Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Jiamiao Hu.
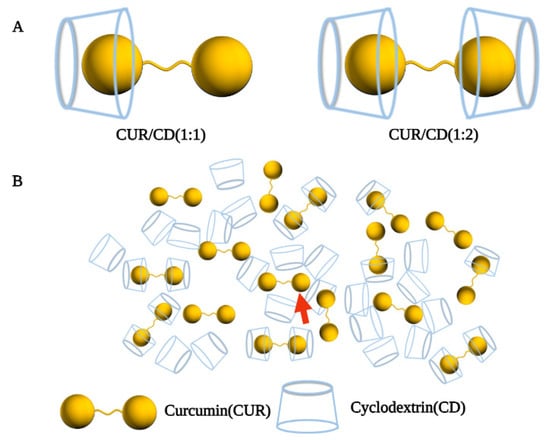
Supramolecular systems are based on molecular recognition, in which two or more molecules are bound by intermolecular non-covalent bonding forces to form complex and ordered entities or aggregates with specific functions and properties.
- cyclodextrin
- curcumin
- supramolecular system
- stimuli-responsive delivery
1. Curcumin–Cyclodextrin Supramolecular System with Cyclodextrin as the Carrier
1.1. Natural Cyclodextrins
The first wave of studies has focused on the interaction of CUR with natural CDs. The researchers used solvent evaporation, freeze-drying, kneading, and other methods to prepare curcumin–cyclodextrin inclusion complexes [19][1]. When inclusion complexes are formed, the crystallinity, solubility, and optical properties of CUR molecules are changed, and the properties are subsequently measured using thermal analysis, spectroscopy, or chromatography to verify the formation of the inclusion complex [30][2].
Solvent evaporation is the most commonly used method for the preparation of curcumin–cyclodextrin complexes due to its simplicity (Figure 1A). For instance, this method was used by Yallapu et al. to prepare CUR/β-CD as a light-yellow fluffy powder with excellent aqueous solubility (Figure 1B). Unlike pure curcumin, which readily precipitates in an aqueous solution, curcumin encapsulated by β-CD showed an increased solubility up to 1.84 mg/mL in water (Figure 1C) [31][3]. In another study, López-Tobar et al. used β-CD and γ-CD as the carriers to encapsulate curcumin and analyzed the stability of the inclusion complexes using Raman spectroscopy. The results illustrated that H-bonds play an important role in the encapsulation process of curcumin, prompting changes in the structure of curcumin from the planar keto-enol tautomer to the non-planar diketone tautomer. These changes may cause an increase in the bioavailability, bioactivity, and chemical stability of curcumin. It is also interesting to note that the authors mention that γ-CD affords better encapsulation than β-CD, which may be due to the fact that the size matching between curcumin and gamma CD cavity is better [32][4]. Similarly, Alizadeh et al. also demonstrated that hydrogen bonds play a key role in enhancing the physiological activity of CUR [33][5]. Their study compared the antioxidant activity of free CUR with CUR/β- or γ-CD. The results indicated that CUR/γ-CD had superior antioxidant activity to that of CUR/β-CD or free CUR. This was attributed to the formation of one or more intermolecular hydrogen bonds upon the complexation of CUR by the CDs, which affected the intramolecular hydrogen bonds of CUR, thus enhancing the hydrogen-donating ability (enhanced antioxidant activity) of CUR molecules. In addition, Jahed et al. further investigated the interaction forces between CUR and CD using NMR spectroscopy [34][6]. The 1H NMR and 2D ROESY spectra confirmed that the chemical shifts of the internal protons of β-CD (H-3 and H-5) were shifted after encapsulation, and there was a cross-peak between the H-3 proton of β-CD and the aromatic rings group of CUR. These studies show that the driving forces involved in the CD encapsulation of CUR include hydrophobic interactions between host and guest, hydrogen bonding, van der Waals forces, and other non-covalent bonding forces. These driving forces sometimes act individually, but in most cases, multiple forces act synergistically to promote the formation of supramolecular systems.
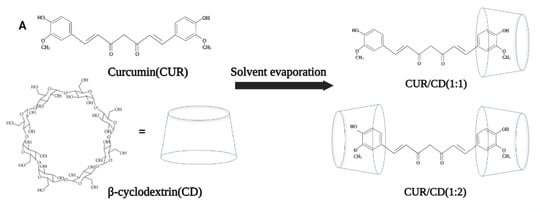
Figure 1. Schematic diagram of the preparation of curcumin/β-cyclodextrin supramolecules by solvent evaporation [31].
Schematic diagram of the preparation of curcumin/β-cyclodextrin supramolecules by solvent evaporation [3].
1.2. Cyclodextrin Derivatives
Natural CDs themselves have many shortcomings, such as the small pore size of α-CD cavities and relatively low water solubility of β-CD, which hinder the further application of CDs. For this reason, researchers have introduced modified groups to obtain cyclodextrin derivatives with different properties or functions, while keeping the basic skeleton of CD macrocycles unchanged. These derivatives are classified as hydrophilic, hydrophobic, ionic, and amphiphilic; and these modified CDs have also been widely investigated in the application of curcumin encapsulation.
Since the main objective of curcumin–cyclodextrin complexation is to obtain an inclusion complex with high water solubility, therefore, hydrophilic cyclodextrin derivatives are the primary choice for encapsulating CUR. For example, hydroxypropyl β-CD (HP-β-CD) is an alkylation product of β-CD. Alkylating the -OH groups on the periphery of β-CD with hydroxypropyl groups breaks the series of hydrogen bonds that these -OH groups make. This improves the solubility of the resulting HP-β-CD [35][7]. Li et al. used HP-β-CD as a carrier to improve the solubility and oral bioavailability of the poorly soluble drug CUR [36][8]. In rats, CUR/HP-β-CD and free CUR had similar pharmacokinetic behaviors after intravenous administration, and both had similar antitumor efficacy. Moreover, the oral bioavailability of CUR was enhanced 2.77-fold by HP-β-CD encapsulation. Additionally, Shityakov et al. demonstrated that the concentration of CUR in distilled water was about 60-fold higher when HP-γ-CD was used as compared to γ-CD due to the better hydrophilicity of HP-γ-CD [37][9]. These articles showed that CUR encapsulated in hydroxypropyl-modified CD resulted in a complex with superior water solubility.
Interestingly, Mai et al. prepared solid dispersions of CUR/HP-β-CD by grinding, freeze-drying, and common solvent evaporation methods [38][10]. The solubility of the inclusion complexes was increased 299, 180, and 489-fold, respectively, as compared with CUR crystals. Surprisingly, this solid dispersion did not consist of pure inclusion complexes but was rather a mixed system. The system consisted of free CUR molecules, inclusion complexes, CUR molecules not in inclusion complexes, and empty HP-β-CD molecules (Figure 2B). One or both of the aromatic rings of curcumin entered into the HP-β-CD cavity to form a 1:1 or 2:1 host-to-guest ratio inclusion complex (Figure 2A). In addition, unlike the free CUR molecules, the CUR molecules that were not in the inclusion complex may have been trapped in the cavities of a three-dimensional network structure formed by the polymerization of multiple cyclodextrin monomers. These results indicate that the actual process of curcumin inclusion in cyclodextrin does not present an ideal state in which the components are independent of each other, but rather is a complex system.
2. Curcumin–Cyclodextrin Supramolecular System with Cyclodextrin Polymer as the Carrier
The structure of CD allows the formation of polymers with different structural characteristics, either covalently or non-covalently bonded [57][11]. These polymers have both the inclusion properties of CD and the favorable properties of polymers and are often used to form complexes with other molecules [58][12].
2.1. Cyclodextrin Self-Assembled Supramolecular Networks with Curcumin Encapsulated
Self-assembly can be defined as the process by which molecules or other assembled substrates spontaneously form ordered structural bodies through weak interactions [59][13]. CDs form amorphous, micelle-like, high-molecular-weight polymers by self-assembly to form a supramolecular system with the guest [27][14]. In this system, changes to molecular structure translate to differences in supramolecular forms, including superlattice crystals, micelles, vesicles, and liquid crystals. Each of these has unique structural features and new physicochemical properties completely different from those of the original constituent molecules [60][15].
Supramolecular vesicles are hollow spheres with hydrophobic membranes and hydrophilic interiors, and the strategy of loading CUR in CD self-assembled supramolecular vesicles may be a good solution [61][16]. In a study by Ma et al., CD molecules encapsulated CUR through host–guest recognition to form a supramolecular amphiphile, which further self-assembled into vesicles due to hydrophobic interactions (Figure 3A). The resulting CUR/CD vesicles were hollow spheres with diameters in the range of 70–130 nm based on TEM and SEM observations, which increased the water solubility of CUR by 7000-fold (solubility up to 2 × 10−4 mol/L) [62][17]. In another study by Bai et al., the β-CD trimer(β-CD3) could form micelles in the presence of CUR as a guest unit by host–guest inclusion interaction and hydrophilic-hydrophobic interactions when the formed supramolecular self-assembly’s concentration was above the critical aggregation concentration. Furthermore, adjusting the ratio of β-CD3 to CUR, the transformation of the supramolecular self-assembled structure from spherical micelles (β-CD3: CUR at 2:3) to multi-compartment vesicles (β-CD3: CUR at 6:3) could be achieved (Figure 3B) [63][18]. Furthermore, in basal cell experiments, spindle-like complex micelles (β-CD3: CUR at 4:3) and multi-compartmental vesicles (β-CD3: CUR at 6:3) exhibited greater cytotoxicity, uptake capacity, and apoptosis rates than spherical complex micelles (β-CD3: CUR at 2:3), suggesting that altered self-assembly morphology somewhat influences the biological performance of the assemblies.
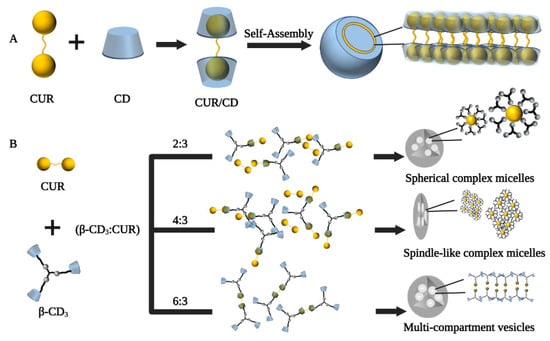
Figure 3. Preparation process of cyclodextrin self-assembled supramolecular networks. (A) Schematic diagram of the proposed mechanism of vesicle formation from CD and CUR [62][17]. (B) Schematic diagram of supramolecular self-assembly of three different shapes of curcumin/β-cyclodextrin trimers [63][18].
2.2. Cross-Linked Cyclodextrin Polymer-Encapsulated Curcumin
Cross-linked CD polymers are formed by the covalent bonding of individual cyclodextrin monomers by cross-linking agents to form a cross-linked network structure, which is different from self-assembled non-covalently bonded polymerization [64][19]. The most common and widely reported method is the cross-linking of CD with epichlorohydrin, which was used by Chen et al. to prepare CD polymers for the encapsulation of CUR [65][20]. The resulting curcumin–cyclodextrin polymers exhibited higher anti-proliferative activity against A375 cells, compared to free CUR. In another study by Haimhoffer et al., polyethylene glycol was used as a cross-linking agent for CDs to form ternary complexes with CUR [66][21]. The resulting CD polymer effectively delivered the complexed CUR to the cell membrane, which improved the CUR permeability significantly more than the CD polymer cross-linked with encapsulation. Interestingly, the reaction of CDs with cross-linking agents such as diphenyl carbonate, diisocyanate, phthalic anhydride, and carbonyl compounds can yield cyclodextrin nano-sponges, cross-linked CD polymers with nano-sized, porous structures. Compared to common cyclodextrins, they form inclusion and non-inclusion complexes with drugs, which can improve drug delivery capacity as well as prolong the release of drug molecules. Mashaqbeh et al. prepared CD-based nano-sponges with diphenyl carbonate as a cross-linking agent, which enhanced the stability and solubility of CUR. The solubility of CUR was enhanced in the CD-based nano-sponges compared to the CUR/CD inclusion complex. In addition, the three-dimensional structure of the nano-sponges imparted higher stability to the complex [67][22]. In another study by Pushpalatha et al., CD-based nano-sponges (CDNS) prepared with two different cross-linking agents—diphenyl carbonate (DPC) and pyromellitic dianhydride (PMDA)—were compared for the delivery of CUR [68][23]. Compared to pure CUR, CUR-DPC-CDNS showed a 5-fold increase in solubility, while CUR-PMDA-CDNS showed a 16-fold increase in solubility. In cytotoxicity assays in MCF-7 cells, CUR-PMDA-CDNS exhibited higher cytotoxicity than CUR-DPC-CDNS. PMDA cross-linking may be a better method to obtain nano-sponges. In a similar study, Rafati et al. used this method to prepare CD nano-sponges, which were complexed with CUR to extend the drug release time, which was sustained over 42 h. The porous structure of the nano-sponges allowed CUR to bind to CD on the surface of the carrier and inside the cavity, exhibiting biphasic drug-release kinetics. The CUR molecules located on the surface of the nano-sponge were first released rapidly, followed by the slow release of CUR molecules located inside the cavity [69][24].
2.3. Diamine-Linked CD Dimer-Encapsulated Curcumin
In contrast to the high molecular weight possessed by cross-linked CDs, γ-CD oligomers could be used as carriers of CUR for the treatment of prostate cancer cells by Harada et al. [70][25]. In this drug-delivery system, two γ-CDs were substituted for each CD glucopyranose unit C6A site by succinamide or urea to form a diamine-linked γ-CD dimer, which then encapsulated the CUR by hydrogen bonding. During drug delivery, the diamine linker can be hydrolyzed by intracellular enzymes, resulting in the intracellular release of the drug CUR [71,72][26][27].
3. Other Novel Cyclodextrin Nano-Supramolecular Systems with Curcumin
3.1. Chitosan-Based Nano-Systems
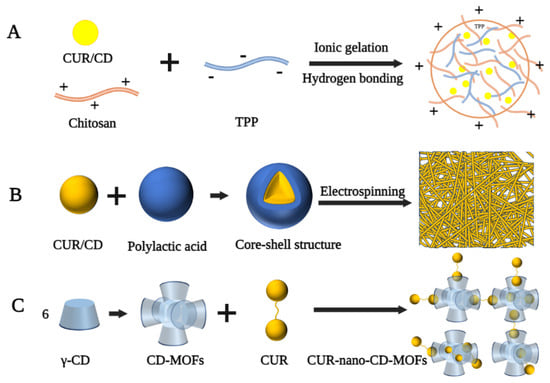
Chitosan (CS) is a linear polysaccharide produced by the deacetylation of chitin and is often used to develop nanomaterials as carriers [82][28]. In the delivery system of CUR and CD, the negative charge of CUR/CD limits its cellular delivery properties and its therapeutic efficacy. CS, as a cationic natural polysaccharide, can form more stable inclusion complexes through ionic interactions to facilitate intracellular drug transport [83,84][29][30]. Popat et al. prepared CUR/CD-CS nanoparticles with a particle size in the range of 180–200 nm, spherical shape, and zeta potential of +15 mv for the treatment of human skin cancer cells (SCC25) [85][31]. Highly soluble CUR/CD hollow spheres were first prepared by a spray drying method, followed by the addition of tripolyphosphate (TPP), thus encapsulating the CUR/CD using hydrogen bonding and ionic gelation of CS with TPP (Figure 4A). The encapsulated nanoparticles were still positively charged and transported CUR into cancer cells via the enhanced permeation and enhanced permeation retention effect, exhibiting higher cytotoxicity against the SCC25 cell line compared to free CUR, CUR-CS, and CUR/CD. In a similar study by Alizadeh et al., CUR/β-CD-CS and CUR/γ-CD-CS exhibited excellent in vitro release properties and high cytotoxicity against human lung cancer cells [86][32]. Similarly, Karpkird et al. synthesized nanocarriers consisting of CD polymers cross-linked by citric acid (pbCD) and CS for the encapsulation of CUR [87][33]. In vitro studies showed that the release rate of CSpbCD-CUR was slower than that of free CUR, resulting in a lower cytotoxicity of CSpbCD-CUR than pbCD-CUR or free CUR.
3.2. Nanofibers
Electrospinning is a common method of preparing nanofibers by using an electrostatic force to stretch the electrospinning fluid [90][36]. Nanofibers made by this technique have many attractive properties, such as easily adjustable structure and size, large specific surface area, and diverse chemical composition, which make them suitable as transport systems for drug molecules [91][37]. Sun et al. prepared CUR/CD inclusion complex-loaded polyvinyl alcohol nanofibers via the electrospinning technique [92][38]. 1H NMR spectra suggested that the chemical integrity of CUR was not altered after electrostatic spinning. Therefore, the resulting nanofibers have the potential for development in drug delivery, wound healing, and cancer treatment. Rezaei et al. instead added almond gum to prepare CUR/CD inclusion complex-loaded almond gum/polyvinyl alcohol composite nanofibers [93][39]. The diameter of these nanofibers was in the range of 98–169 nm, and the inclusion complexes were present in a non-crystalline form. In addition, the solubility of the inclusion complex in the nanofibers was increased by 160-fold compared to that of pure CUR. In another study by Aytac et al., electrospinning was used to prepare core-shell nanofibers for the slow release of CUR [88][34]. In this formulation, CUR and HP-β-CD inclusion complexes were used as the core and polylactic acid (PLA) as the shell to form nanofibers with an average diameter of 695 nm (Figure 4B). In vitro release experiments showed that CUR/HP-β-CD-PLA nanofibers released CUR more slowly than CUR-PLA nanofibers during simulated gastric acid and intestinal fluid digestion due to the incorporation of a shell structure.
3.3. Cyclodextrin-Based Metal–Organic Framework Nanoparticle
CD-based metal–organic frameworks (CD-MOFs) are practical multifunctional materials with a porous structure and good biocompatibility, which can also be used as carriers to transport drugs [94][40]. Chen et al. used a modified solvothermal method and PEG to prepare a nano-CD-based organic backbone for the encapsulation of CUR [89][35]. In this nano-system, the CD-MOFs consisted of an extended body central framework of (γ-CD)6 cubic units, while CUR was present in the amorphous form in the hydrophobic cavity of (γ-CD)2 and the cycloidal cavity of (γ-CD)6 (Figure 4C). The resulting CUR-Nano-CD-MOFs still exhibited high antioxidant activity compared to free CUR after 120 min of continuous UV irradiation.
References
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467.
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238.
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Beta-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B. 2010, 79, 113–125.
- López-Tobar, E.; Blanch, G.; del Castillo, M.R.; Sanchez-Cortes, S. Encapsulation and isomerization of curcumin with cyclodextrins characterized by electronic and vibrational spectroscopy. Vib. Spectrosc. 2012, 62, 292–298.
- Alizadeh, N.; Malakzadeh, S. Changes in chemical stability and bioactivities of curcumin by forming inclusion complexes of beta- and Gama-cyclodextrins. J. Polym. Res. 2020, 27, 42.
- Jahed, V.; Zarrabi, A.; Bordbar, A.K.; Hafezi, M.S. Nmr (1H, Roesy) Spectroscopic and Molecular Modelling Investigations of Supramolecular Complex of Beta-Cyclodextrin and Curcumin. Food Chem. 2014, 165, 241–246.
- Song, L.T.; Jiang, X.Y.; Tang, K.W.; Miao, J.B. Study on Inclusion Interaction of Ibuprofen with B-Cyclodextrin Derivatives. Lat. Am. Appl. Res. 2011, 41, 147–151.
- Li, N.; Wang, N.; Wu, T.; Qiu, C.; Wang, X.; Jiang, S.; Zhang, Z.; Liu, T.; Wei, C.; Wang, T. Preparation of Curcumin-Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex by Cosolvency-Lyophilization Procedure to Enhance Oral Bioavailability of the Drug. Drug Dev. Ind. Pharm. 2018, 44, 1966–1974.
- Shityakov, S.; Salmas, R.E.; Durdagi, S.; Roewer, N.; Förster, C.; Broscheit, J. Solubility Profiles, Hydration and Desolvation of Curcumin Complexed with Γ-Cyclodextrin and Hydroxypropyl-Γ-Cyclodextrin. J. Mol. Struct. 2017, 1134, 91–98.
- Mai, N.N.S.; Nakai, R.; Kawano, Y.; Hanawa, T. Enhancing the Solubility of Curcumin Using a Solid Dispersion System with Hydroxypropyl-Beta-Cyclodextrin Prepared by Grinding, Freeze-Drying, and Common Solvent Evaporation Methods. Pharmacy 2020, 8, 203.
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Prog. Polym. Sci. 2019, 93, 1–35.
- Matencio, A.; Pedrazzo, A.R.; Difalco, A.; Navarro-Orcajada, S.; Monfared, Y.K.; Conesa, I.; Rezayat, A.; López-Nicolás, J.M.; Trotta, F. Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers 2021, 13, 4226.
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289.
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049.
- Wei, P.; Yan, X.; Huang, F. Supramolecular polymers constructed by orthogonal self-assembly based on host–guest and metal–ligand interactions. Chem. Soc. Rev. 2014, 44, 815–832.
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22.
- Ma, M.; Sun, T.; Xing, P.; Li, Z.; Li, S.; Su, J.; Chu, X.; Hao, A. A supramolecular curcumin vesicle and its application in controlling curcumin release. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 157–165.
- Bai, Y.; An, N.; Chen, D.; Liu, Y.Z.; Liu, C.P.; Yao, H.; Wang, C.; Song, X.; Tian, W. Facile Construction of Shape-Regulated Beta-Cyclodextrin-Based Supramolecular Self-Assemblies for Drug Delivery. Carbohydr. Polym. 2020, 231, 115714.
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408.
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179.
- Haimhoffer, A.; Dossi, E.; Beresova, M.; Bacskay, I.; Varadi, J.; Afsar, A.; Rusznyak, A.; Vasvari, G.; Fenyvesi, F. Preformulation Studies and Bioavailability Enhancement of Curcumin with a ‘Two in One’ Peg-Beta-Cyclodextrin Polymer. Pharmaceutics 2021, 13, 1710.
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-Beta-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073.
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53.
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727.
- Harada, T.; Giorgio, L.; Harris, T.J.; Pham, D.T.; Ngo, H.T.; Need, E.F.; Coventry, B.J.; Lincoln, S.F.; Easton, C.J.; Buchanan, G.; et al. Diamide Linked Gamma-Cyclodextrin Dimers as Molecular-Scale Delivery Systems for the Medicinal Pigment Curcumin to Prostate Cancer Cells. Mol. Pharm. 2013, 10, 4481–4490.
- Harada, T.; McTernan, H.L.; Pham, D.T.; Lincoln, S.F.; Kee, T.W. Femtosecond Transient Absorption Spectroscopy of the Medicinal Agent Curcumin in Diamide Linked Gamma-Cyclodextrin Dimers. J. Phys. Chem. B 2015, 119, 2425–2433.
- Harada, T.; Pham, D.T.; Leung, M.H.; Ngo, H.T.; Lincoln, S.F.; Easton, C.J.; Kee, T.W. Cooperative Binding and Stabilization of the Medicinal Pigment Curcumin by Diamide Linked Gamma-Cyclodextrin Dimers: A Spectroscopic Characterization. J. Phys. Chem. B 2011, 115, 1268–1274.
- Elgadir, M.; Uddin, M.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629.
- Negm, N.A.; Hefni, H.H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267.
- Popat, A.; Karmakar, S.; Jambhrunkar, S.; Xu, C.; Yu, C. Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf. B 2014, 117, 520–527.
- Alizadeh, N.; Malakzadeh, S. Antioxidant, Antibacterial and Anti-Cancer Activities of Beta-and Gamma-Cds/Curcumin Loaded in Chitosan Nanoparticles. Int. J. Biol. Macromol. 2020, 147, 778–791.
- Karpkird, T.; Manaprasertsak, A.; Penkitti, A.; Sinthuvanich, C.; Singchuwong, T.; Leepasert, T. A novel chitosan-citric acid crosslinked beta-cyclodextrin nanocarriers for insoluble drug delivery. Carbohydr. Res. 2020, 498, 108184.
- Aytac, Z.; Uyar, T. Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: Enhanced water solubility and slow release of curcumin. Int. J. Pharm. 2017, 518, 177–184.
- Chen, Y.; Su, J.; Dong, W.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Cyclodextrin-Based Metal-Organic Framework Nanoparticles as Superior Carriers for Curcumin: Study of Encapsulation Mechanism, Solubility, Release Kinetics, and Antioxidative Stability. Food Chem. 2022, 383, 132605.
- Nie, G.; Li, S.; Lu, X.; Wang, C. Progress on Applications of Inorganic Nanofibers Synthesized by Electrospinning Technique. Chem. J. Chin. Univ.-Chin. 2013, 34, 15–29.
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729.
- Sun, X.-Z.; Williams, G.R.; Hou, X.-X.; Zhu, L.-M. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr. Polym. 2013, 94, 147–153.
- Rezaei, A.; Nasirpour, A. Encapsulation of curcumin using electrospun almond gum nanofibers: Fabrication and characterization. Int. J. Food Prop. 2018, 21, 1608–1618.
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal–Organic Frameworks and Their Applications. Accounts Chem. Res. 2021, 54, 1440–1453.
More