Plant pathogens are responsible for causing economic and production losses in several crops worldwide, thus reducing the quality and quantity of agricultural supplies. To reduce the usage of chemically synthesized pesticides, strategies and approaches using endophytic microorganisms are being used in plant disease management. Although the term “endophyte” was originally introduced by de Bary in 1866, the most used definition of endophytes was proposed by Petrini in 1991. It refers to a group of organisms “inhabiting plant organs that at some time in their life can colonize internal plant tissues without causing apparent harm to the host”. These endophytes are usually fungi or bacteria that are present in the phyllosphere, endosphere or rhizosphere. These microorganisms live in the tissues of plants without causing any symptoms of disease, leading to beneficial effects for the hosts.
- antibacterial
- antifungal
- biofertilizers
- endophytes
- mycoherbicides
1. Introduction
2. Fungal Endophytes and Their Benefits for Plants
Although the term “endophyte” was originally introduced by de Bary in 1866, the most used definition of endophytes was proposed by Petrini in 1991 [18]. It refers to a group of organisms “inhabiting plant organs that at some time in their life can colonize internal plant tissues without causing apparent harm to the host” [19]. These endophytes are usually fungi or bacteria that are present in the phyllosphere, endosphere or rhizosphere. These microorganisms live in the tissues of plants without causing any symptoms of disease, leading to beneficial effects for the hosts (Figure 1) by: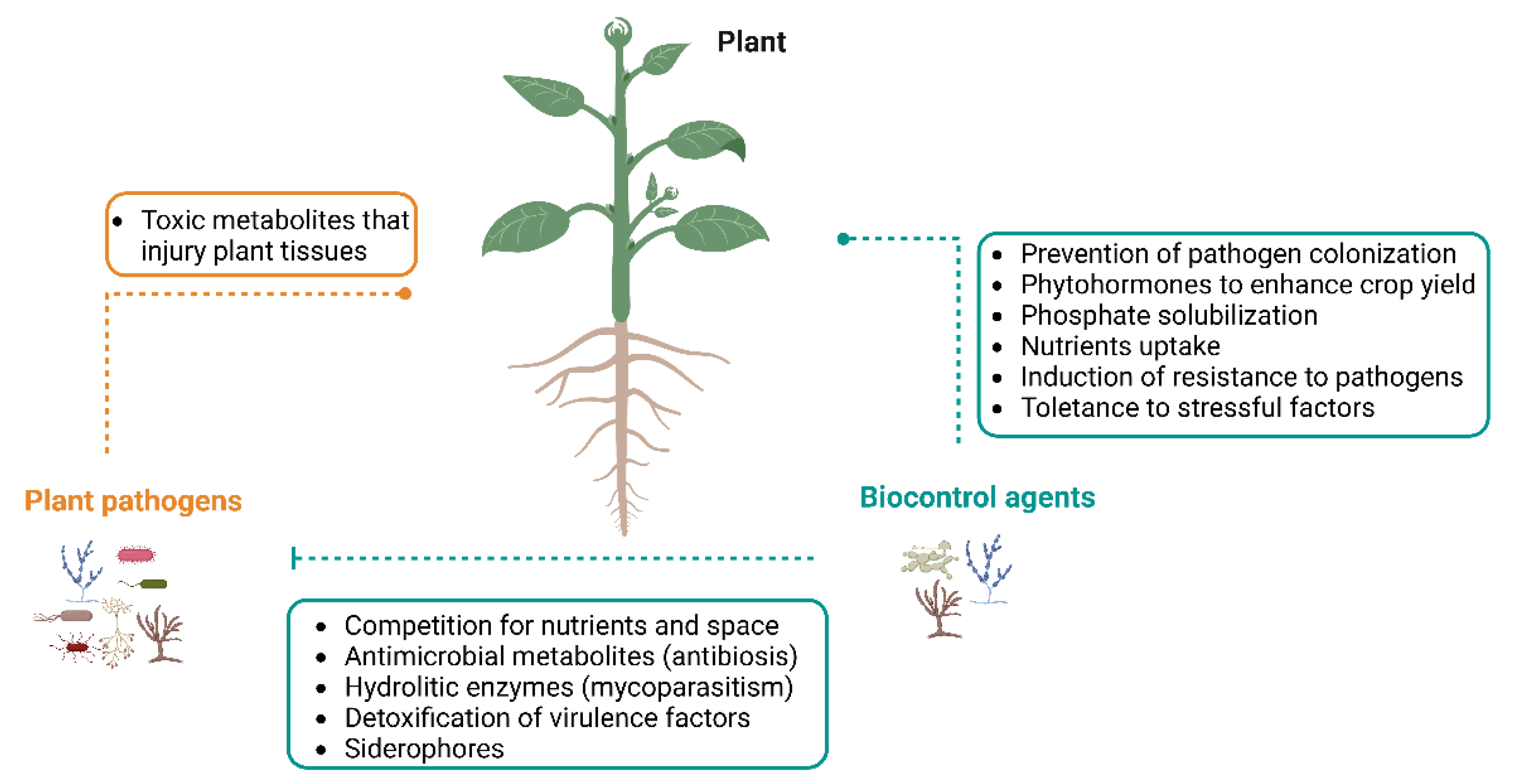
- (1)
-
Facilitating the acquisition of limited nutrients (e.g., nitrogen) [3];
- (2)
-
Producing phytohormones (e.g., gibberellins and indole acetic acid) that enhance crop yield and quality [20,21,22][20][21][22];
- (3)
-
Providing plant tolerance to environmental stresses factors (e.g., salinity, drought, heavy metal presence) [3,20][3][20];
- (4)
-
Improving resistance to pathogens [3].
- (1)
-
Competing for nutrients and space [35,36][35][36];
- (2)
-
Antibiosis-production of inhibitory metabolites or antibiotics [33,35,37][33][35][37];
- (3)
-
Induction of plant defense response against plant pathogens [35,38][35][38];
- (4)
-
Secretion of extracellular hydrolytic enzymes [38];
- (5)
-
Detoxification of virulence factors [38].
3. Conclusions
Regardless of the recognized benefits of endophytic fungi on plants and their potential in both biocontrol and biofertilization, they have been rarely studied regarding their application in agriculture. Nevertheless, due to the actual climate change scenarios (e.g., drought and high levels of soil salinity), it is crucial to understand the impacts of these environmental stresses on agriculture, as well as to unravel adaptation patterns of the endophytic community. Moreover, an effective utilization of endophytic fungi aids in promoting a sustainable agriculture for a safe environment and a positive impact on human health. In recent years, accumulating evidence has provided important advances regarding biological control agents for the development of commercialized bacterial and fungal-based biopesticides to control plant diseases. However, the implementation of large-scale studies to expand the knowledge on the usage of biopesticides is still hampered by the high cost of commercial products, the standard methods of preparations, the dose determination of active substances and the susceptibility of biopesticides to environmental conditions. In this regard, and taking into consideration the possibility of using endophytic microorganisms as promising leads for the development of biopesticides and biofertilizers, some strategies should be adopted to improve the performance of these endophytic fungi. For instance, the development of specific delivery systems such as biopriming, encapsulation or foliar spraying should be favored to support the success of biocontrol and biofertilization programs. Moreover, the development of effective microbial consortium composed of endophytic fungi could also be a promising strategy, not only to ensure the microbial diversity in the soil, but also in the phylosphere; phylosphere colonization is of paramount importance to ensure crop development and plant health management, regulating plant physiology under climate change scenarios.
References
- Large, E.C. Control of Potato Blight (Phytophthora infestans) by Spraying with Suspensions of Metallic Copper. Nature 1943, 151, 80–81.
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal agents in agriculture: Friends and foes of public health. Biomolecules 2019, 9, 521.
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527.
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148.
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17.
- Wang, Z.; Li, C.; White, J. Effects of Epichloë endophyte infection on growth, physiological properties and seed germination of wild barley under saline conditions. J. Agron. Crop Sci. 2019, 206, 43–51.
- Zhang, H.; Zhang, C.; Xiang, X.; Zhang, Q.; Zhao, W.; Wei, G.; Hu, A. Uptake and transport of antibiotic kasugamycin in castor bean (Ricinus communis L.) seedlings. Front. Microbiol. 2022, 13, 948171.
- Osada, H. Discovery and applications of nucleoside antibiotics beyond polyoxin. J. Antibiot. 2019, 72, 855–864.
- El-Naggar, N.E.A. Streptomyces-based cell factories for production of biomolecules and bioactive metabolites. In Microbial Cell Factories Engineering for Production of Biomolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 183–234.
- Chakraborty, A.; Ray, P. Mycoherbicides for the Noxious Meddlesome: Can Colletotrichum be a Budding Candidate? Front. Microbiol. 2021, 12, 754048.
- Suebrasri, T.; Harada, H.; Jogloy, S.; Ekprasert, J.; Boonlue, S. Auxin-producing fungal endophytes promote growth of sunchoke. Rhizosphere 2020, 16, 100271.
- Domka, A.M.; Rozpaadek, P.; Turnau, K. Are fungal endophytes merely mycorrhizal copycats? The role of fungal endophytes in the adaptation of plants to metal toxicity. Front. Microbiol. 2019, 10, 371.
- Yi, Y.; Luan, P.; Wang, K.; Li, G.; Yin, Y.; Yang, Y.; Zhang, Q.; Liu, Y. Antifungal Activity and Plant Growth-Promoting Properties of Bacillus mojovensis B1302 against Rhizoctonia cerealis. Microorganisms 2022, 10, 1682.
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923.
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 2022, 52, 395–435.
- Xu, T.C.; Lu, Y.H.; Wang, J.F.; Song, Z.Q.; Hou, Y.G.; Liu, S.S.; Liu, C.S.; Wu, S.H. Bioactive secondary metabolites of the genus Diaporthe and anamorph Phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms 2021, 9, 217.
- Abramczyk, B.; Marzec-Grządziel, A.; Grządziel, J.; Król, E.; Gałązka, A.; Oleszek, W. Biocontrol Potential and Catabolic Profile of Endophytic Diaporthe eres Strain 1420S from Prunus domestica L. in Poland—A Preliminary Study. Agronomy 2022, 12, 165.
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, e173.
- Chitnis, V.R.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Oelmüller, R.; Shaanker, R.U. Fungal endophyte-mediated crop improvement: The way ahead. Front. Plant Sci. 2020, 11, 1588.
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55.
- Khan, A.L.; Hamayun, M.; Kim, Y.-H.; Kang, S.-M.; Lee, J.-H.; Lee, I.-J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Proc. Biochem. 2011, 46, 440–447.
- Khan, A.L.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Jung, H.-Y.; Lee, J.-H.; Lee, I.-J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3.
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Effect of fungal endophytes on plant growth and nutrient uptake in Trifolium subterraneum and Poa pratensis as affected by plant host specificity. Mycol. Prog. 2021, 20, 1217–1231.
- Baron, N.C.; Costa, N.T.A.; Mochi, D.A.; Rigobelo, E.C. First report of Aspergillus sydowii and Aspergillus brasiliensis as phosphorus solubilizers in maize. Ann Microbiol. 2018, 68, 863–870.
- Christian, N.; Herre, E.A.; Clay, K. Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol. 2019, 222, 1573–1583.
- Rajini, S.B.; Nandhini, M.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Prakash, H.S. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant Pathol. 2020, 69, 642–654.
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 1–10.
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005.
- Ismail, H.M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.J. Aspergillus niger boosted heat stress tolerance in sunflower and soybean via regulating their metabolic and antioxidant system. J. Plant Interact. 2020, 15, 223–232.
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058.
- Adhikari, K.; Bhandari, S.; Niraula, D.; Shrestha, J. Use of neem (Azadirachta indica A. Juss) as a biopesticide in agriculture: A review. J. Agric. Appl. Biol. 2021, 1, 100–117.
- Kalpana, T.; Anil, K. A review of biopesticides and their plant phytochemicals information. Ann. Romanian Soc. Cell Biol. 2021, 25, 3576–3588.
- Santos, C.M.; da Silva, R.A.; Garcia, A.; Polli, A.D.; Polonio, J.C.; Azevedo, J.L.; Pamphile, J.A. Enzymatic and antagonist activity of endophytic fungi from Sapindus saponaria L. (Sapindaceae). Acta Biol. Colomb. 2019, 24, 322.
- Ribeiro, A.D.S.; Polonio, J.C.; Costa, A.T.; Dos Santos, C.M.; Rhoden, S.A.; Azevedo, J.L.; Pamphile, J.A. Bioprospection of culturable endophytic fungi associated with the ornamental plant Pachystachys lutea. Curr. Microbiol. 2018, 75, 588–596.
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45.
- Landum, M.; Felix, M.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108.
- Rakshith, D.; Santosh, P.; Satish, S. Isolation and characterization of antimicrobial metabolite producing endophytic Phomopsis sp. from Ficus pumila Linn. (Moraceae). Int. J. Chem. Anal. Sci. 2013, 4, 156–160.
- Deketelaere, S.; Tyvaert, L.; França, S.C.; Höfte, M. Desirable traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 2017, 8, 1186.
- Gupta, S.K.; Sharma, M. Approaches and Trends in Plant Disease Management; Scientific Publishers: Jodhpur, India, 2014; p. 429.
- Roberts, W. Studies on biogenesis. Philos. Trans. R. Soc. 1874, 164, 466.
- Baker, K.F. Evolving concepts of biological control of plant pathogens. Annu. Rev. Phytopathol. 1987, 25, 67–85.
- Weindling, R. Experimental consideration of the mold toxins of Gliocladium and Trichoderma. Phytopathology 1941, 31, 991.
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10.
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens 2021, 10, 1311.
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538.
- Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Saxena, S. Endophytic Fungi: A Source of Potential Antifungal Compounds. J. Fungi 2018, 4, 77.
- Wu, S.-H.; He, J.; Li, X.-N.; Huang, R.; Song, F.; Chen, Y.-W.; Miao, C.-P. Guaiane sesquiterpenes and isopimarane diterpenes from an endophytic fungus Xylaria sp. Phytochemistry 2014, 105, 197–204.
- Sangeetha, G.; Usharani, S.; Muthukumar, A. Biocontrol with Trichoderma species for the management of postharvest crown rot of banana. Phytopathol. Mediterr. 2009, 48, 214–225.
- Bai, Y.B.; Gao, Y.Q.; Nie, X.D.; Tuong, T.M.; Li, D.; Gao, J.M. Antifungal activity of griseofulvin derivatives against phytopathogenic fungi in vitro and in vivo and three-dimensional quantitative structure-activity relationship analysis. J. Agric. Food Chem. 2019, 67, 6125–6132.
- Segaran, G.; Sathiavelu, M. Fungal endophytes: A potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol. 2019, 21, 101284.
