Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Pavlo Petakh and Version 2 by Vivi Li.
Leptospirosis is one of the most widespread and dangerous zoonoses in the world. The main cause of death in leptospirosis is the development of a severe form of leptospirosis—Weil’s disease, which affects the kidneys, lungs, and liver, which are a “triad” of target organs in leptospirosis.
- leptospirosis
- dysbiosis
- Weil’s disease
1. Introduction
Leptospirosis is a re-emerging zoonosis, caused by Leptospira spp. It is estimated to infect more than a million people with approximately 60,000 deaths annually [1]. Disease is usually transmitted by contact with the urine of a host carrying the pathogen in water or soil, causing infection in humans through the skin or the gastrointestinal tract (GI) [2]. Human leptospirosis has a wide range of clinical manifestations, including mild, asymptomatic infections, as well as serious and life-threatening complications with multi-organ dysfunction, e.g., when kidneys, lungs, and liver are seriously damaged [3][4][3,4]. Weil’s syndrome (10% of cases), is a severe form of leptospirosis with a high mortality rate; it is characterized by hepatic dysfunctions associated with renal failure and hemorrhages [5]. Severe leptospirosis patients should receive early recognition and intensive medical care. The pathology of leptospirosis and the factors that cause severe leptospirosis are currently unclear [6][7][6,7] and antibiotic therapy is still the preferred treatment. However, due to the difficulty of early diagnosis of leptospirosis, some patients infected with L. interrogans often develop multiorgan dysfunction [8].
Both host factors and pathogens may play an important role in the pathogenesis of leptospirosis [9]. Induction of an inflammatory response by a pathogen can initiate destructive immune mechanisms leading to host tissue damage, sepsis, and death [10]. The extensive release of cytokines, including interleukin 6 (IL-6), interleukin 1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), is known as a cytokine storm. Several studies have shown how cytokines contribute to pathogenesis and clinical manifestations of leptospirosis [11][12][11,12].
Gut dysbiosis is one of the potential factors that can affect the clinical course of leptospirosis [13]. Gut microbiota interacts with the host immune system in ways that influence the development of different diseases [14]. The gut microbiota generates a wide range of metabolites, such as short-chain fatty acids (SCFAs) or secondary bile acids [15]. SCFA has anti-inflammatory effects by inducing apoptosis and preserving the mucosal barriers to endotoxin infiltration [16]. There is growing evidence that there is a critical link between the gut microbiome and other organs most affected by leptospirosis, such as the liver, lungs, and kidneys [17][18][17,18].
2. Leptospira Immunity
The innate immune system is the first line of host defense and is essential in the early recognition and elimination of leptospires [9]. Microbial Pathogen-Associated Molecular Patterns (PAMPs) will be recognized by the Pattern Recognition Receptors (PRRs) expressed at the surface of innate immune cells, such as macrophages and dendritic cells (DCs), mainly the Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [19]. Among TLRs, TLR2 and TLR4 are the most studied in leptospirosis now. TLR4 can be activated by Lipopolysaccharides (LPS) from Gram-negative bacteria, which causes a pro-inflammatory cytokine- and chemokine-dependent response [20]. Leptospiral LPS is less endotoxic than Gram-negative LPS and activates human macrophages via TLR2 rather than TLR4 [21] (Figure 1).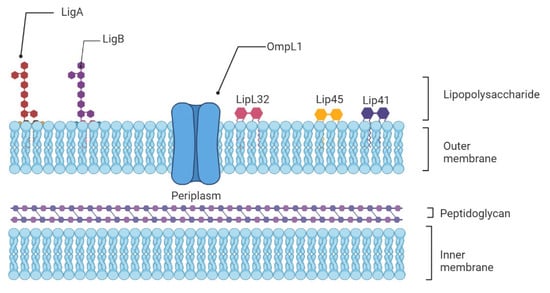
Figure 1. Schematic representation of the architecture of the leptospiral membrane. The inner membrane is closely associated with the peptidoglycan cell wall, which is overlaid by the outer membrane. Surface-exposed lipoproteins (LipL32, LigA, LigB), the transmembrane outer membrane protein porin L1 (OmpL1), and lipopolysaccharide are among the main components of the outer membrane.
3. Cytokines, Chemokines, Genetics, and Their Role in Severe Leptospirosis
Inflammatory cytokines and cytokine regulators participate in pathogen clearance without excessive inflammation-induced organ damage. Severe infectious diseases are often associated with a prolonged increase in pro-inflammatory IL-1β, TNF-α, IL-6 expression, or “cytokine storm”, causing persistent inflammation and followed by a massive and systemic production of anti-inflammatory cytokines [34]. Interestingly, the clinical hallmarks of severe leptospirosis can resemble septic shock, with multi-organ failure, hypotension, and death, which suggest that the development of severe leptospirosis could be associated with dysregulated inflammation [35]. There have been data obtained during clinical studies that confirm this hypothesis. The very first research of cytokines in human leptospirosis showed a significant increase in TNF-α levels from patient sera [36][37][36,37]. However, as TNF-α is an early phase cytokine, its levels are reduced or undetectable with disease progression [9]. Further investigations reported higher production of cytokines in severe compared to mild disease [10][11][10,11]. Serum levels of pro-inflammatory IL-6, chemokine IL-8, and anti-inflammatory IL-10 were significantly higher among patients that developed SPHS compared to non-SPHS leptospirosis patients [10]. In addition, concentrations of these cytokines were elevated in sera from patients with organ dysfunction compared to mild cases without organ involvement [38]. Additionally, in human leptospirosis, contradictory findings showed either greater IL-10 levels or no significant change in IL-10 levels [10][39][10,39]. Inflammatory response is restricted by regulatory cytokines, such as IL-4 or IL-13, promoting T helper (Th) lymphocyte differentiation toward Th2, suppressing tissue-damaging effects of sustained inflammation [40]. Serum levels of IL-4 were increased in human patients and frequencies in IL-4 and IL-4R receptor gene polymorphisms were significantly higher in leptospirosis patients compared to healthy subjects [41]. High levels of chemokines are found in susceptible hamsters, and are associated with organ damage and poor outcome [10][42][10,42]. Notably, patients with severe clinical signs had higher levels of CXCL8/IL-8 expression, and these patients had a higher mortality rate [39]. In addition, high concentrations of adhesion molecules (ICAM-1 or VCAM) are associated with leptospirosis-induced organ damage [43]. Chemokines along with endothelial adhesion molecules are induced by TNF-α, which promotes leukocyte attraction and extravasation into injured tissue. Pentraxins are a class of evolutionarily conserved proteins that trace their evolutionary roots back to early invertebrates. C-reactive protein (CRP) and serum amyloid P component (SAP) are short pentraxins, and Pentraxin 3 (PTX-3) is a long one. In leptospirosis patients, increasing serum levels of PTX-3 were associated with mortality and disease severity [44]. The study of genetic susceptibility to infectious illness has experienced a revolutionary transformation in the previous decade [45]. Variations in the genetic makeup of the host may result in changes in susceptibility to leptospirosis [46]. Human leukocyte antigen (HLA), cytokine genes, and killer-cell immunoglobulin-like receptors (KIR) polymorphisms were studied in leptospirosis patients and healthy controls [41]. According to the researchers, susceptibility to leptospiral infection was significantly associated with alleles in the HLA-A and B loci and various HLA haplotypes. Patients with a history of leptospirosis exhibited considerably more polymorphisms in the IL-4 and IL-4R genes. Other researchers, on the other hand, discovered that leptospirosis infection was responsive to genetic diversity in the IL12RB1, IL1, and CISH genes [47]. TLR1 Ile602Ser and TLR2 Arg753Gln gene polymorphisms have also been found to substantially influence the development of severe leptospirosis with jaundice and hepatic insufficiency [48].4. Immune Evasion in Leptospirosis
4.1. Neutrophils Are Poor Phagocytes
Neutrophils are key cells that act against extracellular pathogens through three major mechanisms, i.e., phagocytosis, degranulation, and the release of extracellular traps (Figure 2). According to a recent study, most pathogenic leptospires were found on the neutrophil surface and were not phagocytized. Saprophytic leptospires, on the other hand, were taken up. Intracellular ROS levels were found to be related to leptospire uptake. Overall, pathogenic leptospires appear to be able to avoid or significantly reduce their uptake by human neutrophils, but the precise mechanisms and in vivo relevance involved are unknown [49].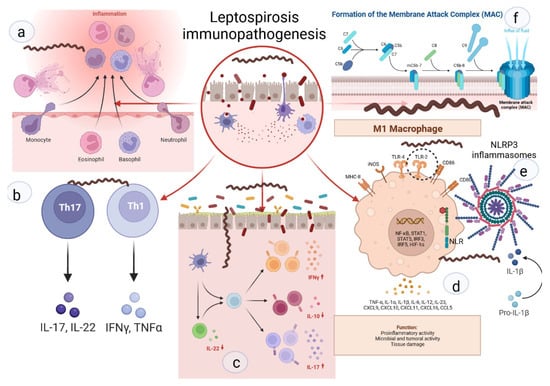
Figure 2. Key links in the immunopathogenesis of leptospirosis: (a) recruitment of neutrophils to the inflammatory zone and formation of NETs; (b) activation of differentiation of pro-inflammatory subpopulations of T-helpers; (c) increase in pro-inflammatory signaling against the background of deficiency of suppressor signals and Treg cells; (d) activation of M1-macrophages, stimulation of TLR2; (e) NLR with subsequent formation of NLRP3-inflammasome and subsequent maturation of IL1b; (f) -formation of MAC and destruction of the cell membrane.
