1. Routes of Breast Milk Contamination with AFM1
1.1. Diet
The diet of the nursing mother appears to be the exclusive source for the contamination of breastfed children with aflatoxins; passive skin penetration and inhalation may have a minor contribution but there is no evidence to
aou
thor'sr knowledge for these routes of contamination. Upon ingestion with contaminated foods, aflatoxins are absorbed in the small intestine and directed through the portal bloodstream to the liver where they undergo various metabolic reactions mainly catalyzed by microsomal cytochrome P450 (CYP 450) oxidases
[1][14]. The resulting intermediate metabolites then follow different pathways whereby they can either generate reactive intermediates, i.e., epoxides, that interact with DNA, RNA, and proteins to form toxic adducts or combine with soluble nucleophilic molecules, e.g., glutathione and glucuronic acid, to form non-toxic conjugates that are eliminated in biological excretions
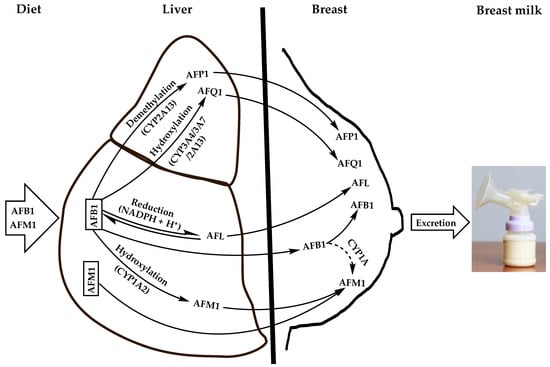
[2][3][4][15,16,17]. Alternatively, they are distributed unmodified via the systemic circulation to various tissues and biological fluids, including milk. Notably, the detoxification of AFB1 in the liver produces 4 major derivatives resulting from hydroxylation, demethylation, or reduction reactions (
Figure 1). The hydroxylation of AFB1 furan ring by CYP1A2 isozyme generates AFM1 while the hydroxylation of its cyclopentenone ring by CYP3A4, CYP3A7, or CYP2A13 forms AFQ1; demethylation of AFB1 with CYP2A13 produces AFP1 and its ketoreduction by cytosolic nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reductase yields aflatoxicol (AFL)
[1][14].
Figure 1. Natural contamination means of breast milk with dietary AFB1 and its major derivatives that are carried over to breast milk. Dashed arrow indicates an uncertain pathway that was demonstrated in vitro in bovine mammary cell-line (BME-UV1) to be likely carried out by a CYP1A isozyme.
Although the latter AFB1 derivatives are considered as detoxified metabolites of AFB1, they retain toxicities to various degrees since they conserve the double bond 8,9 of the furan ring being the main precursor of toxic adducts with DNA, RNA, and proteins upon epoxidation
[1][5][14,18]. It is worth mentioning, however, that AFL, the most toxic AFB1 derivative (20–50% of AFB1 potency)
[6][19], is a short-lived metabolite, which is readily converted back to the parent molecule and which appears to not react directly with DNA to form mutagenic adducts
[7][20]. Indeed, despite its demonstrated ability to form DNA adducts in vitro in isolated rainbow trout hepatocytes, when dosed in vivo in fry rainbow trout, the DNA adducts formed were largely dominated by AFB1-DNA, suggesting that AFL is converted into AFB1 prior to its activation by epoxidation and the formation of DNA adducts
[7][8][20,21]. Nonetheless, detection of AFL in milk from different mammals, including humans, at significant levels suggests that it is not completely converted into the parent aflatoxin during lactation and that it can exert toxicological effects on consumers in its own right
[9][22]. On the other hand, AFQ1 and AFP1 are weekly toxic to non-toxic metabolites
[1][10][11][14,23,24], and unlike AFM1 they are essentially excreted in urine and feces
[12][13][25,26]; therefore they are of less concern to the mother and child health. Consequently, AFM1 remains the main toxic derivative of AFB1 that can contaminate human breast milk owing to its relatively high toxicity (retains up to 10% of AFB1 toxicity)
[14][15][16][27,28,29], relative abundance among the AFB1 metabolites
[17][30], and established carcinogenicity in animals
[18][31]. In addition, it has a high affinity to the mammary glands making breast milk the preferred route of excretion, as it represents 95% of AFB1 metabolites excreted in milk
[13][19][26,32]. Hence, the prime importance given to the contamination of milk, including human breast milk, with this aflatoxin from the scientific and public health standpoints. Therefore, th
is re
focus here iview will focus on AFM1 in breast milk and the health risks it poses to infants and young children. It should be emphasized, however, that the occurrence of other aflatoxins and mycotoxins in general in breast milk should not be disregarded as they can have their own toxicities and/or act in synergy with AFM1 or with each other to increase the health risks on the mothers and the nursing children. For example, AFL was shown to contaminate 13% of 290 analyzed bovine milk samples in Mexico at levels varying between <0.05 and 12.4 mg/L
[20][33]. Another study on Oaxaca cheese marketed in Mexico city showed that 97% of 30 analyzed samples were contaminated with AFL at average level of 13.1 ng/g (range <0.01–25.5 ng/g)
[9][22]. This is especially alarming that AFL has the same tumorigenic potency as its parent AFB1
[15][28] and warrants to be studied separately as a public health hazard in milks, including human milk. Occurrence of mycotoxin mixtures in breast milk with different toxicity potencies and to different levels and frequencies are well documented
[21][22][11,12].
Apart from the contamination of breast milk with the AFM1 via the liver as an AFB1 carry-over metabolite, it can also be directly ingested with dairy products and distributed unmodified through the liver to breast milk via blood circulation (
Figure 1). Moreover, AFB1 can also transit from the liver unmodified to the breast where it can be hydroxylated to AFM1 by the epithelial cells of the mammary glands (
Figure 1), as was evidenced in vitro in the bovine epithelial cell line BME-UV1 where about 1% of the AFB1 was transformed into AFM1 most likely by a cytochrome enzyme of the CYP1A subfamily
[5][17][18,30]. This appears to apply to the human mammary glands whose epithelial cells were reported to produce CYP1A1 isoform
[23][24][8,34]. However, the actual bioconversion of AFB1 into AFM1 in the mammary glands and the enzyme(s) involved remain to be clarified, especially that CYP1A2 responsible for this bioconversion in the liver and in other tissues was not detected in the mammary epithelial cells
[25][35]. Moreover, the capacity of bovine mammary epithelial cells to convert AFB1 into AFM1 was shown to be six-fold weaker than that of the hepatocytes
[26][36], suggesting that the mammary glands would have a minor contribution to the accumulation of AFM1 in milk.
It appears from the above discussion, that AFM1 may contaminate breast milk at toxic levels for the mother and her child, especially when the dietary exposure of the nursing mother to AFB1 or AFM1 is too high. Although the extent of breast milk contamination with AFM1 is dictated by its direct intake from dairy products as well as by AFB1 carry-over, the latter means would represent the major route in countries where the diet is characterized by a high consumption pattern of foods prone to AFB1 contamination (e.g., cereals, dry fruits, spices, and nuts)
[27][37]. Additionally, the contribution of AFB1 carry-over to the extent of breast milk contamination with AFM1 is dependent on the carry-over rate (COR), i.e., the proportion of AFB1 converted into AFM1 in the liver and possibly in the mammary glands. While the carry-over rate has been extensively studied in lactating domestic animals exposed to different amounts of aflatoxins
[9][28][29][30][31][22,38,39,40,41], such studies are lacking in lactating mothers due the ethical considerations severely restricting the use of human subjects in experiments where they can be intentionally given aflatoxin-contaminated foods
[32][33][34][42,43,44]. Nevertheless, the correlation between AFB1 dietary intake and human milk contamination with AFM1 is well established
[19][35][36][37][32,45,46,47]. A survey was conducted on 50 volunteer Nigerian nursing mothers from three districts of Ogun State (South Western Nigeria) who provided samples of the foods they consumed most frequently along with samples of the breast milk they produced during the period of the study
[36][46]. The analysis of the food samples revealed a high prevalence (93.75–100%) of AFB1, but the contamination levels (0.07–0.89 ng/g) were below the European Union (EU) Maximum Tolerable Limit (MTL) of 2 ng/g. Meanwhile, 82% of the milk samples they produced was contaminated with AFM1 at concentrations ranging between 3.49 and 35 ng/L, with 16% exceeding the EU MTL for infant milk formula of 0.025 ng/g
[38][48], with a significant correlation (r = 0.33) between AFB1 intake and AFM1 excretion into milk.
1.2. Feed as an Indirect Source of Human Breast Milk Contamination with AFM1
Feed contamination with AFB1 contributes indirectly, but significantly, to increase the levels of AFM1 in human breast milk
[37][39][47,49]. A portion of the AFB1 ingested by lactating domestic animals is carried over as AFM1 into the milk they produce, which will in turn be transferred to breast milk of nursing mothers consuming such contaminated milk or its derivatives. The persistence of aflatoxins in processed dairy products owing to their resistance to technological processes, such as heat treatments and fermentation
[40][50], represents an important contributing factor to the increase of AFM1 levels in the breast milk
[30][37][41][40,47,51] fed to suckling infants, thereby exposing them to various health conditions.
Dairy product contamination with AFM1 vary greatly depending on the level of feed contamination with AFB1 and the rate of its transfer, i.e., the carry-over rate (COR), as AFM1 to milk. The COR has been intensively studied in domestic lactating animals where it was reported to vary depending on many factors, including the species, the breed, the stage of lactation, the season of the year, the feed composition, the health status of the lactating animal, the milk yield, and individual variability
[9][28][29][30][31][42][43][22,38,39,40,41,52,53]. Different mathematical models have been proposed to predict AFM1 concentration in milk based on AFB1 intake by lactating animals via feed and, hence, conformity/non-conformity of the milk to the regulatory standards. This would in turn allow setting the appropriate MTL of AFB1 in feed to guarantee that the concentration of AFM1 in milk do not exceed safe levels. However, none of these models could apply to all cases and under all milk production conditions for the same lactating animal species. In most cases, the experimental data did not fit those calculated by the models developed for the same or similar experimental settings
[42][44][52,54]. For example, according to a steady-state model developed by van Eijkeren et al.
[45][55], the maximum COR of ingested AFB1 into AFM1 of 3.2% was about two-fold lower than that predicted by using other models
[28][31][42][38,41,52]. Additionally, the application of this model to observed data from similar studies did not fit and have yielded AFM1 concentrations differing by a factor of 0.9 to 1.3
[44][54]. Regardless of modelling studies, experimental data reported mean COR values ranging between 0.032 and 6.2% depending on the animal species and the breed among other experimental parameters.
COR values for humans have not received enough research interest to provide a clear idea on the portion of dietary AFB1 converted to AFM1 and be transferred into breast milk. In one study on a limited number of lactating women (
n = 5) and for a short follow-up duration (4 and 5 days), 0.1–0.4% of the ingested AFB1 was carried over to breast milk as AFM1
[46][56]. Another study demonstrated a significant correlation between the ingested AFB1 in 50 lactating mothers from Ogun State (Nigeria) and the excretion of AFM1 in breast milk
[36][46] without calculating the carry-over ratio. Instead, it demonstrated that mothers fed on feed contaminated with AFB1 at levels varying between 0.16 and 0.33 ng/g have yielded breast milk containing 3.49 to 35 ng/L of AFM1.
Considering the experimental and model-calculated data on the carry-over of feed AFB1 into milk AFM1, there is a general agreement that the EU MTL of 5 ng/g for dairy feed can adequately prevent the levels of AFM1 in the milk of lactating animals from exceeding the EU MTL of 0.05 ng/g
[45][55]. Nonetheless, reduction of the AFB1 MTL in dairy feeds to 1.4–4.0 ng/g were suggested to ensure higher guarantee for AFM1 levels in milk to be kept below 0.05 ng/g
[28][31][43][38,41,53]. Conversely, the AFB1 EU MTL of 20 ng/g in feeds other than those destined for lactating animals resulted in higher frequencies of AFM1 levels exceeding the MTL of 0.05 ng/g in milk
[31][41][41,51] but remained below the US Food and Drug Administration (USFDA) MTL of 0.5 ng/g
[41][43][51,53]. It should be emphasized, however, that these MTL values remain too high for baby foods, which should be kept below 0.025 ng/g according to the UE regulations
[38][48]. Moreover, the more relaxed the regulations for AFM1 MTL in milk, the higher is its intake by the nursing mothers consuming such milk and derivatives eventually leading to higher exposure of suckling infants.
2. Adverse Health Effects of AFM1 on Infants and Young Children
Infants can be exposed to AFM1 from their in utero life throughout the nursing period where breastfeeding can be the main or exclusive food source
[47][48][49][95,96,97]. Under these conditions, the extent of exposure is highly dependent on AFM1 intake by the mother as well as the rate of its transfer to the baby through the umbilical cord, as a fetus, and then through breast milk, as a suckling newborn and infant. Such continuous exposure can cause teratogenicity, stillbirth, or miscarriage during pregnancy
[49][50][97,98] or lead to physiological and neurological disorders that the child would suffer the consequences for the rest of his/her life, such as stunting, malnutrition diseases (e.g., kwashiorkor and marasmus), autism, nodding syndrome, and related cognitive disorders
[51][99]. The highest incidence of these diseases is recorded in low-income and middle-income countries of the endemic regions, especially those of Asia and Africa under subtropical climate conditions
[32][47][52][42,95,100].
Breast milk contamination with AFM1 is of serious concern to public health for three main reasons: (i) the potential high intake of AFM1 by neonates and infants who are fed mainly or exclusively on breast milk in case of a high contamination; (ii) the weaker detoxifying capacity due to their immature organs, mainly the liver, and higher metabolic activity; and (iii) the possible continued exposure after infancy and childhood to aflatoxins through various foods
[53][54][101,102], which increases the risk for the onset of severe chronic endpoints, such as cancer, at younger ages. These conditions result in an overall increase in the susceptibility of infants and young children to aflatoxins by about three-fold more than adults
[53][55][101,103]. Therefore, high exposure of pregnant and nursing women to AFM1 can be anticipated to increase the rates of disabilities, morbidities, and mortalities within a society resulting in a heavy economic and social burden.
2.1. Growth Impairment
Stunting, underweight, or wasting of children are the most documented growth impairments associated with the exposure to AFM1 during fetal life and infancy through umbilical cord blood and breast milk, respectively
[50][56][57][98,104,105]. In the year 2020, 149.2 million (22%) and 45 million (6.7%) of the world children under 5 of age were affected by stunting (low height for age ratio) and wasting (low weight for height ratio), respectively
[58][106]. In the sub-Saharan countries of Africa, notorious for the incidence of aflatoxins in their foods and feeds
[59][107], the prevalence of stunting and underweight in children below 5 years of age were reported to be as high as 38% and 22%, respectively in 2015/2016
[60][108]. Although aflatoxin contamination is not the only etiology that would explain such a high incidence of growth impairment, its contribution cannot be overlooked.
From the public health standpoint, these growth disorders have life threatening or life lasting consequences on affected children. Stunting alone was estimated to cause the death of children by 14–17% and was considered to be an underlaying cause of poor cognitive, motor development, and educational performances; it was even suggested to be congenitally transmitted to the offspring
[33][58][43,106]. Wasting, on the other hand, can be treated and weight gain can resume normally, but it increases the death risk depending on the severity or leads to stunting upon prolonged exposure or after recurrent episodes
[47][58][95,106]. Despite the prevailing belief that under-nutrition, inadequate dietary intake, and gastrointestinal illness are the primary etiologies of growth impairment
[61][109], it is now well established that exposure to mycotoxins, e.g., aflatoxins and fumonisins, in utero and throughout infancy and early childhood hinders the linear growth and weight gain
[33][47][49][53][56][57][62][63][64][43,95,97,101,104,105,110,111,112]. This was further corroborated by the failure of interventions, such as the provision of appropriate education on nutrition and complementary feeding in addition to the implementation of proper water, sanitation, and hygiene (WASH) to improve significantly the linear growth and normal weight gain in undernourished children
[65][66][67][68][69][70][71][113,114,115,116,117,118,119].
One of the earliest studies that demonstrated the causal relationship between growth impairment and exposure of children to aflatoxins was conducted in Benin and Togo of Western Africa
[62][110].
In Hereinthis study, the Z scores of the height-for-age (HAZ), weight-for-age (WAZ), and height-for-weight (HWZ) were determined in children below 5 years of age whose diet consisted of breast milk only (exclusive breast feeding), breast milk and weaning foods, breast milk and household foods, and weaning and household foods. When matched with aflatoxin-albumin (AF-Alb) adduct levels in the blood, the outcome revealed that the levels of AF-Alb were inversely related to HAZ and WAZ scores, i.e., the higher these levels were, the more severe the stunting and the underweight occurred in children. A subsequent longitudinal study on Gambian children showed that high exposure to aflatoxins from the perinatal period to the age of one year, as evidenced by high levels of AF-Alb adducts in the maternal, umbilical cord, and children blood, reduced significantly the height and weight gains in children from 6 months to one year of age
[57][105]. Conversely,
thi
t iss study demonstrated that a reduction of AF-Alb levels in the maternal blood from 110 pg/mg to 10 pg/mg during pregnancy resulted in an increased height and weight gains of 0.8 kg and 2 cm, respectively by the children during the first year of growth. According to the authors
' understanding, this effect stems from the maternal exposure to aflatoxins during pregnancy and extends throughout the first year of the children’s life, which has established the association between aflatoxin exposure during infancy and growth impairment. However, in the first 16 weeks after birth where the children were essentially breastfed, the serum AF-Alb was detected at low levels in only few infants (13 out of 115 babies: 11%), suggesting that were residual aflatoxin adducts from the maternal exposure
[57][105]. Moreover, no direct causal effect between the growth impairment and the presence of AFM1 in the breast milk of the surveyed mothers was specifically demonstrated
in th
ereis study, as the exposure to aflatoxins was measured indirectly by the blood levels of AF-Alb, which typically evokes AFB1 exposure
[72][120]. However, since AFM1 can form albumin adducts, albeit at lower extents than does AFB1, AF-Alb can also indicate exposure to AFM1
[73][121]. In addition, AF-Alb levels in the children’s blood were shown to correlate highly with AFM1 levels in their urine
[74][122], suggesting that the urinary AFM1 most likely originates from the diet (mother’s milk and/or complementary food) or from the maternal and cord blood in the case of neonates
[50][98] rather than being an AFB1 metabolite due to the low AFB1-detoxifying activity in the young children
[75][80].
The inverse relationship between AFM1 in breast milk and growth impairment in nursing children has been reported in different countries around the world. A study on lactating women from Tehran (Iran) showed that breast milk contamination at levels varying between 0.3 and 26.7 ng/kg (median of 8.2 ng/kg) was inversely correlated with height at birth but not with weight
[76][123]. Another study conducted in the same country on exclusively breastfed children of 90–120 days old from the city of Tabriz showed that their exposure to an average AFM1-concentration of 6.96 ng/L (range of 5.1 to 8.1 ng/L) retarded both height and weight gain compared with children fed on AFM1-free breast milk
[77][124]. The growth impairment of suckling children despite the generally low levels of AFM1 in breast milk was attributed to a chronic exposure starting in utero from the carry-over of AFB1 and/or AFM1 from the mother’s diet, as substantiated by the under-height at birth
[76][123], and continuing exposure after birth through the maternal milk and/or weaning and household foods
[47][76][78][95,123,125].
The association of exposure to AFM1 from mothers’ milk with growth impairment in children from fetal to early life is well documented
[62][76][77][79][80][110,123,124,126,127]. However, most of the studies were observational (cohort or cross-sectional) with less power as evidence and each of them has its own pitfalls and limitations. To the best, they may make the causal link circumstantial rather than direct. In addition, no study has demonstrated the mechanism of action to elucidate how do aflatoxins, including AFM1, act to cause stunting, weight gain, or wasting in children. The demonstration of the mechanism of action is required by the IARC to consider the causal link being direct
[81][131]. Therefore, there is a need for specifically designed studies to provide unequivocal clinical evidence for the association between AFM1 exposure and growth impairment. Longitudinal studies using cluster randomized controlled clinical trials on children intentionally given low doses of AFM1 appear to be best fit for such a purpose, but they are challenged with the dilemma of the ethical considerations for the use of human subjects. Prior to performing these trials, the experimental design should be described in detail and submitted for review and approval to national and international organizations concerned by the ethical questions of scientific research. Once approved, the research team should be committed to provide updates on a regular basis and to communicate to the authorizing body any amendments introduced into the protocol in due course. This procedure is intended to ensure that the study meets strictly the ethical principles, e.g., maintaining a favorable balance of risk/benefit and respecting participants among other provisions, while making a significant contribution to the advance of the scientific knowledge in the field. So far, only two studies have been done in this framework, and they have used AFB1
[71][119] and total aflatoxins
[32][42] on partially breastfed children. None of them has specifically investigated the effect of AFM1 on breastfed children. The first of these studies was conducted in Kenya on infants who have been recruited before birth (starting from the fifth month of pregnancy) and followed until the age of 22 months for linear growth and the concentrations of serum AFB1-Alb
[71][119]. Recruited mothers and their children were split into two cluster-randomized groups consisting of an intervention group, receiving aflatoxin-safe maize (<10 ng/g of AFB1) as complementary food, and a control group receiving a regular household maize known to be usually contaminated with higher levels of AFB1. The clinical trials of study were approved in 2013 by Institutional Review Board for Research of the International Food Policy Research Institute (
https://www.socialscienceregistry.org/trials/105 (accessed on 10 June 2022)) and ended in 2016 revealing the absence of causal link between AFB1 intake and stunting at 22 months of age
[71][119]. These results should be interpreted with caution, as the study suffered many limitations, the most prominent of which was the high rate of follow-up loss and incomplete data collection in both the intervention and the control groups. Additionally, at the midline of the experiment (13 months of age), a-7% decrease in the stunting rate was observed but the exposure (serum AF-Alb) did not decrease. Conversely, at the endline (22 months of age), no improvement in linear growth was observed despite a significant decrease in serum AF-Alb. These results, which remain unexplained, suggest that interfering factors not considered in the design of the study, such as seasonal variation, environmental enteric dysfunction, immunomodulation, and hepatic metabolism of micronutrients, may have affected its outcome. The second study of the kind (Cluster randomized controlled trial) being conducted on Tanzanian children was approved for clinical trials in 2019 by the Institutional Review Board (IRB) and the Tanzanian National Institute for Medical Research (NIMR) (
https://www.clinicaltrials.gov/ct2/show/NCT03940547 (accessed on 12 June 2022)).
HereIn thi
ns study, children were recruited at birth and followed for linear growth and serum AF-Alb for one year, including 6 months of exclusive breastfeeding followed by mixed feeding (breastfeeding was not interrupted). Recruited children were split into two-group clusters: the intervention group were intentionally exposed to low doses of total aflatoxins (up to 5 ng/g) after the sixth month of age via complementary food on a continuous basis for 18 additional months. The research team has published yearly updates in scientific journals
[32][33][34][42,43,44] and in the ClinicalTrials.gov website
[82][132]; the final outcome is as yet to be disclosed. Although the latter study did not address AFM1 in breast milk, it may serve as a model for future studies on exclusively breastfed children in areas where nursing mothers are fed on staple foods highly contaminated with aflatoxins, hence likely to secrete AFM1-contaminated breast milk for the control cluster group. The intervention cluster randomized group should comprise mothers fed on aflatoxin-safe foods and their breast milk be tested for the absence or safe levels of AFM1.
2.2. Other AFM1-Related Health Issues
Several adverse health effects of aflatoxins on infants and young children have been proposed as standalone diseases or as possible underlying mechanisms of action for growth impairment. These include immunomodulation causing chronic immune activation; gastrointestinal diseases; nutrient maldigestion and malabsorption; and impaired bone growth and remodeling
[83][84][85][86][133,134,135,136]. The common feature to all these diseases is that they are related to the disruption of the small intestine functions mediated by damaging its lining epithelium. Additionally, the pathological and clinical features associated with aflatoxin intoxications have been attributed to a sub-clinical condition known as environmental enteric dysfunction (EED) being an underlying cause of stunting and anemia
[84][86][87][134,136,137]. Although EED is primarily associated with the ingestion of high load of fecal bacteria under poor water, sanitation, and hygiene conditions that characterize developing countries
[88][89][138,139], it was reported to share overlapping pathways with aflatoxin-mediated diseases
[85][135]. For example, like EED, aflatoxins were hypothesized to impair protein synthesis thereby promoting gastrointestinal infections and liver toxicity, ultimately leading to growth impairment
[84][90][134,140].
Few and fragmentary studies have been done on the immunomodulatory effects of aflatoxins on children and their impact on liver toxicity and protein synthesis. Turner et al.
[83][133] first reported that a high exposure of Gambian children to aflatoxins suppresses selectively their humoral immunity. A drastic reduction in salivary IgA titers of these children with a concomitant increase in the levels of serum AF-Lys biomarkers was observed.
IThe aut
ihors concluded that a high exposure of children to aflatoxins compromises their immunity, which explains the frequency of their gastrointestinal infections and hence the burden of infant infection-related mortality in West African countries. However, the same study showed that the levels of serum AF-Lys did not correlate with the Cell Mediated Immunity (CMI) response
of to test antigens (tetanus, diphtheria,
Streptococcus, tuberculin, candida,
Trichophyton, and
Proteus), nor did it with the antibody response to rabies vaccines. Further studies are thus needed to substantiate the relationship between exposure of young children to aflatoxins and the immunomodulatory effects on one hand and to gastrointestinal infection-related mortality on the other hand.
A recent longitudinal study investigated the hypothesis that exposure to aflatoxins impairs protein synthesis in children with an emphasis on the proteins used as biomarkers of inflammatory reactions (C-reactive protein, α-1-glycoprotein) as well as other serum proteins directly or indirectly involved in growth development, such as transthyretin, lysine, tryptophan, and Insulin-like growth factor-1 (IGF-1)
[85][135]. The study was conducted on 102 Ethiopian children (6–35 months of age), including 50 stunted children, living in an aeras with highly contaminated staple foods (>10 mg/g). The study aiming to relate chronic exposure to aflatoxins (AFB1, AFB2, AFG1, AFG2, and AFM1) to linear growth impairment showed no clear correlation between exposure to aflatoxins, separately or in combination, to the protein status, inflammation, or linear growth.
Exposure of children to aflatoxins has also been suggested as a possible etiology for the onset or the aggravation of the clinical manifestations of neurological disorders, such as autism and nodding. A cross-sectional study conducted in Italy on autistic children (
n = 172) revealed a significant difference between the levels of AFM1 in their blood compared with control group of non-autistic children or those at a risk (genetic relatedness to autistic parents). Nevertheless, other studies suggested that, like EED, the neurological disorders associated with exposure to aflatoxins relate to intestinal lining damage and to microbiota–gut–brain axis imbalance (dysbiosis)
[91][141]. This microbiota is known to play a central role in the regulation of the metabolism and homeostasis as well as in controlling the CNS functions via neural, endocrine, and immune pathways. Therefore, its disturbance causes inflammatory bowel diseases and systemic inflammation leading to the alteration of the central nervous system (CNS) functions as is the case in some neuropsychiatric conditions, including autism
[92][142].
Malnutrition-related diseases, such as kwashiorkor, marasmic kwashiorkor and marasmus, faltering, stunting, nodding, organomegaly, and retarded mental and physical activities have been ascribed to chronic exposure of children below 5 years of age to aflatoxins since their in utero life
[47][93][94][95][96][97][95,143,144,145,146,147]. However, the exact relationship between the exposure of children to aflatoxins and the occurrence of these diseases as well as the specific contribution of breast milk AFM1 to their onset has been poorly investigated and remain to be substantiated.
The occurrence of AFM1 in breast milk to different extents depending on the countries and the agroclimatic zones within the same country as well as the socio-economic conditions is well documented
[36][98][99][46,148,149]. Due to the well-established toxicity of AFM1 in humans and animals
[47][95], its occurrence in breast milk is of serious concern to the public health, as it affects individuals in their early life causing either immediate or delayed death or inducing lifetime disabilities. Therefore, there is an urgent need to address this issue at national level and globally regardless of the paucity of scientific evidence for a direct causal link between exposure to this toxicant and the claimed health effects it may cause to children in utero, during infancy, childhood, and even at later stages of their lives.