang, Y.; Wiaderek, K.M.; Borkiewicz, O.J.; Ren, Y.; Zhang, J.; Ren, J.; Fan, L.; Li, C.C.; Li, D.; et al. Spontaneous strain buffer enables superior cycling stability in single-crystal nickel-rich NCM cathode. Nano Lett. 2021, 21, 9997–10005.
- Saleem, A.; Hussain, A.; Ashfaq, M.Z.; Javed, M.S.; Rauf, S.; Hussain, M.M.; Saad, A.; Shen, J.; Majeed, M.K.; Iqbal, R. A well-controlled cracks and gliding-free single-crystal Ni-rich cathode for long-cycle-life lithium-ion batteries. J. Alloys Compd. 2022, 924, 166375.
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450.
- Han, G.M.; Kim, Y.S.; Ryu, H.H.; Sun, Y.K.; Yoon, C.S. Structural stability of single-crystalline ni-rich layered cathode upon delithiation. ACS Energy Lett. 2022, 7, 2919–2926.
- Zhu, J.; Chen, G. Single-crystal based studies for correlating the properties and high-voltage performance of LiO2 cathodes. J. Mater. Chem. A 2019, 7, 5463–5474.
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149.
- Azhari, L.; Meng, Z.; Yang, Z.; Gao, G.; Han, Y.; Wang, Y. Underlying limitations behind impedance rise and capacity fade of single crystalline Ni-rich cathodes synthesized via a molten-salt route. J. Power Sources 2022, 545, 231963.
- Guo, Z.; Jian, Z.; Zhang, S.; Feng, Y.; Kou, W.; Ji, H.; Yang, G. The effect of Ni oxidation state on the crystal structure and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material for highly reversible lithium storage. J. Alloys Compd. 2021, 882, 160642.
- Lv, F.; Zhang, Y.; Wu, M.; Gu, Y. A Molten-salt method to synthesize ultrahigh-nickel single-crystalline LiNi0.92Co0.06Mn0.02O2 with superior electrochemical performance as cathode material for lithium-ion batteries. Small 2022, 18, 2201946.
- Ma, X.; Hou, J.; Vanaphuti, P.; Yao, Z.; Fu, J.; Azhari, L.; Liu, Y.; Wang, Y. Direct upcycling of mixed Ni-lean polycrystals to single-crystal Ni-rich cathode materials. Chem 2022, 8, 1944–1955.
- Ni, L.; Guo, R.; Fang, S.; Chen, J.; Gao, J.; Mei, Y.; Zhang, S.; Deng, W.; Zou, G.; Hou, H.; et al. Crack-free single-crystalline Co-free Ni-rich LiNi0.95Mn0.05O2 layered cathode. eScience 2022, 2, 116–124.
- Huang, C.; Xia, X.; Chi, Z.; Yang, Z.; Huang, H.; Chen, Z.; Tang, W.; Wu, G.; Chen, H.; Zhang, W. Preparation of single-crystal ternary cathode materials via recycling spent cathodes for high performance lithium-ion batteries. Nanoscale 2022, 14, 9724–9735.
- Liu, L.; Zhang, Y.; Zhao, Y.; Jiang, G.; Gong, R.; Li, Y.; Meng, Q.; Dong, P. Surface growth and intergranular separation of polycrystalline particles for regeneration of stable single-crystal cathode materials. ACS Appl. Mater. Interfaces 2022, 14, 29886–29895.
- Yan, W.; Jia, X.; Yang, S.; Huang, Y.; Yang, Y.; Yuan, G. Synthesis of single crystal LiNi0.92Co0.06Mn0.01Al0.01O2 cathode materials with superior electrochemical performance for lithium ion batteries. J. Electrochem. Soc. 2020, 167, 120541.
- Ren, S.; Tian, L.; Shao, Q.; Chen, J. Synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 by flux method. Energy Storage Sci. Technol. 2020, 9, 1702–1713.
- Lee, S.H.; Sim, S.J.; Jin, B.S.; Kim, H.S. High performance well-developed single crystal LiNi0.91Co0.06Mn0.03O2 cathode via LiCl-NaCl flux method. Mater. Lett. 2020, 270, 127615.
- Kimijima, T.; Zettsu, N.; Yubuta, K.; Hirata, K.; Kami, K.; Teshima, K. Molybdate flux growth of idiomorphic Li(Ni1/3Co1/3Mn1/3)O2 single crystals and characterization of their capabilities as cathode materials for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 7289–7296.
- Li, Y.; He, J.; Luo, L.; Li, X.; Chen, Z.; Zhang, Y.; Deng, L.; Dong, P.; Yang, S.; Wu, K.; et al. Highly dispersed micrometer nickel-rich single-crystal construction: Benefits of supercritical reconstruction during hydrothermal synthesis. ACS Appl. Energy Mater. 2022, 5, 6302–6312.
- Guo, F.; Xie, Y.; Zhang, Y. Low-temperature strategy to synthesize single-crystal LiNi0.8Co0.1Mn0.1O2 with enhanced cycling performances as cathode material for lithium-ion batteries. Nano Res. 2021, 15, 2052–2059.
- Du, K.; Zhu, F.; Sun, Q.; Hu, G.; Peng, Z.; Cao, Y.; Zhang, Y.; Li, L.; Huang, J.; Zhang, S. Ni0.6Co0.2Mn0.2(OH)2 with dispersed hexagonal slabs enables synthesis of single crystal LiNi0.6Co0.2Mn0.2O2 with enhanced electrochemical performance for lithium-ion batteries. J. Alloys Compd. 2021, 873, 159839.
- Zhang, D.; Liu, Y.; Feng, L.; Qin, W. Evolution effect of Ti-based modifiers awards improved lithium ion diffusion rate of single crystal nickel-rich cathode. J. Solid State Chem. 2022, 306, 122796.
- Chen, S.; Zhang, X.; Xia, M.; Wei, K.; Zhang, L.; Zhang, X.; Cui, Y.; Shu, J. Issues and challenges of layered lithium nickel cobalt manganese oxides for lithium-ion batteries. J. Electroanal. Chem. 2021, 895, 115412.
- Zhang, Z.; Bai, M.; Fan, X.; Yi, M.; Zhao, Y.; Zhang, J.; Hong, B.; Zhang, Z.; Hu, G.; Lai, Y. A low cost single-crystalline LiNi0.60Co0.10Mn0.30O2 layered cathode enables remarkable cycling performance of lithium-ion batteries at elevated temperature. J. Power Sources 2021, 503, 230028.
- Zhao, W.; Zou, L.; Zhang, L.; Fan, X.; Zhang, H.; Pagani, F.; Brack, E.; Seidl, L.; Ou, X.; Egorov, K.; et al. Assessing long-term cycling stability of single-crystal versus polycrystalline nickel-rich NCM in pouch cells with 6 mAh cm(-2) electrodes. Small 2022, 18, 2107357.
- Ryu, H.H.; Lee, S.B.; Sun, Y.K. Promoting grain growth in Ni-rich single-crystal cathodes for high-performance lithium-ion batteries through Ce doping. J. Solid State Electrochem. 2022, 26, 2097–2105.
- Weber, R.; Li, H.; Chen, W.; Kim, C.-Y.; Plucknett, K.; Dahn, J.R. In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials. J. Electrochem. Soc. 2020, 167, 100501.
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-free Ni-rich single crystal positive electrode materials for lithium ion batteries: Part I. Two-step lithiation method for Al- or Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 040531.
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-free Ni-rich single crystal positive electrode materials for lithium ion batteries: Part II. One-step lithiation method of Mg-doped LiNiO2. J. Electrochem. Soc. 2021, 168, 050506.
- Han, Y.; Heng, S.; Wang, Y.; Qu, Q.; Zheng, H. anchoring interfacial nickel cations on single-crystal LiNi0.8Co0.1Mn0.1O2 cathode surface via controllable electron transfer. ACS Energy Lett. 2020, 5, 2421–2433.
- Guo, J.; Li, W. Synthesis of single-crystal LiNi0.7Co0.15Mn0.15O2 materials for Li-ion batteries by a sol–gel method. ACS Appl. Energy Mater. 2021, 5, 397–406.
- Ma, X.; Vanaphuti, P.; Fu, J.; Hou, J.; Liu, Y.; Zhang, R.; Bong, S.; Yao, Z.; Yang, Z.; Wang, Y. A universal etching method for synthesizing high-performance single crystal cathode materials. Nano Energy 2021, 87, 106194.
- Malik, M.; Chan, K.H.; Azimi, G. Review on the synthesis of LiNixMnyCo1-x-yO2 (NMC) cathodes for lithium-ion batteries. Mater. Today Energy 2022, 28, 101066.
- Zhu, J.; Zheng, J.; Cao, G.; Li, Y.; Zhou, Y.; Deng, S.; Hai, C. Flux-free synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 boosts its electrochemical performance in lithium batteries. J. Power Sources 2020, 464, 228207.
- Huang, Z.; Liu, X.; Zheng, Y.; Wang, Q.; Liu, J.; Xu, S. Boosting efficient and low-energy solid phase regeneration for single crystal LiNi0.6Co0.2Mn0.2O2 via highly selective leaching and its industrial application. Chem. Eng. J. 2023, 451, 139039.
- Gao, D.; Yang, J.; Zhang, D.; Chang, C. An effective strategy to enhance the electrochemical performance of LiNi0.6Mn0.2Co0.2O2: Optimizing a Li diffusion pathway via magnetic alignment of single-crystal cathode material under an ordinary 0.4-T magnetic field. Ceram. Int. 2022, 48, 31598–31605.
More
