Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 3 by Peter Tang.
Obstructive sleep apnea (OSA) is a common disease that is often under-diagnosed and under-treated in all ages. This is due to differences in morphology, diversity in clinical phenotypes, and differences in diagnosis and treatment of OSA in children and adults, even among individuals of the same age.
- OSA
- CPAP
- apnea–hypopnea index
- personalized management
1. Introduction
Obstructive sleep apnea (OSA) is characterized by a partial or complete obstruction of the upper airways for at least 10 s, while exerting respiratory effort conducted by chest and abdomen movements. Symptoms of OSA include excessive daytime sleepiness, fatigue, snoring, repeated microarousals, and headache upon awakening. Diagnosis of OSA is based on clinical symptoms and polysomnography (PSG) at sleep lab or at home (home sleep apnea testing—HSAT). Treatment of OSA includes continuous positive airway pressure (CPAP), oral appliances, and surgery, in addition to weight loss and sleep hygiene.
However, OSA is a sleep disorder with high prevalence and comorbidities, and diverse clinical manifestations or phenotypes. Conventional approaches to diagnosis and treatment of OSA are no longer fully appropriate for all cases of OSA. To optimize the diagnosis and treatment of this disease, a more tailored approach is needed. The personalization (or individualization) in diagnosis and treatment of OSA is an optimal solution for all diseases in general and for OSA in particular.
2. Personalization of Diagnosis and Treatment of Children with OSA
2.1. General Consideration
OSA in children is common. Although the prevalence of snoring in children is 3–12%, OSA affects 1% to 10% of children [1]. OSA affects the development of the child in terms of behavior, brain development, metabolism, and overall health. These harmful effects continue to affect children to adulthood. Therefore, the early diagnosis and appropriate treatment of OSA will help children to develop normally and to prevent future detrimental effects [2][3]. The most common age group with OSA is 2 to 8 years old, related to adenotonsillar hypertrophy which causes upper airway narrowing. Other causes, such as premature birth and Down syndrome, also increase the risk of OSA. Boys have a higher risk of OSA than girls after puberty, but before puberty it is equal in the two sexes [4]. Severity of OSA is increased when children are obese, exposed to cigarette smoke, or have family with difficult economic conditions [5][6].2.2. Personalization of Clinical Approach for Children with OSA
2.2.1. Overview
The history and symptoms of children with OSA are varied and complex. There are many validated questionnaires which have been used to screen children with OSA. However, the current treatment guidelines recommend that physicians should ask about sleep duration and quality of sleep, as well as snoring, in suspected children with OSA. Evaluation of the child’s sleep quality should include issues such as number of microarousals, abnormal sleeping positions, and sleep disruptions with unusual movements [4]. It is important to inform parents about sleep symptoms related to OSA because they may not perceive the problems as unusual or harmful to the child’s health [7]. Clinically, OSA in children often presents differently from adults with OSA. Children’s parents might discover OSA in their children with snoring, mouth breathing, pause of breathing, waking in the middle of the night, bedwetting, or enuresis. Children with OSA often experience sleep disruptions that affect daytime behaviors such as hyperactivity, inability to concentrate well, irritability, anger, aggression, or decreased memory. Parents of young children may pay more attention to their child’s daytime problems, and complain to the clinician about them, than their child’s nocturnal symptoms [8].2.2.2. Clinical Manifestations of Children with OSA
The symptoms of OSA in children vary by age group during childhood. From 3 to 12 months of age, newborn babies with OSA show signs of sleep disruption, poor day–night cycle discrimination, loud breathing or snoring, night sweats, poor feeding, physical retardation, ear infections, or persistent recurrent respiratory infections [1]. From 1 to 3 years of age, children with OSA often cry at night or panic during sleep, loudly breathing or snoring, mouth breathing, with short apnea, agitation at sleep, daytime fatigue, irritability, aggression, recurrent respiratory infections, or growth retardation [1]. Children from 3 to 6 years old have much more complicated and varied manifestations; in addition to the symptoms of younger children, they have additional symptoms such as abnormal sleeping positions, bedwetting, parasomnia, poor concentration, headache on waking, sleepiness during the day, or difficulty waking up in the morning [1]. School-age children with OSA usually show all the symptoms of younger children and others which are typical at this age, including loudly snoring, bruxism sleep, poor school study results, abnormal growth, delayed puberty, shyness and low self-esteem, depression, dental problems, and maxillofacial structure deformation [1][9]. In children, there are four main causes of OSA: obesity, adenotonsillar hypertrophy, abnormalities of craniofacial structure, and neuromuscular dysfunction. All of these cause narrowing and obstruction of the upper airway. Children with neuromuscular dysfunction may present with OSA and central sleep apnea (CSA). Structural craniofacial abnormalities are common in hereditary syndromes such as Crouzon, Pierre Robin, or Apert. Some other craniofacial abnormalities that cause short anterior pharynx space also increase the risk of OSA, such as chin hypoplasia, small chin or large tongue, and midfacial hypoplasia [4].2.2.3. Clinical and Laboratory Considerations for Children with OSA
Clinical examination of children with OSA may reveal excessive fatigue or hyperactivity. ENT (ear–nose–throat) examination may reveal allergic rhinitis with blocked nose, small chin, large tongue, narrow palate, or large tonsils. Similar to adults, obesity can be seen in children with OSA [8]. Blood pressure should be assessed for children with OSA because some children may develop hypertension due to prolonged OSA. Clinical examination also focuses on abnormal craniofacial structure with congenital syndromes. Physicians should ask carefully about a history of preterm birth as this is a risk factor for OSA [10]. Polysomnography (PSG) is the gold standard for the diagnosis of OSA in children and adults. Some other tests can support the diagnosis of OSA in children, such as respiratory polysomnography (RPG) or nocturnal oxygen saturation measurement, but the latter is not a substitute for PSG or RPG [4]. It is necessary to conduct more comprehensive tests for general consideration before developing specific treatments for OSA. These tests include electrocardiogram, chest X-ray, 24 h blood pressure monitoring, especially for children with severe OSA [11]. Imaging tests used to evaluate craniofacial anatomy are also necessary to contribute to the diagnosis and treatment of OSA in children [11]. All-night sleep polysomnography for children should be conducted with a full range of sensors on electroencephalogram, electro-optical, electromyography, electrocardiogram, respiratory exertion, nasal-to-mouth flow, nasal–oral temperature sensor, snoring, SpO2, and leg movements. The ideal case needs an additional CO2 sensor, which can be used either at the end of exhalation or under the skin. Measurement results need to be full of parameters of waking time, sleep quality, sleep potential, and sleep stages. The AHI (apnea–hypopnea index—the number of apnea events in 1 h) needs to be determined accurately. The OSA severity grade is as follows: 1/h ≤ AHI < 5/h mild; 5/h ≤ AHI < 10/h moderate; and 10/h ≤ severe AHI [4]. This classification only applies to children under 13 years old. Because the airway structure of infants is often highly stable and intact, the most common respiratory events are hypoventilation without apnea, as in adults. Hypoxia is also not as common as in adults and often, when determining respiratory events, an EEG will be needed to evaluate microarousal to identify hypoventilation related to microarousal. Therefore, if conducting respiratory polygraphs in children, the probability of missing diagnosis will be very high.2.3. Personalized Treatment of Children with OSA
Treatment of OSA in children needs to follow a personalized approach within a general consideration. Weight loss is essential for obese children because it reduces the severity of OSA [12]. In obese children, the encouragement to physical activities is also very important: participating in sports may help to stabilize weight and improve the quality of sleep. Sleep hygiene is also important for healthy physiologic function and to reduce the risk of OSA. Specific treatment for children with OSA includes surgical or non-surgical therapy. In OSA children with adenotonsillar hypertrophy and/or blocked nasal passages due to allergic rhinitis, the effectiveness of leukotriene receptor antagonists (LRAs) has been demonstrated for those with mild-to-moderate OSA [13]. LRAs might reduce the adenotonsillar size after 3 months of treatment and therefore improve the apnea–hypopnea index (AHI) results in children with OSA [14][15]. In cases of nasal problems, topical corticosteroid use might improve AHI in mild-to-moderate OSA [8]. The combination of LRAs and topical nasal corticosteroids may result in a significant reduction in AHI in children with OSA [12]. The recent systematic review and meta-analysis on the safety and adverse effects of intranasal corticosteroid therapy in children stated that the use of topical nasal corticosteroids which have been approved by Food and Drug Administration (FDA) are generally safe in the pediatric population: growth velocity reduction, hypothalamic–pituitary–adrenal axis suppression, and visual changes are uncommon [16]. For OSA children with adenotonsillar hypertrophy, adenotonsillectomy remains an optimal therapy for OSA. This intervention could be indicated for those with AHI ≥ 10 events/h or for those with mild to moderate OSA (5 < AHI < 10 events/h) but with severe symptoms [12][14][17][18]. Other interventions such as partial adenotonsillectomy or oropharyngeal orthopedic surgery have been ineffective and data are limited [12]. Tonsillectomy can be total or partial. The total tonsil and capsule are removed in total tonsillectomy; when the capsule or portion of the tonsil is left in situ this is known as intracapsular tonsillectomy [19]. However, when tonsillectomy fails, drug-induced sleep endoscopy (DISE) may provide a more personalized surgical plan and limit unsuccessful interventions [20]. Children should have a careful follow-up after tonsillectomy. Regarding surgical intervention for adenotonsillar hypertrophy, children with obesity and allergic rhinitis must have those health issues addressed before surgery. Adenotonsillectomy is effective in reducing AHI in children who are not obese and without nasopharyngeal problems or other causes [21]. The results of the recent publication, based on a series of 114 children (mean age of 5.5 ± 2.1 years) with OSA who had adenotonsillar hypertrophy, demonstrated that treatment with ALR for moderate OSA or surgery (Figure 1) for severe OSA might reduce the symptoms related to OSA at night and during the day, and the mean AHI post-intervention [22].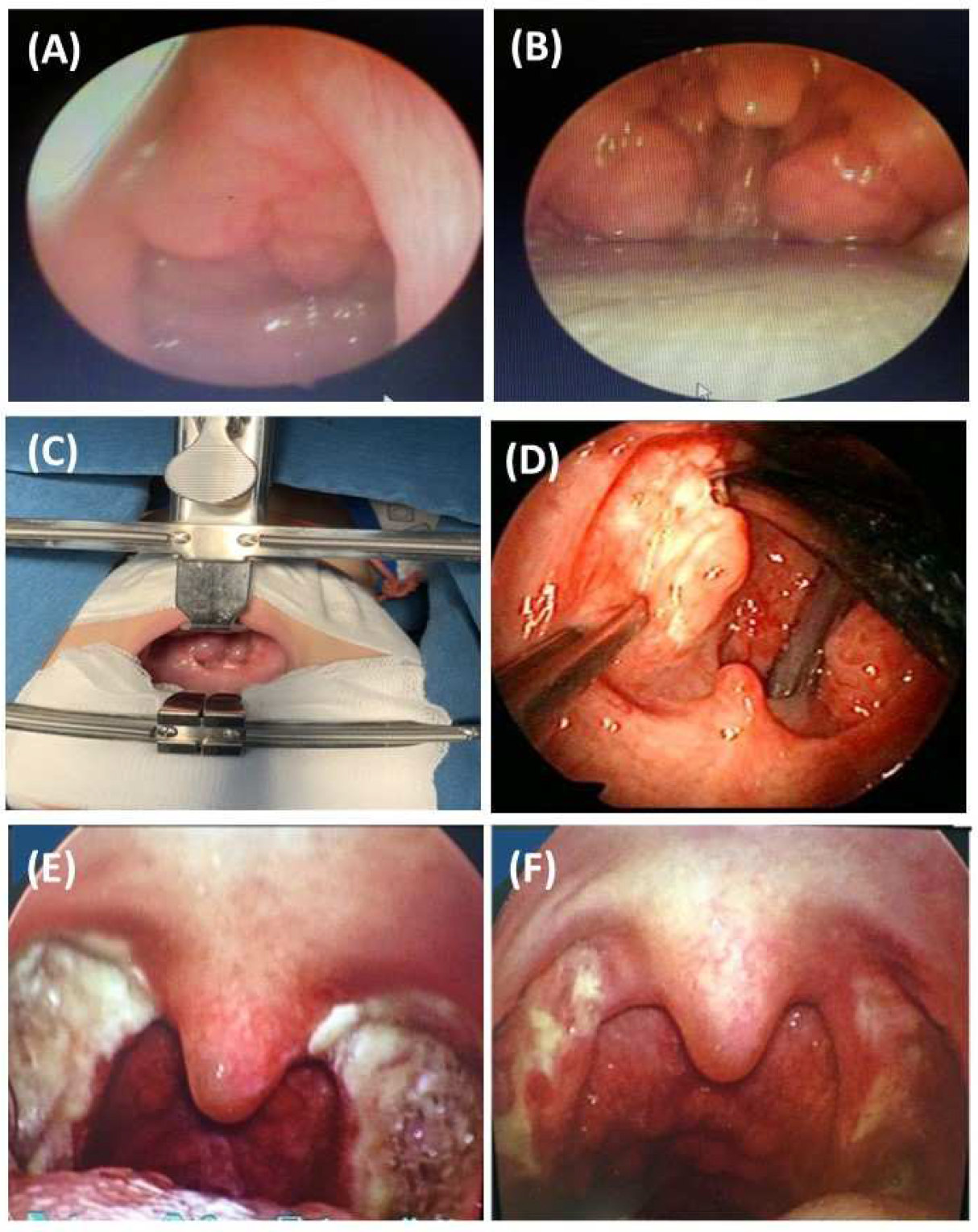
References
- Capdevila, O.S.; Gozal, L.; Dayyat, E.; Gozal, D. Pediatric Obstructive Sleep Apnea: Complications, Management, and Long-term Outcomes. Proc. Am. Thorac. Soc. 2008, 5, 274–282.
- Garg, R.K.; Afifi, A.M.; Garland, C.B.; Sanchez, R.; Mount, D.L. Pediatric Obstructive Sleep Apnea: Consensus, Controversy, and Craniofacial Considerations. Plast. Reconstr. Surg. 2017, 140, 987–997.
- Bazzano, L.A.; Hu, T.; Bertisch, S.M.; Yao, L.; Harville, E.W.; Gustat, J.; Chen, W.; Webber, L.S.; Redline, S. Childhood obesity patterns and relation to middle-age sleep apnoea risk: The Bogalusa Heart Study. Pediatr. Obes. 2016, 11, 535–542.
- Schwengel, D.A.; Dalesio, N.M.; Stierer, T.L. Pediatric obstructive sleep apnea. Anesthesiol. Clin. 2014, 32, 237–261.
- Ramirez, F.D.; Groner, J.A.; Ramirez, J.L.; McEvoy, C.T.; Owens, J.A.; McCulloch, C.E.; Cabana, M.D.; Abuabara, K. Prenatal and Childhood Tobacco Smoke Exposure Are Associated with Sleep-Disordered Breathing Throughout Early Childhood. Acad. Pediatr. 2020, 21, 654–662.
- Xiao, L.; Su, S.; Liang, J.; Jiang, Y.; Shu, Y.; Ding, L. Analysis of the Risk Factors Associated with Obstructive Sleep Apnea Syndrome in Chinese Children. Front. Pediatr. 2022, 10, 216.
- Honaker, S.M.; Meltzer, L.J. Sleep in pediatric primary care: A review of the literature. Sleep Med. Rev. 2016, 25, 31–39.
- Li, Z.; Celestin, J.; Lockey, R.F. Pediatric Sleep Apnea Syndrome: An Update. J. Allergy Clin. Immunol. Pr. 2016, 4, 852–861.
- Segù, M.; Pollis, M.; Santagostini, A.; Meola, F.; Manfredini, D. Correlation between Parental-Reported Tooth Grinding and Sleep Disorders: Investigation in a Cohort of 741 Consecutive Children. Pain Res. Manag. 2020, 2020, 1–5.
- El Mallah, M.; Bailey, E.; Trivedi, M.; Kremer, T.; Rhein, L.M. Pediatric Obstructive Sleep Apnea in High-Risk Populations: Clinical Implications. Pediatr. Ann. 2017, 46, e336–e339.
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270.
- Cielo, C.M.; Gungor, A. Treatment Options for Pediatric Obstructive Sleep Apnea. Curr. Problems Pediatr. Adoles. Health Care 2016, 46, 27–33.
- Kheirandish-Gozal, L.; Bandla, H.P.R.; Gozal, D. Montelukast for Children with Obstructive Sleep Apnea: Results of a Double-blind Randomized Placebo-controlled Trial. Ann. Am. Thorac. Soc. 2016, 13, 1736–1741.
- Goldbart, A.D.; Greenberg-Dotan, S.; Tal, A. Montelukast for Children with Obstructive Sleep Apnea: A Double-blind, Placebo-Controlled Study. Pediatrics 2012, 130, e575–e580.
- Goldbart, A.D.; Goldman, J.L.; Veling, M.C.; Gozal, D. Leukotriene Modifier Therapy for Mild Sleep-disordered Breathing in Children. Am. J. Respir. Crit. Care Med. 2005, 172, 364–370.
- Donaldson, A.M.; Choby, G.; Kim, D.H.; Marks, L.A.; Lal, D. Intranasal Corticosteroid Therapy: Systematic Review and Meta-analysis of Reported Safety and Adverse Effects in Children. Otolaryngol. Neck Surg. 2020, 163, 1087–1096.
- Hua, F.; Zhao, T.; Walsh, T.; Sun, Q.; Chen, X.; Worthington, H.; Jiang, F.; He, H. Effects of adenotonsillectomy on the growth of children with obstructive sleep apnoea-hypopnea syndrome (OSAHS): Protocol for a systematic review. BMJ Open 2019, 9, e030866.
- Shan, S.; Wang, S.; Yang, X.; Liu, F.; Xiu, L. Effect of adenotonsillectomy on the growth, development, and comprehensive cognitive abilities of children with obstructive sleep apnea: A prospective single-arm study. BMC Pediatr. 2022, 22, 1–7.
- Uwiera, T.C. Considerations in Surgical Management of Pediatric Obstructive Sleep Apnea: Tonsillectomy and Beyond. Children 2021, 8, 944.
- Gazzaz, M.J.; Isaac, A.; Anderson, S.; Alsufyani, N.; Alrajhi, Y.; El-Hakim, H. Does drug-induced sleep endoscopy change the surgical decision in surgically naïve non-syndromic children with snoring/sleep disordered breathing from the standard adenotonsillectomy? A retrospective cohort study. J. Otolaryngol.-Head Neck Surg. 2017, 46, 1–8.
- Trosman, S.J.; Eleff, D.J.; Krishna, J.; Anne, S. Polysomnography results in pediatric patients with mild obstructive sleep apnea: Adenotonsillectomy vs. watchful waiting. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 25–30.
- Tran-Minh, D.; Phi-Thi-Quynh, A.; Nguyen-Dinh, P.; Duong-Quy, S. Efficacy of obstructive sleep apnea treatment by antileu-kotriene receptor and surgery therapy in children with adenotonsillar hypertrophy: A descriptive and cohort study. Front. Neurol. 2022, 13, 1008310.
- Guilleminault, C.; Monteyrol, P.-J.; Huynh, N.T.; Pirelli, P.; Quo, S.; Li, K. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath. 2011, 15, 173–177.
- Ahn, H.W.; Lee, B.S.; Kim, S.W.; Kim, S.J. Stability of Modified Maxillomandibular Advancement Surgery in a Patient with Preadolescent Refractory Obstructive Sleep Apnea. J. Oral. Maxillofac. Surg. 2015, 73, 1827–1841.
- Li, Y.; Lu, Y.; Li, X.; Zhao, L.; Guo, J.; Yu, L.; Feng, J.; Li, B.; Li, X.; Liu, Y. Efficacy of orthodontic treatment versus adenotonsillectomy in children with moderate obstructive sleep apnoea and mandibular retrognathia: Study design and protocol for a non-inferiority randomised controlled trial. BMJ Open 2022, 12, e055964.
- Fagundes, N.C.F.; Perez-Garcia, A.; Graf, D.; Flores-Mir, C.; Heo, G. Orthodontic interventions as a management option for children with residual obstructive sleep apnea: A cohort study protocol. BMJ Open 2022, 12, e061651.
More
