Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Beatrix Zheng and Version 5 by Beatrix Zheng.
Mucormycosis is a rare infection caused the members of the order Mucorales. Its prevalence ranges from 0.005 to 1.7 per million people worldwide, while in India, it reaches 14 cases per 100,000 inhabitants . During the COVID-19 pandemic, a surge in mucormycosis cases has been observed, especially in India, where the Government of India portal reported 47,508 cases from 5 May 2021 to 3 August 2021. Characteristically, Samir Joshi et al. reported 160 cases of COVID-19-associated mucormycosis (CAM) from April to May 2021 in the Ear, Nose, Throat Department of BJGMC-SGH hospital in India, compared with 3–8 cases of mucormycosis detected each year from 2016 to 2020.
- mucorales
- invasive fungal infections
- SARS-CoV-2
1. Clinical Presentation
CAM was diagnosed after a median of 17.4 days (Q1:14.4, Q3:21.8, IQR 7.5 days) post COVID-19 diagnosis but simultaneous manifestation with acute COVID-19 is also reported. Mucormycosis may be associated with neuroinflammation of the acute phase or be integrated in the post-COVID-19 syndrome [1]. However, the long period that is mediated between COVID-19 positivity and CAM diagnosis may actually reflect a delay in diagnosis, that may be associated with a higher mortality [2].
Mucormycosis most commonly affects the head and neck region. ROCM is the commonest form globally and was also the most frequent form associated with COVID-19. ROCM was diagnosed in 8082/8218 (98.3%) CAM patients and pulmonary infection in 98/8218 (1.2%), of whom 30.6% were in Europe. (Table 1). Mucormycosis of the gastrointestinal tract was found in 5/8218 (0.06%) CAM patients, cutaneous in 11/8218 (0.13%), disseminated in 11/8218 (0.13%) and renal in 1/8218 (0.01%) (Table 1). In Europe 3/40 (7.5%) of CAM patients had ROCM, 30/40 (75%) had pulmonary mucormycosis, 4/40 (10%) mucormycosis of the gastrointestinal tract and 3/40 (7.5%) disseminated (Figure 1).
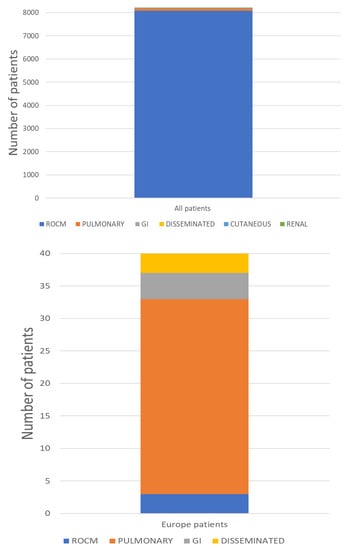
Figure 1.
Clinical presentation of patients with CAM in total and in Europe.
Table 1. Incidence of CAM among hospitalized COVID-19 patients, type of infection, invasive or non-invasive mechanical ventilation, risk factors and all-cause mortality.
| Study | Incidence of CAM (%) | Type of Infection (%) |
Cerebral Involvement /ROCM pts, n (%) |
IMV or NIV n (%) |
DM (% of CAM pts) |
Steroids Intake (% of CAM pts) |
All-Cause Mortality (%) |
|---|---|---|---|---|---|---|---|
| Said Ahmed WM et al. [3] | NA | Maxillary osteomyelitis | 0/14 (0%) | NA | 64.2% DM 35.7% with temporary post-COVID-19 hyperglycemia |
NA | NA |
| Murthy R et al. [4] | NA | RO | 0/111 (0%) | NA | NA | NA | NA |
| Walia S et al. [5] | NA | SN (100%), O (51.85%), C (9.44%), Cu (1.85%), P (0.18%). | 51/529 (9.6%) | NA | 97.96% | 84.85% | 9.25% |
| Vare AA et al. [6] | 1.36% | ROCM | 3/67 (5%) | 18/67 (27%) | 90% | 84% | 34% |
| Fouad YA et al. [2] | NA | O | 0/26 (0%) | NA | 96.2% | 76.9% | 46,2% |
| Soni K et al. [7] | NA | ROCM | 29/145 (20%) | NA | 86.2% | 65% | 18% |
| Metwally MI et al. [8] | NA | Head and neck | 8/63 (12.7%) | NA | 80.9% | 82.5% | 17.5% |
| Arora U et al. [9] | NA | RS (29%), RO (47.3%), ROCM (14.5%), O (1.3%), RO/palatal (5.3%), Cu (0.6%), P (1.3%), D (0.6%) | 22/148 (14.9%) | NA | 92.1% | 65.8% | NA |
| Jindal G et al. [10] | NA | ROCM | 9/15 (60%) | NA | 100% | 80% | 6.6% |
| Syed-Abdul S et al. [11] | NA | NA | NA | NA | NA | NA | NA |
| Patel A et al. [12] | NA | RO (96.5%), P (3.4%) | 0/28 (0%) | NA | NA | NA | NA |
| Pruthi H et al. [13] | NA | P | NA | 0/5 (0%) | 100% | NA | 80% |
| Bansal SB et al. [14] | 10.8% | RO (91%), P (9%) | NA | 0/11 (0%) | 64%, 36% developed transient hyperglycemia |
100% | 18.2% |
| Dulski TM et al. [15] | NA | RO (10%), ROCM (30%), P (30%), D (20%), GI (10%) | 3/4 (75%) | 5/10 (50%) | 80% | 90% | 60% |
| Meshram HS et al. [16] | 4.4% | ROCM (91.8%), P (8.2%) | 11/42 (26.2%) | 0/61 (0%) | 24.6% | 44% | 26.2% |
| Aggarwal SK et al. [17] | NA | ROCM | 4/13 (30.8%) | NA | 92.3% | 92.3% | 15.4% |
| Kulkarni R et al. [18] | 2.1% (1 centre) | ROCM | 12/102 (11.8%) | NA | 81.6% | NA | 51%. |
| Choksi T et al. [19] | NA | ROCM | 6/73 (2%) | 17/73 (23.3%) | 74% | 98% | 36% |
| Kumar S et al. [20] | NA | ROCM | 60/287 (21%) | NA | 80% | NA | NA |
| Mehta R et al. [21] | NA | ROCM | 0/17 (0%) | NA | 100% | NA | NA |
| Panwar P et al. [22] | NA | ROCM | 0/7 (0%) | NA | 100% | 42.8% | 0% |
| Patel DD et al. [23] | NA | ROCM | 21/96 (21.9%) | 6/96 (6.3%) | 71.8% | 82.3% | NA |
| Vaid N et al. [24] | NA | ROCM | NA | NA | 33.8% | 100% | 10.7% |
| Goddanti N et al. [25] | NA | ROCM | NA | NA | 95.7% | 79% | NA |
| Yadav T et al. [26] | NA | ROCM | 25/50 (50%) | NA | 86% | 44% | NA |
| Meshram VB et al. [27] | NA | ROCM (90.9%), P (9%) | 3/10 (30%) | 0/11 (0%) | 54.5% | 100% | 27% |
| Zirpe K et al. [28] | NA | ROCM | 20/84 (23.8%) | NA | 64.3% | 83.3% | 15.5% |
| Alloush TK et al. [29] | NA | ROCM | 9/14 (64.2%) | 0/14 (0%) | 92.8% | NA | 21.4% |
| Pal P et al. [30] | NA | ROCM | 3/10 (30%) | ΝA | 70% | 80% | 30% |
| Danion F et al. [31] | NA | P(53%), GI (18%), ROCM (12%), D (18%) | NA | 13/17 (76.5%) | 47% | 76.5% | 88% |
| Nehara HR et al. [32] | NA | ROCM | 18/105 (17.1%) | NA | 78.1% | 66.3% | 19.05% |
| Pandiar D et al. [33] | NA | Oral | 0/12 (0%) | NA | 66,7% | NA | NA |
| Kumar S et al. [34] | NA | NA | NA | 0/55 (0%) | 83.6% | 98.2% | 16% |
| Bilgic A et al. [35] | 2.5% | ROCM | NA | 6/38 (16%) | 50% | 100% | 5% |
| Guemas E et al. [36] | 7.1% | P | NA | NA | 20% | 90% | 50% |
| Kumar SG et al. [37] | NA | ROCM | 44/101 (43.6%) | NA | 94% | 80.1% | 17.8% |
| Mani S et al. [38] | NA | ROCM | 4/89 (4.5%) | NA | 96% | 92% | 3.4% |
| Dravid A et al. [39] | NA | ROCM (98.3%), D (1.7%) | 26/58 (44.8%) | 3/59 (5.1%) | 89.8% | 100% | 25.4% |
| Naruka S et al. [40] | NA | ROCM | 9/79 (11.4%) | NA | 100% | NA | 18.18% |
| Jain K et al. [41] | NA | ROCM | 3/95 (3.2%) | NA | 77% | 100% | 5.2% |
| Bhanuprasad K et al. [42] | NA | ROCM | 39 (29.5%) | 3/132 (2.3%) | 97.7% | 55.3% | 9.8% |
| Desai EJ et al. [43] | NA | ROCM | 0/100 (0%) | NA | 80% | NA | 20% |
| Nasir\n et al. [44] | 0.35% | P (60%), ROCM (40%) | 4/4 (100%) | 3/10 (30%) | 70% | 80% | 70% |
| Gupta \s et al. [45] | NA | ROCM | 4/56 (7.1%) | NA | 85% | 66% | 16% |
| Joshi S et al. [46] | NA | ROCM | 22/178 (12.4%) | 5/178 (2.8%) | 74.2% | 52.8% | 15% |
| Pradhan P et al. [47] | NA | ROCM | 10/46 (21.7%) | NA | 95.65% | 89.1% | 19.5% |
| Mehta RNM et al. [48] | NA | ROCM | 33/215 (15.3%) | NA | 91% | 88% | 12.1% |
| Riad A et al. [49] | NA | ROCM | 7/7 (100%) | NA | 85.7% | 100% | 0% |
| Guzmán-Castro S et al. [50] | 0.04% | ROCM (83.3%), P(16.6%) | 5/5 (100%) | 2/6 (33.3%) | 83.3% | 100% | 83.3% |
| Seidel D et al. [51] | 2 centres: 0.67%, 0.58% ICU: 1.47%, 1.78% |
P(84.6%), ROCM (7.7%), GI (7.7%) | 1/1 (100%) | 11/13 (84.6%) | 23.07% | 84.6% | 53.8% |
| Gupta R et al. [52] | NA | ROCM | 25/115 (21.7%) | 13/115 (11.3%) | 85.2% | 100% | 21.7% |
| Alfishawy M et al. [53] | NA | ROCM (95.2%), P (4.8%) | 5/20 (25%) | NA | 90% | 100% | 33.3% |
| Dave TV et al. [54] | NA | ROCM | 19/58 (33%) | NA | 74% | NA | 34% |
| Selarka L et al. [55] | 1.8% | ROCM | 9/47 (19.1%) | 20/47 (42.6%) | 76.6% | 100% | 23.4% |
| Avatef Fazeli M et al. [56] | NA | ROCM | 0/12 (0%) | 1/12 (8.3%) | 83.33% | 75% | 66.7% |
| Mishra Y et al. [57] | 3.36% | ROCM | 0/32 (0%) | NA | 87.5% | 93% | 12.5% |
| Sen M et al. [58] | NA | ROCM | 539/2826 (19.1%) | 114/1602 (7.1) | 78% | 87% | 14% |
| Pakdel F et al. [59] | NA | ROCM | 7/15 (46%) | 1/15 (6.7%) | 86% | 46.6% | 47% |
| Y M. Reddy et al. [60] | NA | RO | 0/6 (0%) | NA | 100% | 66.7% | 100% |
| R. Arora et al. [61] | NA | ROCM | 6/60 (10%) | NA | 98.3% | 63.3% | NA |
| D.P Gupta et al. [62] | NA | ROCM | NA | NA | 100% | NA | 5.7% |
| M.Gautam et al. [63] | NA | ROCM | NA | NA | 100% | 66.7% | 0% |
| R.M.Mehta et al. [64] | NA | P | NA | 4/5 (80%) | 80% | 100% | 80% |
| Y.M.Reddy et al. [65] | NA | ROCM | NA | NA | 100% | 80.6% | 35.5% |
| S.P.Singh et al. [66] | NA | RO | 0/6 (0%) | 0/6 (0%) | 100% | 66.7% | 16.7% |
| M.Hada et al. [67] | NA | ROCM | 54/270 (20%) | NA | 92.2% | 72% | NA |
| M. Kumar H et al. [68] | NA | ROCM (85.7%), P (14.3%) | 15/24 (62.5%) | 6/28 (21.4%) | 75% | 70.4% | 73.9% |
| S. Bhandari et al. [69] | NA | NA | NA | NA | 86.8% | 84.3% | NA |
| M Chouhan et al. [70] | NA | ROCM | 9/41 (21.9%) | NA | 97.6% | 87.8% | 9.8% |
| Y. Singh et al. [71] | NA | ROCM (92.3%), P (7.7%) | 2/12 (16.7%) | 10/13 (76.9%) | 61.5% | 84.6% | 69.2% |
| S M Desai et al. [72] | NA | ROCM | 3/50 (6%) | NA | 82% | 84% | 30% |
| A. Kumari et al. [73] | NA | ROCM | 4/20 (20%) | NA | 80% | 80% | 30% |
| S. Mitra et al. [74] | NA | ROCM | NA | NA | 100% | 78.1% | NA |
| A Ramaswami et al. [75] | NA | ROCM | 17/70 (24.3%) | NA | 70% | 70% | NA |
| A.R. Joshi et al. [76] | NA | ROCM | 7/25 (28%) | 12/25 (48%) | 88% | 100% | 56% |
| A. Patel et al. [77] | 7 centers: 0.27% (general wards) | ROCM (86.1%), P (8.6%), renal (0.5%), other (e.g., Cu, GI) (2.7%), D (2.1%) | 44/161 (27.3%) | NA | 60.4% | 78.1% | 44.1% |
| S Sharma et al. [78] | NA | ROCM | 2/23 (8.7%) | NA | 91.3% | 100% | NA |
| R. Kant et al. [79] | NA | ROCM (96%), P (4%) | 11/96 (11.5%) | NA | 95% | 81% | 13% |
| C. Eker et al. [80] | NA | ROCM | 9/15 (60%) | NA | 100% | NA | 33.3% |
| A.K. Pandit et al. [81] | NA | ROCM | 30/56 (53.6%) | NA | 85.7% | 53.6% | 30.6% |
| S.F. Youssif et al. [82] | 7.6% | ROCM | 32/33 (97%) | NA | 63.6% | NA | 90.9% |
| A. Sekaran, et al. [83] | NA | ROCM | 6/30 (20%) | 8/30 (26.7%) | 100% | 90% | 16.7% |
| R. R. Shabana et al. [84] | NA | ROCM | 4/30 (13.3%) | 1/30 (3.3%) | 90% | 66.6% | 20% |
| A. K Patel et al. [85] | NA | ROCM (92.2%), P (7.8%) | 5/59 (8.5%) | NA | 75% | 90.6% | 4.7% |
| H. D.D. Martins et al. [86] | NA | ROCM | 0/6 (0%) | NA | 83.3% | NA | 16.7% |
| S. Iqtadar et al. [87] | NA | ROCM | NA | 0/7 (0%) | 71.4% | 100% | 14.3% |
| A. Al Balushi et al. [88] | NA | ROCM | 3/10 (30%) | 6/10 (60%) | 100% | 30% | 60% |
| R. Soman et al. [89] | NA | ROCM (78.6%), P (21.4%) | 5/22 (22.7%) | NA | NA | NA | 25% |
C: Cerebral, Cu: Cutaneous, DM: Diabetes Mellitus, D: Disseminated, GI: gastrointestinal, IMV: Invasive Mechanical Ventilation, NA: Not Available, NIV: Non-invasive ventilation, O: Orbital, P: Pulmonary, RO: Rhino Orbital, ROCM: Rhino-Orbital-Cerebral Mucormycosis, RS: Rhino-sinus, SN: Sinonasal.
Clinical manifestations of head and neck mucormycosis include headache, loosening of teeth, black necrotic turbinate, facial pain, facial palsy, peri-orbital or facial swelling, skin induration and blackish discoloration [8]. Symptoms attributed to nasal and oral cavity invasion include epistaxis, bloody nasal discharge and palate destruction. Orbital extension may lead to destruction of the ophthalmic artery and optic nerves resulting in ptosis of the eyelid, proptosis, vision disturbances and blindness. In a large retrospective study from India, 519/2716 (19%) patients with CAM presented with vision loss [58]. Cavernous sinus involvement occurs due to extension from the orbit and manifests as diplopia and ophthalmoplegia [90].
Cerebral involvement was noted in 1400/7388 (18.9%) patients with COVID-19 associated ROCM, reported in 72 studies (Table 2). Cerebral involvement may manifest as cavernous sinus thrombosis, fungal abscess, meningitis and cerebrovascular disease [48]. Rahul Kulkarni et al. noted that 45/49 (91.8%) of patients with cerebral involvement presented with ischemic stroke, which concerned large artery infracts, followed by intracranial hemorrhage in 3/49 (6.1%) and sub-arachnoid hemorrhage in 1/49 (2.0%) [18].
Ninety-eight patients with pulmonary mucormycosis are described in the literature (57 in India, 30 in Europe, 6 in Pakistan, 3 in USA, 1 in Egypt and 1 in Mexico) (Table S3). Ten studies contained data on ventilatory support, with invasive or non-invasive mechanical ventilation reported in 26/45 (57.8%) of patients with COVID-19-associated pulmonary mucormycosis. In 7 studies where CAPA was sought, 19/43 (44.2%) patients with pulmonary mucormycosis were found with positive microbiological testing for Aspergillus. Symptoms of pulmonary mucormycosis in non-ventilated patients include fever, dyspnea, cough, chest pain and hemoptysis [91]. Pruthi et al. reported five cases of pulmonary mucormycosis associated with COVID-19 that were complicated by pulmonary artery pseudoaneurysm [13]. In mechanically ventilated patients, identification of an agent of mucormycosis from respiratory specimens in combination with compatible radiographic findings support the diagnosis.
Symptoms of mucormycosis of the gastrointestinal tract are non-specific and consist of abdominal pain and distension, diarrhea and gastrointestinal bleed [91], while disseminated mucormycosis may affect any organ, but mainly the brain and lungs, and is a result of bloodstream invasion in severely immunocompromised patients [91].
2. Diagnosis
Early recognition of CAM is crucial, as delay in therapy is associated with higher mortality [16]. A high index of suspicion should be maintained when clinical symptoms and radiological features appear in a patient with predisposing factors. According to criteria proposed by the European Confederation of Medical Mycology and the Mycoses Study Group Education and Research Consortium [92], the diagnosis of mucormycosis is based on clinical and imaging characteristics and confirmed with direct microscopy, histopathologic analysis and culture of samples obtained with biopsy.
Diagnosis is challenging, as appropriate specimens are obtained through invasive procedures and specific stains are needed to identify Mucorales. Direct microscopy with potassium hydroxide (KOH) mount is usually used for the rapid diagnosis of mucormycosis, as results are delayed with culture and histopathology. Direct microscopy reveals wide, non-septate, ribbon-like hyaline hyphae, with irregular right-angled branching that are characteristic of Mucorales [93]. Ιnfarcts, angioinvasion and perineural invasion are usually present in the histological analysis. Preceding antifungal therapy may alter morphological characteristics of the fungus, while specimens’ processing must be carefully undertaken to keep hyphae intact [94]. Even when fungal hyphae are recognized in histopathological analysis, cultures may be negative in 50% of cases, due to the fragility of fungal hyphae [93]. Characteristically, among 2175 patients with CAM, direct microscopy with KOH/calcofluor white was performed in 89% (1931), and culture in 19% (432), of cases [58].
Molecular techniques are promising, as rapid detection is needed and cultures are time-consuming and may be false-negative [91]. However, results should be cautiously evaluated due to ubiquitous nature of Mucorales. PCR was performed in five studies in the literature (two French, two from India and one from Egypt) and concerned 174 patients, of which 31 were positive (Table S4). In the French study [31], PCR was positive in 15/17 (88%) patients with CAM in serum (n = 14), BAL (n = 7), tissues (n = 3) and peritoneal fluid (n = 1) [31]. It is of interest that, in another French study [36], Mucorales was detected with PCR in respiratory samples of 10 COVID-19 patients, of which 80% simultaneously tested positive for Aspergillus. This cluster of cases was possibly attributed to environmental exposure, due to construction work near the hospital [36].
Few studies in the literature report on the species isolated, reflecting the difficulties encountered with culture-based identification and the infrequent use of PCR. Rhizopus sp. were the most common species isolated. In a study including 203 cases of mucormycosis with positive cultures during the second wave of the pandemic in India, Rhizopus oryzae, followed by R. microspores, were most frequently identified [95].
Mixed infections with Aspergillus and Candida are detected both in pulmonary and rhino-orbital-cerebral form. Eighteen studies in the literature (12 from India, 3 from Europe, 2 from Pakistan and 1 from Egypt) refer to Aspergillus possible co-infection, with Aspergillus being isolated in 89/863 (10.3%) CAM patients. Danion et al., reported 5 mixed fungal infections with Aspergillus in 17 (29%) CAM cases, of which 2 exhibited pulmonary involvement, 1 ROCM, 1 disseminated and 1 GI disease. All patients were mechanically ventilated and COVID-19-associated pulmonary aspergillosis (CAPA) was diagnosed at a median of 2 days before CAM. Four out of five patients with CAM and CAPA received L-Amphotericin B (one was diagnosed after death) and 5/5 died [31]. In Toulouse, France, eight cases of concomitant infection with Mucor and Aspergillus were detected in the ICU and were attributed to construction work that was undertaken near the hospital. All patients had pulmonary involvement, 3/8 were treated with L-Amphotericin B, 4/8 with a combination of L-Amphotericin B and Posaconazole and/or isavuconazole and 1/8 with isavuconazole. Four out of eight (50%) patients died [36]. Aspergillus fumigatus, Aspergillus niger and Aspergillus nidulans have been isolated [44], while mixed mold infections with Candida are also described in the literature [40][41]. Nidhya Ganesan et al. reported that among 60 biopsy samples from suspected rhino-maxillary/rhino-orbital mucormycosis post COVID-19, mucorales was isolated in 58 (96.67%) samples, aspergillus along with mucorales in 12 (20%) and a combination of mucorales and candida in 8 (13.33%) [96].
Neither 1,3-beta-D-glucan assay and galactomannan are positive in mucormycosis but can aid in the diagnosis of invasive pulmonary aspergillosis, which is recognized as a severe superinfection of COVID-19 pneumonia resulting in higher mortality. A positive serum or BAL galactomannan in a patient with compatible clinical presentation and imaging findings is indicative of invasive aspergillosis [97]. BAL galactomannan was measured in the study of R.H. Mehta et al. and was found positive (≥1) in 4/5 cases of COVID-19-associated pulmonary mucormycosis. In two cases, Aspergillus fumigatus was isolated in fungal culture, while in three cases, Aspergillus was identified in histopathological analysis [64]. Ultimately, mixed infection should be actively searched and isavuconazole is a potential empirical choice if mixed infection is suspected.
References
- Leal-Buitrago, A.; Mondragon-Ángulo, D.; Cely-Aldana, N.A.; Ortega-Sierra, M.G.; Bolaño-Romero, M.P. Letter: Spectrum of hospitalized NeuroCOVID diagnoses from a tertiary care neurology centre in Eastern India. J. Clin. Neurosci. 2022, 96, 227–228.
- Fouad, Y.A.; Bakre, H.M.; Nassar, M.A.; Gad, M.O.A.; Shaat, A.A.K. Characteristics and Outcomes of a Series of COVID-Associated Mucormycosis Patients in Two Different Settings in Egypt Through the Third Pandemic Wave. Clin. Ophthalmol. 2021, 15, 4795–4800.
- Ahmed, W.M.S.; Elsherbini, A.M.; Elsherbiny, N.M.; El-Sherbiny, M.; Ramzy, N.I.; Arafa, F.A. Maxillary Mucormycosis Osteomyelitis in Post COVID-19 Patients: A Series of Fourteen Cases. Diagnostics 2021, 11, 2050.
- Murthy, R.; Bagchi, A.; Gote, Y.S. Role of medial orbital wall decompression in COVID-19-associated rhino-orbital mucormycosis management. Indian J. Ophthalmol. 2021, 69, 3795–3796.
- Walia, S.; Bhaisare, V.; Rawat, P.; Kori, N.; Sharma, M.; Gupta, N.; Urdhwareshwar, S.; Thakur, S.; Arya, N. COVID-19-associated mucormycosis: Preliminary report from a tertiary eye care centre. Indian J. Ophthalmol. 2021, 69, 3685–3689.
- Vare, A.A.; Yellambkar, S.; Farheen, A.; Nandedkar, V.; Bhombe, S.S.; Shah, R. Incidence, cumulative mortality and factors affecting the outcome of COVID-19-associated mucormycosis from Western India. Indian J. Ophthalmol. 2021, 69, 3678–3683.
- Soni, K.; Das, A.; Sharma, V.; Goyal, A.; Choudhury, B.; Chugh, A.; Kumar, D.; Yadav, T.; Jain, V.; Agarwal, A.; et al. Sanje Surgical & medical management of ROCM (Rhino-orbito-cerebral mucormycosis) epidemic in COVID-19 era and its outcomes—A tertiary care center experience. J. Mycol. Med. 2021, 32, 101238.
- Metwally, M.I.; Mobashir, M.; Sweed, A.H.; Mahmoud, S.M.; Hassan, A.G.; ElKashishy, K.; Eesa, M.; Elnashar, I.; Elmalt, A.; Elsayed, A.I.; et al. Post COVID-19 Head and Neck Mucormycosis: MR Imaging Spectrum and Staging. Acad. Radiol. 2021, 29, 674–684.
- Arora, U.; Priyadarshi, M.; Katiyar, V.; Soneja, M.; Garg, P.; Gupta, I.; Bharadiya, V.; Berry, P.; Ghosh, T.; Patel, L.; et al. Risk factors for Coronavirus disease-associated mucormycosis. J. Infect. 2021, 84, 383–390.
- Jindal, G.; Sethi, A.; Bhargarva, K.; Sethi, S.; Mittal, A.; Singh, U.; Singh, S.; Shrivastava, A. Imaging findings in invasive rhino-orbito-cerebral mucormycosis in post-COVID-19 patients. Bayl. Univ. Med. Cent. Proc. 2021, 351, 32–34.
- Syed-Abdul, S.; Babu, A.S.; Bellamkonda, R.S.; Itumalla, R.; Acharyulu, G.; Krishnamurthy, S.; Ramana, Y.V.S.; Mogilicharla, N.; Malwade, S.; Li, Y.-C. Using artificial intelligence-based models to predict the risk of mucormycosis among COVID-19 survivors: An experience from a public hospital in India. J. Infect. 2021, 84, 351–354.
- Patel, A.; Patel, K.; Patel, K.; Shah, K.; Chakrabarti, A. Therapeutic drug monitoring of posaconazole delayed release tablet while managing COVID-19-associated mucormycosis in a real-life setting. Mycoses 2021, 65, 312–316.
- Pruthi, H.; Muthu, V.; Bhujade, H.; Sharma, A.; Baloji, A.; Ratnakara, R.G.; Bal, A.; Singh, H.; Sandhu, M.S.; Negi, S.; et al. Pulmonary Artery Pseudoaneurysm in COVID-19-Associated Pulmonary Mucormycosis: Case Series and Systematic Review of the Literature. Mycopathologia 2021, 187, 31–37.
- Bansal, S.B.; Rana, A.; Babras, M.; Yadav, D.; Jha, P.; Jain, M.; Sethi, S.K. Risk factors and outcomes of COVID associated mucormycosis in kidney transplant recipients. Transpl. Infect. Dis. 2021, 24, e13777.
- Dulski, T.M.; DeLong, M.; Garner, K.; Patil, N.; Cima, M.J.; Rothfeldt, L.; Gulley, T.; Porter, A.; Vyas, K.S.; Liverett, H.K.; et al. Notes from the Field: COVID-19-Associated Mucormycosis—Arkansas, July–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1750–1751.
- Meshram, H.S.; Kute, V.B.; Yadav, D.K.; Godara, S.; Dalal, S.; Guleria, S.; Bhalla, A.K.; Pathak, V.; Anandh, U.; Bansal, S.; et al. Impact of COVID-19-associated Mucormycosis in Kidney Transplant Recipients: A Multicenter Cohort Study. Transplant. Direct. 2021, 8, e1255.
- Aggarwal, S.K.; Kaur, U.; Talda, D.; Pandey, A.; Jaiswal, S.; Kanakan, A.; Singh, A.; Chakrabarti, S.S. Case Report: Rhino-orbital Mucormycosis Related to COVID-19: A Case Series Exploring Risk Factors. Am. J. Trop. Med. Hyg. 2022, 106, 566–570.
- Kulkarni, R.; Pujari, S.S.; Gupta, D.; Ojha, P.; Dhamne, M.; Bolegave, V.; Dhonde, P.; Soni, A.; Adwani, S.; Diwan, A.; et al. Cerebrovascular Involvement in Mucormycosis in COVID-19 Pandemic. J. Stroke Cerebrovasc. Dis. 2021, 31, 106231.
- Choksi, T.; Agrawal, A.; Date, P.; Rathod, D.; Gharat, A.; Ingole, A.; Chaudhari, B.; Pawar, N. Cumulative Mortality and Factors Associated with Outcomes of Mucormycosis after COVID-19 at a Multispecialty Tertiary Care Center in India. JAMA Ophthalmol. 2022, 140, 66–72.
- Kumar, S.; Choudhary, R.; Pandey, V.P. “MuCovid-21” study: Mucormycosis at an Indian tertiary care centre during the COVID-19 pandemic. J. R. Coll. Physicians Edinb. 2021, 51, 352–358.
- Mehta, R.; Nagarkar, N.M.; Ksbs, K.S.; Ty, S.S.; Arora, R.D.; Aggarwal, A. Facial Nerve Palsy in COVID-19-Associated Mucormycosis Patients: A Case Series. Cureus 2021, 13, e19208.
- Panwar, P.; Gupta, A.; Kumar, A.; Gupta, B.; Navriya, S.C. Mucormycosis in COVID Diabetic Patients: A Horrifying Triad! Indian J. Crit. Care Med. 2021, 25, 1314–1317.
- Patel, D.D.; Adke, S.; Badhe, P.V.; Lamture, S.; Marfatia, H.; Mhatre, P. COVID-19 associated Rhino-Orbito-Cerebral Mucormycosis: Imaging spectrum and Clinico-radiological correlation—A single Centre experience. Clin. Imaging 2021, 82, 172–178.
- Vaid, N.; Mishra, P.; Gokhale, N.; Vaid, S.; Vaze, V.; Kothadiya, A.; Deka, T.; Agarwal, R. A Proposed Grading System and Experience of COVID-19 Associated Rhino Orbito Cerebral Mucormycosis from an Indian Tertiary Care Cente. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3505–3512.
- Goddanti, N.; Reddy, Y.M.; Kumar, M.K.; Rajesh, M.; Reddy, L.S. Role of COVID 19 Inflammatory Markers in Rhino-Orbito-Cerebral Mucormycosis: A Case Study in Predisposed Patients at a Designated Nodal Centre. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3498–3504.
- Yadav, T.; Tiwari, S.; Gupta, A.; Garg, P.K.; Khera, P.S.; Rajagopal, R.; Goyal, A.; Soni, K.; Chugh, A.; Jain, V.; et al. Magnetic Resonance Imaging in Coronavirus Disease—2019 Associated Rhino-Orbital-Cerebral Mucormycosis (CA-ROCM)—Imaging Analysis of 50 Consecutive Patients. Curr. Probl. Diagn. Radiol. 2022, 51, 112–120.
- Meshram, H.S.; Kute, V.B.; Chauhan, S.; Dave, R.; Patel, H.; Banerjee, S.; Desai, S.; Kumar, D.; Navadiya, V.; Mishra, V. Mucormycosis as SARS-CoV2 sequelae in kidney transplant recipients: A single-center experience from India. Int. Urol. Nephrol. 2021, 54, 1693–1703.
- Zirpe, K.; Pote, P.; Deshmukh, A.; Gurav, S.K.; Tiwari, A.M.; Suryawanshi, P. A Retrospective Analysis of Risk Factors of COVID-19 Associated Mucormycosis and Mortality Predictors: A Single-Center Study. Cureus 2021, 13, e18718.
- Alloush, T.K.; Mansour, O.; Alloush, A.T.; Roushd, T.; Hamid, E.; El-Shamy, M.; Shokri, H.M. Rhino-orbito-cerebral mucormycosis during the COVID-19 third wave in 2021: An Egyptian preliminary report from a single tertiary hospital. Neurol. Sci. 2021, 43, 799–809.
- Pal, P.; Chatterjee, N.; Ghosh, S.; Ray, B.K.; Mukhopadhyay, P.; Bhunia, K.; Srivastava, S.R.; Adhikari, S.; Barman, D.; Banerjee, B.; et al. COVID Associated Mucormycosis: A Study on the Spectrum of Clinical, Biochemical and Radiological Findings in A Series of Ten Patients. J. Assoc. Physicians India 2021, 69, 11–12.
- Danion, F.; Letscher-Bru, V.; Guitard, J.; Sitbon, K.; Dellière, S.; Angoulvant, A.; Desoubeaux, G.; Botterel, F.; Bellanger, A.-P.; Gargala, G.; et al. Coronavirus Disease 2019-Associated Mucormycosis in France: A Rare but Deadly Complication. Open Forum Infect. Dis. 2021, 9, ofab566.
- Nehara, H.R.; Kumawat, S.; Gupta, J.; Gupta, G.; Sirohi, P.; Ih, S.; Gupta, B. Coronavirus Disease, Diabetes and Glucocorticoid a Terrible Trio for Invasive Mucormycosis: An Observational Study from Northwest Rajasthan. J. Assoc. Physicians India 2022, 69, 11–12.
- Pandiar, D.; Ramani, P.; Krishnan, R.P.; Dinesh, Y. Histopathological analysis of soft tissue changes in gingival biopsied specimen from patients with underlying corona virus disease associated mucormycosis (CAM). Med. Oral Patol. Oral Cir. Bucal. 2022, 27, e216–e222.
- Kumar, S.; Acharya, S.; Jain, S.; Shukla, S.; Talwar, D.; Shah, D.; Hulkoti, V.; Parveen, S.; Patel, M.; Patel, S. Role of Zinc and Clinicopathological Factors for COVID-19-Associated Mucormycosis (CAM) in a Rural Hospital of Central India: A Case-Control Study. Cureus 2022, 14, e22528.
- Bilgic, A.; Kodjikian, L.; Sudhalkar, A.; Dwivedi, S.; Vasavada, V.; Shah, A.; Dziadzko, M.; Mathis, T. Risk Factors for COVID-19 Associated Mucormycosis: The Ophthalmologist’s Perspective. J. Fungi 2022, 8, 271.
- Guemas, E.; Cassaing, S.; Malavaud, S.; Fillaux, J.; Chauvin, P.; Lelièvre, L.; Ruiz, S.; Riu, B.; Berry, A.; Iriart, X. A Clustered Case Series of Mucorales Detection in Respiratory Samples from COVID-19 Patients in Intensive Care, France, August to September 2021. J. Fungi 2022, 8, 258.
- Gg, S.K.; Deepalam, S.; Siddiqui, A.; Adiga, C.P.; Kumar, S.; Shivalingappa, S.S.; Acharya, U.V.; Goolahally, L.N.; Sharma, S.; Andrew, D.; et al. Coronavirus Disease 2019 (COVID-19)-Associated Rhino-Orbito-Cerebral Mucormycosis: A Multi-Institutional Retrospective Study of Imaging Patterns. World Neurosurg. 2022, 162, e131–e140.
- Mani, S.; Thirunavukkarasu, A. A clinico-pathological study of COVID-19 associated rhino-orbital-cerebral mucormycosis. Indian J. Ophthalmol. 2022, 70, 1013–1018.
- Dravid, A.; Kashiva, R.; Khan, Z.; Bande, B.; Memon, D.; Kodre, A.; Mane, M.; Pawar, V.; Patil, D.; Kalyani, S.; et al. Epidemiology, clinical presentation and management of COVID-19 associated mucormycosis: A single centre experience from Pune, Western India. Mycoses 2022, 65, 526–540.
- Naruka, S.; Rana, N.; Singh, N.; Kishore, A.; Nagpal, K. COVID-19 associated rhino-orbital-cerebral mucormycosis-an institutional series. Ear Nose Throat J. 2022.
- Jain, K.; Surana, A.; Choudhary, T.S.; Vaidya, S.; Nandedkar, S.; Purohit, M. Clinical and histology features as predictor of severity of mucormycosis in post-COVID-19 patients: An experience from a rural tertiary setting in Central India. SAGE Open Med. 2022, 10.
- Bhanuprasad, K.; Manesh, A.; Devasagayam, E.; Varghese, L.; Cherian, L.M.; Kurien, R.; Karthik, R.; Deodhar, D.; Vanjare, H.; Peter, J.; et al. Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 111, 267–270.
- Desai, E.J.; Pandya, A.; Upadhya, I.; Patel, T.; Banerjee, S.; Jain, V. Epidemiology, Clinical Features and Management of Rhino Orbital Mucormycosis in Post COVID 19 Patients. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 103–107.
- Nasir, N.; Farooqi, J.; Mahmood, S.F.; Jabeen, K. COVID-19 associated mucormycosis: A life-threatening complication in patients admitted with severe to critical COVID-19 from Pakistan. Clin. Microbiol. Infect. 2021, 27, 1704–1707.
- Gupta, S.; Ahuja, P. Risk Factors for Procurence of Mucormycosis and its Manifestations Post Covid-19: A Single Arm Retrospective Unicentric Clinical Study. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3131–3138.
- Joshi, S.; Telang, R.; Tambe, M.; Havaldar, R.; Sane, M.; Shaikh, A.; Roy, C.; Yathati, K.; Sonawale, S.; Borkar, R.; et al. Outbreak of Mucormycosis in Coronavirus Disease Patients, Pune, India. Emerg. Infect. Dis. 2022, 28, 1–8.
- Pradhan, P.; Shaikh, Z.; Mishra, A.; Preetam, C.; Parida, P.K.; Sarkar, S.; Samal, D.K.; Nayak, A.; Chadaram, S.; Das, K.K.; et al. Predisposing factors of rhino-orbital-cerebral mucormycosis in patients with COVID 19 infection. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3151–3157.
- Mehta, R.; Nagarkar, N.M.; Jindal, A.; Rao, K.N.; Nidhin, S.B.; Arora, R.D.; Sharma, A.; Wankhede, A.; Satpute, S.; Chakravarty, S.; et al. Multidisciplinary Management of COVID-Associated Mucormycosis Syndemic in India. Indian J. Surg. 2021, 84, 934–942.
- Riad, A.; Shabaan, A.A.; Issa, J.; Ibrahim, S.; Amer, H.; Mansy, Y.; Kassem, I.; Kassem, A.B.; Howaldt, H.; Klugar, M.; et al. COVID-19-Associated Mucormycosis (CAM): Case-Series and Global Analysis of Mortality Risk Factors. J. Fungi 2021, 7, 837.
- Guzmán-Castro, S.; Chora-Hernandez, L.D.; Trujillo-Alonso, G.; Calvo-Villalobos, I.; Sanchez-Rangel, A.; Ferrer-Alpuin, E.; Ruiz-Jimenez, M.; Corzo-Leon, D.E. COVID-19-associated mucormycosis, diabetes and steroid therapy: Experience in a single centre in Western Mexico. Mycoses 2021, 65, 65–70.
- Seidel, D.; Simon, M.; Sprute, R.; Lubnow, M.; Evert, K.; Speer, C.; Seeßle, J.; Khatamzas, E.; Merle, U.; Behrens, C.; et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses 2021, 65, 103–109.
- Gupta, R.; Kesavadev, J.; Krishnan, G.; Agarwal, S.; Saboo, B.; Shah, M.; Mittal, A.; Durani, S.; Luthra, A.; Singhal, A.; et al. COVID-19 associated mucormycosis: A Descriptive Multisite Study from India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102322.
- Alfishawy, M.; Elbendary, A.; Younes, A.; Negm, A.; Hassan, W.S.; Osman, S.H.; Nassar, M.; Elanany, M.G. Diabetes mellitus and Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): A wake-up call from Egypt. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102195.
- Dave, T.V.; Nair, A.G.; Hegde, R.; Vithalani, N.; Desai, S.; Adulkar, N.; Kamal, S.; Mittal, R.; Bradoo, R.A. Clinical Presentations, Management and Outcomes of Rhino-Orbital-Cerebral Mucormycosis (ROCM) Following COVID-19: A Multi-Centric Study. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 488–495.
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260.
- Fazeli, M.A.; Rezaei, L.; Javadirad, E.; Iranfar, K.; Khosravi, A.; Saman, J.A.; Poursabbagh, P.; Ghadami, M.R.; Parandin, M.M.; Dehghani, A.; et al. Increased incidence of rhino-orbital mucormycosis in an educational therapeutic hospital during the COVID-19 pandemic in western Iran: An observational study. Mycoses 2021, 64, 1366–1377.
- Mishra, Y.; Prashar, M.; Sharma, D.; Akash; Kumar, V.P.; Tilak, T. Diabetes, COVID 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab. Syndr. 2021, 15, 102196.
- Honavar, S.; Sen, M.; Sengupta, S.; Rao, R.; Kim, U.; Sharma, M.; Sachdev, M.; Grover, A.; Surve, A.; Budharapu, A.; et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021, 69, 1670–1692.
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses 2021, 64, 1238–1252.
- Reddy, Y.M.; Parida, S.; Reddy, S.B.; Yeduguri, S.; Pidaparthi, L.; Jaiswal, S.K.; Sadhvani, B.; Murthy, J.M.K. Decoding “guitar pick sign” in COVID-19-associated mucormycosis: A case series. Indian J. Ophthalmol. 2022, 70, 1425–1427.
- Arora, R.; Goel, R.; Khanam, S.; Kumar, S.; Shah, S.; Singh, S.; Chhabra, M.; Meher, R.; Khurana, N.; Sagar, T.; et al. Rhino-Orbito-Cerebral-Mucormycosis During the COVID-19 Second Wave in 2021—A Preliminary Report from a Single Hospital. Clin. Ophthalmol. 2021, 15, 3505–3514.
- Gupta, D.P.; Gupta, S.; Shah, C.K.; Sreevidya, S.R. Clinical Study of Surge of Mucormycosis in COVID-19 Pandemic: A Tertiary Care Center Study. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3455–3462.
- Gautam, M.; Soni, M.; Bhaisare, V.; Rawat, P.; Walia, S.; Kori, N. Complete and incomplete lower motor neuron facial palsy in post-COVID-19 mucormycosis. Indian J. Ophthalmol. 2022, 70, 1365–1370.
- Mehta, R.M.; Bansal, S.; Kalpakkam, H. Critical COVID-19-associated pulmonary mucormycosis: The underreported life-threatening spectrum of the mucormycosis epidemic. Lung India 2022, 39, 187–190.
- Reddy, Y.M.; Yeduguri, S.; Reddy, N.V.S.; Parida, S.; Kamatham, S.N.; Pidaparthi, L.; Jaiswal, S.K.; Sadhvani, B.; Tourani, V.; Kumar, S.; et al. Pathogenetic factors fanning the flames of COVID-19 to cause rhino-orbito-cerebral mucormycosis: An observational study. J. Med. Mycol. 2022, 32, 101252.
- Singh, S.P.; Rana, J.; Singh, V.K.; Singh, R.; Sachan, R.; Singh, S.; Jain, S. Rhino-orbital mucormycosis: Our experiences with clinical features and management in a tertiary care center. Rom. J. Ophthalmol. 2021, 65, 339–353.
- Hada, M.; Gupta, P.; Bagarhatta, M.; Tripathy, K.; Harsh, A.; Khilnani, K.; Mendiratta, K.; Agarwal, S.; Chouhan, J.K.; Bhandari, S. Orbital magnetic resonance imaging profile and clinicoradiological correlation in COVID-19-associated rhino-orbital-cerebral mucormycosis: A single-center study of 270 patients from North India. Indian J. Ophthalmol. 2022, 70, 641–648.
- Kumar, H.M.; Sharma, P.; Rudramurthy, S.M.; Sehgal, I.S.; Prasad, K.T.; Pannu, A.K.; Das, R.; Panda, N.K.; Sharma, N.; Chakrabarti, A.; et al. Serum iron indices in COVID-19-associated mucormycosis: A case-control study. Mycoses 2021, 65, 120–127.
- Bhandari, S.; Bhargava, S.; Samdhani, S.; Singh, S.N.; Sharma, B.B.; Agarwal, S.; Sharma, M.P.; Sharma, S.; Sharma, V.; Kakkar, S.; et al. COVID-19, Diabetes and Steroids: The Demonic Trident for Mucormycosis. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3469–3472.
- Chouhan, M.; Solanki, B.; Shakrawal, N. Rhino-orbital-cerebral mucormycosis: Fungal epidemic in a viral pandemic. J. Laryngol. Otol. 2021, 135, 981–986.
- Singh, Y.; Ganesh, V.; Kumar, S.; Patel, N.; Aggarwala, R.; Soni, K.D.; Trikha, A. Coronavirus Disease-Associated Mucormycosis from a Tertiary Care Hospital in India: A Case Series. Cureus 2021, 13, e16152.
- Desai, S.M.; Gujarathi-Saraf, A.; Agarwal, E.A. Imaging findings using a combined MRI/CT protocol to identify the “entire iceberg” in post-COVID-19 mucormycosis presenting clinically as only “the tip”. Clin. Radiol. 2021, 76, 784.e27–784.e33.
- Kumari, A.; Rao, N.P.; Patnaik, U.; Malik, V.; Tevatia, M.S.; Thakur, S.; Jaydevan, J.; Saxena, P. Management outcomes of mucormycosis in COVID-19 patients: A preliminary report from a tertiary care hospital. Med. J. Armed Forces India 2021, 77 (Suppl. 2), S289–S295.
- Mitra, S.; Janweja, M.; Sengupta, A. Post-COVID-19 rhino-orbito-cerebral mucormycosis: A new addition to challenges in pandemic control. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 2417–2422.
- Ramaswami, A.; Sahu, A.K.; Kumar, A.; Suresh, S.; Nair, A.; Gupta, D.; Chouhan, R.; Bhat, R.; Mathew, R.; Majeed, J.A.; et al. COVID-19-associated mucormycosis presenting to the Emergency Department-an observational study of 70 patients. Qjm Int. J. Med. 2021, 114, 464–470.
- Joshi, A.R.; Muthe, M.M.; Patankar, S.H.; Athawale, A.; Achhapalia, Y. CT and MRI Findings of Invasive Mucormycosis in the Setting of COVID-19: Experience from a Single Center in India. Am. J. Roentgenol. 2021, 217, 1431–1432.
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359.
- Sharma, S.; Grover, M.; Bhargava, S.; Samdani, S.; Kataria, T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021, 135, 442–447.
- Kant, R.; Totaganti, M.; Mohan, B.; Bairwa, M.; Panda, P.K.; Tyagi, A.; Prasad, A.; Bahurupi, Y. Clinical Characteristics of 100 Patients With COVID-19-Associated Mucormycosis From a Tertiary Care Center in North India. Cureus 2022, 14, e25652.
- Eker, C.; Tarkan, O.; Surmelioglu, O.; Dagkiran, M.; Tanrisever, I.; Karakaya, S.P.Y.; Ulas, B.; Onan, E.; Uguz, A.H.; Ozdemir, S. Alternating pattern of rhino-orbital-cerebral mucormycosis with COVID-19 in diabetic patients. Eur. Arch. Otorhinolaryngol. 2022.
- Pandit, A.K.; Tangri, P.; Misra, S.; Srivastava, M.V.P.; Bhatnagar, S.; Thakar, A.; Sikka, K.; Panda, S.; Vishnu, V.Y.; Singh, R.K.; et al. Mucormycosis in COVID-19 Patients: A Case-Control Study. Microorganisms 2022, 10, 1209.
- Youssif, S.F.; Abdelrady, M.M.; Thabet, A.A.; Abdelhamed, M.A.; Gad, M.O.A.; Abu-Elfatth, A.M.; Saied, G.M.; Goda, I.; Algammal, A.M.; Batiha, G.E.-S.; et al. COVID-19 associated mucormycosis in Assiut University Hospitals: A multidisciplinary dilemma. Sci. Rep. 2022, 12, 10494.
- Sekaran, A.; Patil, N.; Sabhapandit, S.; Sistla, S.K.; Reddy, D.N. Rhino-orbito-cerebral mucormycosis: An epidemic in a pandemic. IJID Reg. 2021, 2, 99–106.
- Shabana, R.R.; Eldesouky, M.A.; Elbedewy, H.A. Exenterate or Not: A Simple Proposed Management Algorithm for Mucormycosis During the Era of COVID-19 in a Tertiary Eye Care Center in Egypt. Clin. Ophthalmol. 2022, 16, 1933–1940.
- Patel, A.K.; Bakshi, H.; Shah, K.; Patel, S.; Patel, T.; Patel, K.; Patel, K.K. Risk factors for COVID-19 associated mucormycosis in India: A case control study. Med. Mycol. 2022, 60, myac044.
- Martins, H.D.; Pares, A.R.; Martínez, A.T.; Guevara, R.A.P.; Inaoka, S.D.; Costa, D.F.; Leal, C.B.; Soares, C.D.; da Paz, A.R.; Perez, D.E.d.C.; et al. A case series of mucormycosis after covid infection in two hospitals. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e757–e759.
- Iqtadar, S.; Hashmat, M.; Chaudhry, M.N.A.; Mumtaz, S.U.; Abaidullah, S.; Pascual-Figal, D.A.; Khan, A. Unnecessary Use of Corticosteroids for managing early mild symptoms of COVID-19 may lead to Rhino-ortibal-cerebral mucormycosis in Patients with Diabetes—A case series from Lahore, Pakistan. Ther. Adv. Infect. Dis. 2022, 9.
- Al Balushi, A.; Al Ajmi, A.; Al Sinani, Q.; Menon, V.; Al Berieki, Z.; Al Shezawi, A.; Al Azri, S.; Al Rashdi, A.; Al Jardani, A.; Al Baluki, T.; et al. COVID-19-Associated Mucormycosis: An Opportunistic Fungal Infection. A Case Series and Review. Int. J. Infect. Dis. 2022, 121, 203–210.
- Soman, R.; Chakraborty, S.; Joe, G. Posaconazole or isavuconazole as sole or predominant antifungal therapy for COVID-19-associated mucormycosis. A retrospective observational case series. Int. J. Infect. Dis. 2022, 120, 177–178.
- Pai, V.; Sansi, R.; Kharche, R.; Bandili, S.C.; Pai, B. Rhino-orbito-cerebral Mucormycosis: Pictorial Review. Insights Imaging 2021, 12, 167.
- Krishna, V.; Bansal, N.; Morjaria, J.; Kaul, S. COVID-19-Associated Pulmonary Mucormycosis. J. Fungi 2022, 8, 11.
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421.
- Samson, R.; Dharne, M. COVID-19 associated mucormycosis: Evolving technologies for early and rapid diagnosis. 3 Biotech. 2021, 12, 6.
- Fathima, A.S.; Mounika, V.L.; Kumar, V.U.; Gupta, A.K.; Garapati, P.; Ravichandiran; Dhingra, S.; Murtia, K. Mucormycosis: A triple burden in patients with diabetes during COVID-19 Pandemic. Health Sci. Rev. 2021, 1, 100005.
- Gupta, P.; Malhotra, H.S.; Saxena, P.; Singh, R.; Shukla, D.; Hasan, M.S.; Verma, V.; Banerjee, G.; Puri, B.; Dandu, H. Utility of itraconazole and terbinafine in mucormycosis: A proof-of-concept analysis. J. Investig. Med. 2022, 70, 914–918.
- Ganesan, N.; Sivanandam, S. Histomorphological features of mucormycosis with rise and fall of COVID-19 pandemic. Pathol. Res. Pract. 2022, 236.
- Dimopoulos, G.; Almyroudi, M.-P.; Myrianthefs, P.; Rello, J. COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Intensive Med. 2021, 1, 71–80.
More
