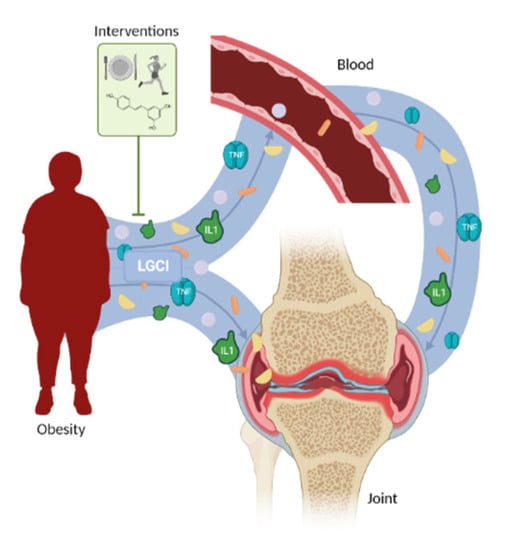
Current lifestyle and environmental factors contribute to obesity development, leading to low grade chronic inflammation (LGCI). Apart from obesity, LGCI is also related to rheumatic diseases like osteoporosis (OP) and osteoarthritis (OA). In these, an excessive accumulation of adipose tissue has been linked to an excessive production proinflammatory factors as adipokines. This work’s aim is to stablish the effect of obesity-associated LGCI in major rheumatic diseases, and to determine optimal strategies to reduce it. Obesity is a risk factor to develop OA, where a systemic LGCI state has been founded. Concretely, obesity associated LGCI has been described as an OA instauration and progression promoter. To avoid this, several therapeutical approaches (as diet control, physical exercise, or nutraceuticals) have been tested. OP is another major rheumatic disease where a basal LGCI has been described, being worsened by obesity. As in OA, diet management and supplementation with vitamin D or probiotics have been proposed as approaches to treat obesity-associated LGCI in this pathology. Currently, the augment of rheumatic diseases prevalence is unstoppable. Nonetheless, obesity is a risk factor that can be controlled. Thus, the study of new interventions to control obesity-associated LGCI impact is a challenge for patients with rheumatic diseases management.
- obesity
- LGCI
- osteoarthritis
- osteoporosis
1. Introduction
Lifestyle and environmental factors play a crucial role in health. It is well known that the diet changes recently adopted in our society have had negative effects on our wellbeing [1]. Among these changes, an excess of caloric intake, the poor quality of microand macronutrients, such as an increase in saturated fatty acids, trans fats, and simple sugars, as well as a decrease in calcium, magnesium, and B vitamin intake should be highlighted [1]. These alterations contribute to chronic stress and low-grade chronic inflammation (LGCI) [1]. Obesity-related LGCI is also known as metainflammation [2]. Moreover, all these modifications of diet intake have also led to an increase in adiposity, which is related to inflammatory status and contributes to LGCI [1]. LGCI is defined as a persistent and unresolved inflammation, accompanied by a subclinical elevation (2- to 4-fold) of inflammatory cytokines or the presence of specific immune cells in peripheral blood [1]. LGCI is related to many chronic diseases including obesity, osteoporosis (OP), osteoarthritis (OA) [3], and sarcopenia [1]. An excessive local or systemic amount of proinflammatory cytokines, such as interleukin-1 (IL1), interleukin-6 (IL6), or tumor necrosis factor alpha (TNF ), alters bone remodeling by promoting osteoclastogenesis [4][5]. Indeed, the deleterious effects of excessive inflammation on bone metabolism have been previously described [6]. However, the inflammatory environment not only affects bone but also other skeletal tissues including cartilage. In cartilage, proinflammatory cytokines condition the expression of cartilage degradation molecules, such as matrix metalloproteinases (MMPs) or aggrecanases [7][8][9][10], among others.
21. Obesity and Obesity-Associated Low-Grade Chronic Inflammation
32. Obesity-Associated Low-Grade Chronic Inflammation in Osteoarthritis
OP is the most prevalent bone pathology. It is characterized by a loss of bone mineral density and the bony architecture, caused primarily by bone remodeling alterations. This mainly leads to bone fragility and fracture proneness. As with most rheumatic diseases, OP is highly related to age and sex, with a higher prevalence in older women [69][70]. In primary OP, caused either by menopause or aging, the regulation exerted by estrogens on IL1, TNF , and IL6 [71][72] is lost [73]. Thus, the increase in the concentration of proinflammatory cytokines has been associated with osteoclastogenesis promotion [4]. These molecules stimulate osteoblast receptor activator of nuclear kappa-B ligand (RANKL) secretion [4][5]. Specifically, it was reported that TNF and IL1 worked synergically to promote bone resorption through the promotion of osteoclastogenesis in a direct and indirect manner [4][5]. Indeed, in several OP animal models, deletion of key cytokines receptors, such as the IL1 receptor or the TNF receptor, drastically diminished bone loss [6]. TNF specifically affects bone growth directly affecting the growth plate, with this effect being reverted by its inhibitor [74]. Prolonged exposure to this cytokine in mice caused bone alterations similar to those presented in rheumatoid arthritis (RA) [75].
It has been suggested that obesity is a protective factor towards OP [76][77][78]. This mistaken interpretation is led by the fact that the mechanical loading increase, caused by the increase in weight in these patients, promotes bone mineral density through the activation of the Wnt-wingless (WNT) pathway, among other mechanisms [79][80][81]. However, it has been described that the presence of obesity in childhood diminishes the bone mass peak critical in this stage [82]. Moreover, in obese mice and humans the increased amount of fat mass is related to a lower bone quality [83][84][85][86]. Thus, the LGCI present in obesity has been suggested as a contributing factor to the reduction in bone mineral density and thus OP development [4][87].
Due to the impact of diet and inflammation on bone health, studies have pointed to diet management as a key factor in the prevention of bone-related diseases such as OP [88][89]. Thus, n-3 polyunsaturated fatty acids’ (PUFAs) consumption has been described as a bone health promoter due to their capacity to decrease LGCI [88][89]. In contrast, n-6 PUFAs intake exhibited deleterious effects on bones, since its consumption contributed to LGCI and an increase in reactive oxygen species. Moreover, these factors impacted mesenchymal stem cells’ (MSC) differentiation towards adipogenesis [88][89]. Hence, the n-6/n-3 PUFAs ratio has been suggested as an interesting tool to manage LGCI in the context of bone health [88][89].
Other studies have proposed vitamin D supplementation during pregnancy as a promoter of bone mineral content [90]. However, its impact on bone structure has not been elucidated yet [90]. Finally, probiotics could be a potential tool to prevent OP due to their capacity to increase calcium absorption and thus improve bone density [91]. Nonetheless, the influence of these treatments in LGCI has not been described so far. Notwithstanding, it is important to notice that a decrease in vitamin D levels has been described in obese patients [92] and recently related to chronic inflammation [93]. Thus, it could be a potential obesity-LGCI treatment in rheumatic diseases.
OP pharmacological tools are scarce. Among them, selective estrogen receptor modulators (SERMs) have demonstrated a positive effect on decreasing body weight and thus obesity [94]. Concretely, Raloxifene and its association with shock waves showed beneficial effects controlling OP in ovariectomized rats by decreasing the obesity-associated LGCI [95]. Surprisingly, the bisphosphonate clodronate liposome also had an antiobesity effect by limiting energy intake. Nonetheless, this process was associated with deleterious side effects that impair its use in this disease [96][97].
1.4. Obesity-Associated Low-Grade Chronic Inflammation in Other Rheumatic Diseases
A good example of inflammatory rheumatic disease is RA. Nonetheless, the inflammatory profile in this pathology significantly differs from the LGCI present in diseases including obesity, OA, and OP. The levels of serum proinflammatory cytokine concentrations are lower in OA patients than in RA patients, as well as in synovial fluid [98][99]. Moreover, the inflammatory environment and the
not successful [102], probably due to the differences between LGCI and acute inflammation in both pathologies.
Gout is another rheumatic pathology with an outstanding inflammatory component. Similarly, it has also been related to obesity, since obesity is one of the major risk factors for this pathology [103]. Surprisingly, in vivo administration of monosodium urate (MSU) crystals to induce gout reduced IL6 and MCP1 macrophage basal and elevated production in obese mice [104]. Nonetheless, in the same subjects, MSU treatment increased the IL1 production similar to that in the control mice [104].
2. Methods
To analyze the impact of diet and its relationship with the inflammatory state, we performed a search in Pubmed using the words “diet”, “inflammation”, “obesity”, and “arthritis” or “osteoporosis” according to Mesh terminology (Tables 1 and 2).
| Inclusion Criteria | Exclusion Criteria |
|
Access to full text available Language: English/Spanish |
Papers not focused on the topic Access to full text unavailable Research letters, pilot studies Different aim |
Table 2. The searches performed, with the total results, the accepted results, and the dismissed articles.
5
| Database |
| Search |
| Total |
| Included |
| Dismissed |
Pubmed |
(("Diet"[Mesh]) AND "Inflammation"[Mesh] AND "Obesity"[Mesh] AND "Arthritis"[Mesh]) |
26 |
23 |
3 |
|
|
Pubmed |
(("Diet"[Mesh]) AND "Inflammation"[Mesh] AND "Obesity"[Mesh] AND "Osteoporosis"[Mesh]) |
5 |
5 |
0 |
| TOTAL | - | 31 | 31 | 3 |
| Database | Search | Total | Included | Dismissed |
|
Pubmed |
(("Diet"[Mesh]) AND "Inflammation"[Mesh] AND "Obesity"[Mesh] AND "Arthritis"[Mesh]) |
26 |
23 |
3 |
|
Pubmed |
(("Diet"[Mesh]) AND "Inflammation"[Mesh] AND "Obesity"[Mesh] AND "Osteoporosis"[Mesh]) |
5 |
5 |
0 |
| TOTAL | - | 31 | 31 | 3 |
LGCI is a common denominator in numerous rheumatic diseases [1][2]. The sustained and slightly increased level of proinflammatory cytokines in these conditions provoke alterations along the musculoskeletal system, affecting cartilage integrity [6][7][8][9], bone remodeling and architecture [4][3], and synovia integrity [40]. However, LGCI is not only
present in rheumatic diseases. It is a characteristic of metabolic pathologies such as type 2 diabetes mellitus and obesity [25][26]. Obesity and being overweight are major risk factors for rheumatic diseases [12]. Traditionally, their deleterious effects were attributed to the increase in the mechanical loading due to the excess weight present in this pathology [39]. Nonetheless, the higher prevalence of pathologies as OA in nonbearing joints in obese individuals versus healthy ones suggested another mechanism for the predisposition to rheumatic diseases in obese patients [45]. LGCI has been understood as the alteration through which obesity affects the musculoskeletal system, with the mechanism of this damage being the excessive production of proinflammatory cytokines and adipokines by adipose tissue [13][14][15][18][19]. In order to control obesity and the LGCI associated with the most frequent rheumatic diseases, both hygienic and pharmacologic approaches were tested. When comparing the reduction in weight obtained through diet control, PE, or the combination of both, diet and diet+PE emerged as the most effective treatments for weight and fat loss, as well as for the control of the excessive production of proinflammatory cytokines and adipokines in OA patients [43][50][51][52][53] It has been also shown that in aiming to reduce obesity-associated LGCI, weight loss is not crucial; yet, it is beneficial for the patients’ health. Regarding pharmacological approaches, several extracts and fatty acids showed effective results in reducing the LGCI secondary to obesity either in osteoarthritic
or in osteoporotic patients [54][55][56][57]. Further studies need to be conducted to evaluate these drugs effect in vivo, even though the obtained results were promising.
As society is aging, all the age-related diseases are increasing in prevalence. Therefore, the increase in rheumatic diseases is, for the moment, unstoppable. Nonetheless, obesity is a risk factor that can be controlled. Thus, the study of new interventions and approaches to control the impact of this disease and its associated LGCI is a challenge for the management of patients with rheumatic diseases.
SERGAS via Miguel Servet II program CPII20/00026).
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest
References
- Ilich, Jasminka Z Gilman, Jennifer C Cvijetic, Selma Boschiero, Dario; Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation... Nutrients 2020, 12, ., 10.3390/nu12040989.
- Chuan, Li Xu, Maria J Kepeng, Wang Adler, Adam J Vella, Anthony T Zhou, Beiyan; Macrophage polarization and Metainflammation. Traslational Research 2018, 191, 29-44, 10.1016/j.trsl.2017.10.004.
- Gómez, Rodolfo Villalvilla, Amanda Largo, Raquel Gualillo, Oreste Herrero-Beaumont, Gabriel; TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs.. Nature reviews rheumathology 2014, 11, 1-12, 10.1038/nrrheum.2014.209.
- Hardy, R. Cooper, M. S.; Bone loss in inflammatory disorders. Bone loss in inflammatory disordes 2009, 201, 309-320, 10.1677/JOE-08-0568.
- Clowes, Jackie A. Riggs, B. Lawrence Khosla, Sundeep; The role of the immune system in the pathophysiology of osteoporosis. Immunological Reviews 2005, 208, 207-227, 10.1111/J.0105-2896.2005.00334.X.
- Vargas, Socorro J. Naprta, Anica Glaccum, Moira Lee, Sun Kyeong Kalinowski, Judith Lorenzo, Joseph A.; Interleukin-6 expression and histomorphometry of bones from mice deficient in receptors for interleukin-1 or tumor necrosis factor. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1996, 11, 1736-1744, 10.1002/JBMR.5650111117.
- Liu-Bryan, Ru Terkeltaub, Robert; Emerging regulators of the inflammatory process in osteoarthritis. Nature Reviews Rheumathology 2015, 11, 35-4, 10.1038/NRRHEUM.2014.162.
- Chevalier, Xavier Eymard, Florent Richette, Pascal; Biologic agents in osteoarthritis: hopes and disappointments. Nature Reviews Rheumathology 2013, 9, 400-410, 10.1038/NRRHEUM.2013.44.
- Husa, Matthew Liu-Bryan, Ru Terkeltaub, Robert; Shifting HIFs in osteoarthritis. Nature medicine 2010, 16, 641-644, 10.1038/NM0610-641.
- Goldring, Mary B Goldring, Steven R; Osteoarthritis. Journal of Cellular Physiology 2007, 2013, 626-34, 10.1002/jcp.21258.
- Chuan, Li Xu, Maria J Kepeng, Wang Adler, Adam J Vella, Anthony T Zhou, Beiyan; Macrophage polarization and Metainflammation. Traslational Research 2018, 191, 19-44, 10.1016/j.trsl.2017.10.004.
- Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 5 September 2022).
- Obesity—NHS. Available online: https://www.nhs.uk/conditions/obesity/ (accessed on 5 September 2022).
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obesity 2022, 32, 211–222.
- Mraz, M.; Lacinova, Z.; Drapalova, J.; Haluzikova, D.; Horinek, A.; Matoulek, M.; Trachta, P.; Kavalkova, P.; Svacina, S. The effect of very-low-calorie diet on mRNA expression of inflammation-related genes in subcutaneous adipose tissue and peripheral monocytes of obese patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 201, 96.
- Buchowski, M.S.; Hongu, N.; Acra, S.; Wang, L.; Warolin, J.; Roberts, L.J. Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS ONE 2012, 7, e47079.
- Canavan, B.; Salem, R.O.; Schurgin, S.; Koutkia, P.; Lipinska, I.; Laposata, M.; Grinspoon, S. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J. Clin. Endocrinol. Metab. 2005, 90, 5779–5785.
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30.
- Gao, F.; Lv, T.R.; Zhou, J.C.; Qin, X.D. Effects of obesity on the healing of bone fracture in mice. J. Orthop. Surg. Res. 2018, 13, 145.
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Taeda, K.; Miyagawa, J.I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83.
- Ahima, R.S. Revisiting leptin’s role in obesity and weight loss. J. Clin. Investig. 2008, 118, 2380–2383.
- Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 1999, 194, 6–11.
- Matsuzawa, Y. Therapy Insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42.
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451.
- Marques, F.; Rodrigues, A.; Sousa, J.; Coppola, G.; Geschwind, D.; Sousa, N.; Correia-Neves, M.; Palla, J.A. Lipocalin 2 is a Choroid Plexus Acute-Phase Protein. J. Cereb. Blood Flow Metab. 2008, 28, 450–455.
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaun, C.; Rauscher, S.; Gröger, M.; Klinger, M.; Wojta, J.; Neumayer, C.; Huk, I.; et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is Associated with Symptomatic Carotid Atherosclerosis and Drives Pro-inflammatory State in Vitro. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 623–631.
- Wu, G.; Li, H.; Zhou, M.; Fang, Q.; Bao, Y.; Xu, A.; Jia, W. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab. Res. Rev. 2014, 30, 447–456.
- Abella, V.; Scotece, M.; Conde, J.; Gómez, R.; Lois, A.; Pino, J.; Gómez-Reino, J.J.; Lago, F.; Mobasheri, A.; Gualillo, O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 2015, 20, 565–571.
- Friebe, D.; Neef, M.; Kratzsch, J.; Erbs, S.; Dittrich, K.; Garten, A.; Petzold-Quinque, S.; Blüher, S.; Reinehr, T.; Stumvoll, M.; et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia 2011, 54, 1200–1211.
- Curat, C.A.; Wegner, V.; Sengenès, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747.
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Association of increased visfatin/PBEF/NAMPT circulating concentrations and gene expression levels in peripheral blood cells with lipid metabolism and fatty liver in human morbid obesity. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 245–253.
- Li, Y.; Zhang, Y.; Dorweiler, B.; Cui, D.; Wang, T.; Woo, C.W.; Brunkan, C.S.; Wolberger, C.; Ima, S.; Tabas, I. Extracellular nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J. Biol. Chem. 2008, 283, 34833–34843.
- Franco-Trepat, E.; Guillán-Fresco, M.; Alonso-Pérez, A.; Jorge-Mora, A.; Francisco, V.; Gualillo, O.; Gómez, R. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. J. Clin. Med. 2019, 8, 1178.
- Findlay, D.M.; Atkins, G.J. Osteoblast-chondrocyte interactions in osteoarthritis. Curr. Osteoporos. Rep. 2014, 12, 127–134.
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2014, 11, 159–170.
- Silvestre, M.P.; Rodrigues, A.M.; Canhão, H.; Teixeira, D.; Calha, C.; Branco, J. Cross-Talk between Diet-Associated Dysbiosis and Hand Osteoarthritis. Nutrients 2020, 12, 3469.
- Sharma, A.R.; Jagga, S.; Lee, S.-S.; Nam, J.-S. Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. Int. J. Mol. Sci. 2013, 14, 19805.
- Sanchez, C.; Pesesse, L.; Gabay, O.; Delcour, J.; Msika, P.; Baudoin, C.; Henrotin, Y. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Care Res. 2012, 64, 1193–1203.
- Zhen, G.; Cao, X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol. Sci. 2014, 35, 227–236.
- Lee, J.; Chang, R.W.; Ehrlich-Jones, L.; Kwoh, C.K.; Nevitt, M.; Semanik, P.A.; Sohn, L.S.M.; Song, J.; Dunlop, D.D. Sedentary Behavior and Physical Function: Objective Evidence From the Osteoarthritis Initiative. Arthritis Care Res. 2015, 67, 366–373.
- Shields, M.; Tremblay, M.S. Sedentary Behaviour and Obesity. 2008. Available online: www.statcan.ca (accessed on 17 April 2022).
- Creamer, P.; Hochberg, M.C. Osteoarthritis. Lancet 1997, 350, 503–509.
- Sun, A.R.J.; Panchal, S.K.; Friis, T.; Sekar, S.; Crawford, R.; Brown, L.; Xiao, Y.; Prasadam, I. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE 2017, 12, e0183693.
- Warmink, K.; Rios, J.L.; van Valkengoed, D.R.; Korthagen, N.M.; Weinans, H. Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage. Int. J. Mol. Sci. 2022, 23, 3725.
- Jang, W.Y.; Jeong, J.; Kim, S.; Kang, M.; Sung, Y.; Choi, M.; Park, S.; Kim, M.; Kim, S.; Ryoo, Z. Serum amyloid A1 levels and amyloid deposition following a high-fat diet challenge in transgenic mice overexpressing hepatic serum amyloid A1. Appl. Physiol. Nutr. Metab. 2016, 41, 640–648.
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Yan, Z.; Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheumatol. 2012, 64, 443–453.
- Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheumatol. 2012, 64, 3220–3230.
- Harasymowicz, N.S.; Choi, Y.R.; Wu, C.L.; Iannucci, L.; Tang, R.; Guilak, F. Intergenerational Transmission of Diet-Induced Obesity, Metabolic Imbalance, and Osteoarthritis in Mice. Arthritis Rheumatol. 2020, 72, 632–644.
- Gierman, L.M.; van der Ham, F.; Koudijs, A.; Wielinga, P.Y.; Kleemann, R.; Kooistra, T.; Stoop, R.; Kloppenburg, M.; van Osch, G.J.V.M.; Stojanovic-Susulic, V.; et al. Metabolic stress-induced inflammation plays a major role in the development of osteoarthritis in mice. Arthritis Rheumatol. 2012, 64, 1172–1181.
- Yusuf, E.; Nelissen, R.; Ioan-Facsinay, A.; Stojanovic-Susulic, V.; DeGroot, J.; van Osch, G.; Middeldorp, S.; Huizinga, T.; Kloppenburg, M. Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann. Rheum. Dis. 2010, 69, 761–765.
- Ioan-Facsinay, A.; Kloppenburg, M. Osteoarthritis: Inflammation and fibrosis in adipose tissue of osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 325–326.
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Herzog, W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J. Orthop. Res. 2016, 34, 1010–1018.
- McLeod, A.; Schiffer, L.; Castellanos, K.; DeMott, A.; Olender, S.; Fitzgibbon, M.; Hughes, S.; Fantuzzi, G.; Tussing-Humphreys, L. Impact of Physical Activity and Weight Loss on Fat Mass, Glucose Metabolism, and Inflammation in Older African Americans with Osteoarthritis. Nutrients 2020, 12, 3299.
- Mears, M.; Tussing-Humphreys, L.; Cerwinske, L.; Tangney, C.; Hughes, S.L.; Fitzgibbons, M.; Gomez-Perez, S. Associations between Alternate Healthy Eating Index-2010, Body Composition, Osteoarthritis Severity, and Interleukin-6 in Older Overweight and Obese African American Females with Self-Reported Osteoarthritis. Nutrients 2018, 11, 26.
- Loeser, R.F.; Beavers, D.P.; Bay-Jensen, A.C.; Karsdal, M.A.; Nicklas, B.J.; Guermazi, A.; Hunter, D.J.; Messiery, S.P. Effects of dietary weight loss with and without exercise on interstitial matrix turnover and tissue inflammation biomarkers in adults with knee osteoarthritis: The Intensive Diet and Exercise for Arthritis trial (IDEA). Osteoarthr. Cartil. 2017, 25, 1822–1828.
- Messier, S.P.; Mhaliko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA 2013, 310, 1263–1273.
- Yu, X.; Zheng, G.; Hu, Z.; Tang, S.; Xu, J.; Shang, P.; Tang, Q.; Liu, H. Asiatic acid ameliorates obesity-related osteoarthritis by inhibiting myeloid differentiation protein-2. Food Funct. 2020, 11, 5513–5524.
- Tang, R.; Harasymowicz, N.S.; Wu, C.L.; Collins, K.H.; Choi, Y.R.; Oswald, S.J.; Guilak, F. Gene therapy for follistatin mitigates systemic metabolic inflammation and post-traumatic arthritis in high-fat diet-induced obesity. Sci. Adv. 2020, 6, eaaz7492.
- Jiang, M.; Li, X.; Yu, X.; Liu, X.; Xu, X.; He, J.; Gu, H.; Liu, L. Oral Administration of Resveratrol Alleviates Osteoarthritis Pathology in C57BL/6J Mice Model Induced by a High-Fat Diet. Mediat. Inflamm. 2017, 2017, 1–11.
- Jhun, J.Y.; Moon, S.J.; Yoon, B.Y.; Byun, J.K.; Kim, E.K.; Yang, E.J.; Ju, J.H.; Hong, Y.S.; Min, J.K.; Park, S.H.; et al. Grape seed proanthocyanidin extract-mediated regulation of STAT3 proteins contributes to Treg differentiation and attenuates inflammation in a murine model of obesity-associated arthritis. PLoS ONE 2013, 8, e78843.
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264.
- Gudbergsen, H. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 314–323.
- Schadler, P. The Effect of Body Mass Index and Metformin on Matrix Gene Expression in Arthritic Primary Human Chondrocytes. Cartilage 2021, 13, 1004S–1018S.
- Wang, C.; Yang, Y.; Zhang, Y.; Liu, J.; Yao, Z.; Zhang, C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends 2019, 12, 605–612.
- Xing, H.; Liang, C.; Wang, C.; Xu, X.; Hu, Y.; Qiu, B. Metformin mitigates cholesterol accumulation via the AMPK/SIRT1 pathway to protect osteoarthritis chondrocytes. Biochem. Biophys. Res. Commun. 2022, 632, 113–121.
- Li, H.; Gou, Y.; Tian, F.; Zhang, Y.; Lian, Q.; Hu, Y.; Zhang, L. Combination of metformin and exercise alleviates osteoarthritis in ovariectomized mice fed a high-fat diet. Bone 2022, 157, 116323.
- Li, H.; Xiang, D.; Terkeltaub, R.; Lin, H.; Zhang, Y.; Zhou, B.; He, K.; Li, K.; Liu, Z.; Wei, J.; et al. Exploration of metformin as novel therapy for osteoarthritis: Preventing cartilage degeneration and reducing pain behavior. Arthritis Res. Ther. 2020, 22, 2020.
- Mei, J.; Sun, J.; Wu, J.; Zheng, X. Liraglutide suppresses TNF-α-induced degradation of extracellular matrix in human chondrocytes: A therapeutic implication in osteoarthritis. Am. J. Transl. Res. 2019, 11, 4800.
- Chen, J.; Xie, J.; Shi, K.; Gu, Y.; Wu, C.; Xuan, J.; Ren, Y.; Chen, L.; Wu, Y.; Zhang, X.; et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 2018, 9, 212.
- Demontiero, Oddom Vidal, Christopher Duque, Gustavo; Aging and bone loss: new insights for the clinician. Therapeutic advances in musculoskeletal disease 2012, 4, 61-76, 10.1177/1759720X11430858.
- Jilka, Robert L.; Cytokines, bone remodeling, and estrogen deficiency: a 1998 update. Bone 1998, 23, 75-81, 10.1016/S8756-3282(98)00077-5.
- Pacifici, Roberto; Cytokines, estrogen, and postmenopausal osteoporosis--the second decade. Endocrinology 1998, 139, 2659-2661, 10.1210/ENDO.139.6.6087.
- Pacifici, R. Rifas, L. Teitelbaum, S. Slatopolsky, E. McCracken, R. Bergfeld, M. Lee, W. Avioli, L. V. Peck, W. A.; Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proceedings of the National Academy of Sciences of the United States of America 1987, 84, 4616-4620, 10.1073/PNAS.84.13.4616.
- Fernandez-Vojvodich, Paola Palmblad, Karin Karimian, Elham Andersson, Ulf Sävendahl, Lars; Pro-Inflammatory Cytokines Produced by Growth Plate Chondrocytes May Act Locally to Modulate Longitudinal Bone Growth. Hormone Research in Paediatrics 2012, 77, 180-187, 10.1159/000337569.
- Guo, Ruolin Yamashita, Motozo Zhang, Qian Zhou, Quan Chen, Di Reynolds, David G. Awad, Hani A. Yanoso, Laura Zhao, Lan Schwarz, Edward M. Zhang, Ying E. Boyce, Brendan F. Xing, Lianping; Ubiquitin Ligase Smurf1 Mediates Tumor Necrosis Factor-induced Systemic Bone Loss by Promoting Proteasomal Degradation of Bone Morphogenetic Signaling Proteins. The Journal of Biological Chemistry 2008, 283, 23084, 10.1074/JBC.M709848200.
- Kopelman, Peter G.; Obesity as a medical problem. Nature 2000, 404, 635-643, 10.1038/35007508.
- Reid, Ian R. Ames, Ruth Evans, Margaret C. Sharpe, Susan Gamble, Gregory France, John T. Lim, Tom M.T. Cundy, Tim F.; Determinants of total body and regional bone mineral density in normal postmenopausal women--a key role for fat mass. The Journal of clinical endocrinology and metabolism 1992, 75, 45-51, 10.1210/JCEM.75.1.1619030.
- Villareal, Dennis T. Apovian, Caroline M. Kushner, Robert F. Klein, Samuel; Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. The American journal of clinical nutrition 2005, 82, 923-934, 10.1093/AJCN/82.5.923.
- Felson, David T. Zhang, Yuqing Hannan, Marian T. Anderson, Jennifer J.; Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1993, 8, 567-573, 10.1002/JBMR.5650080507.
- Reid, Ian R. Plank, Lindsay D. Evans, Margaret C.; Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. The Journal of clinical endocrinology and metabolism 1992, 75, 779-782, 10.1210/JCEM.75.3.1517366.
- Ravn, Pernille Cizza, G. Bjarnason, N. H. Thompson, D. Daley, M. Wasnich, R. D. McClung, M. Hosking, D. Yates, A. J. Christiansen, C.; Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1999, 14, 1622-1627, 10.1359/JBMR.1999.14.9.1622.
- Weiler, H. A. Janzen, L. Green, K. Grabowski, J. Seshia, M. M. Yuen, K. C.; Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone 2000, 27, 203-207, 10.1016/S8756-3282(00)00314-8.
- Hamrick, M. W. Pennington, C. Newton, D. Xie, D. Isales, C.; Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004, 34, 376-383, 10.1016/J.BONE.2003.11.020.
- Goulding, A. Taylor, R. W. Jones, I. E. McAuley, K. A. Manning, P. J. Williams, S. M.; Overweight and obese children have low bone mass and area for their weight. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2000, 24, 627-632, 10.1038/SJ.IJO.0801207.
- Pollock, Norman K. Laing, Emma M. Baile, Clifton A. Hamrick, Mark W. Hall, Daniel B. Lewis, Richard D.; Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. The American Journal of Clinical Nutrition 2007, 89, 1530-1538, 10.1093/AJCN/86.5.1530.
- Blum, M. Harris, S. S. Must, A. Naumova, E. N. Phillips, S. M. Rand, W. M. Dawson-Hughes, B.; Leptin, body composition and bone mineral density in premenopausal women. Calcified tissue international 2003, 73, 27-32, 10.1007/S00223-002-1019-4.
- Haas, Stephan Krins, Stefan Knauerhase, Andreas Löhr, Matthias; Altered bone metabolism and bone density in patients with chronic pancreatitis and pancreatic exocrine insufficiency. JOP : Journal of the pancreas 2015, 16, 58-62, 10.6092/1590-8577/2898.
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada Toksikol. 2014, 65, 139–148. [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr. Res. 2013, 33, 521–533. [CrossRef]
- Villa, C.R.; Chen, J.; Wen, B.; Sacco, S.; Taibi, A.; Ward, W.; Comelli, E. Maternal Dietary Vitamin D Does Not Program Systemic Inflammation and Bone Health in Adult Female Mice Fed an Obesogenic Diet. Nutrients 2016, 8, 675. [CrossRef]
- Florowska, A.; Krygier, K.; Florowski, T.; Dłuz˙ ewska, E. Prebiotics as functional food ingredients preventing diet-related diseases. Food Funct. 2016, 7, 2147–2155. [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [CrossRef]
- Zhou, A.; Nen, E.H. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2022, dyac087. [CrossRef]
- Xu, B.; Lovre, D.; Mauvais-Jarvis, F. The effect of selective estrogen receptor modulators on type 2 diabetes onset in women: Basic and clinical insights. J. Diabetes Its Complicat. 2017, 31, 773–779. [CrossRef] [PubMed]
- Lama, A.; Santoro, A.; Corrado, B.; Pirozzi, C.; Paciello, O.; Pagano, T.B.; Russo, S.; Calignano, A.; Raso, G.M.; Meli, R. Extracorporeal shock waves alone or combined with raloxifene promote bone formation and suppress resorption in ovariectomized rats. PLoS ONE 2017, 12, e0171276. [CrossRef] [PubMed]
- Liu, P.; Gao, Y.; Luo, P.; Yu, H.; Guo, S.; Liu, F.; Gao, J.; Xu, J.;Wang, S.; Zhang, C. Glucocorticoid-induced expansion of classical monocytes contributes to bone loss. Exp. Mol. Med. 2022, 54, 765–776. [CrossRef] [PubMed]
- Bader, J.E.; Emos, R.T.; Velázquez, K.T.; Carson, M.S.; Sougiannis, A.T.; McGuiness, O.P.; Robinson, C.M.; Murphy, E.A. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E358–E372. [CrossRef] [PubMed]
- Nettelbladt Erik, G.; Sundblad Lars, K.M. Protein Patterns in Synovial Fluid and Serum in Rheumatoid Arthritis and Osteoarthritis. Arthritis Rheumatol. 1959, 2, 144–151. [CrossRef]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Ehart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriachi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [CrossRef]
- Naghashian, F.; Hosseinzadeh-Attar, M.J.; Akhlaghi, M.; Yekaninejad, M.S.; Aryaeian, N.; Derakhshanian, H. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [CrossRef]
- Naghashian, F.; Hosseinzadeh-Attar, M.J.; Akhlaghi, M.; Yekaninejad, M.S.; Aryaeian, N.; Derakhshanian, H. The relationship between anthropometric status and rheumatoid arthritis. Exploring the role of nesfatin and asymmetric dimethylarginine. Acta Reumatol. Port. 2019, 44, 126–131.
- Stavropoulos-Kalinoglou, A.; Metsios, G.; Smith, J.; Panoulas, V.; Douglas, K.; Jamurtas, A.; Koutedakis, Y.; Kitas, G. What predicts obesity in patients with rheumatoid arthritis? An investigation of the interactions between lifestyle and inflammation. Int. J. Obes. 2010, 34, 295–301. [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of gout. Ann. Intern. Med. 2005, 143, 499–516. [CrossRef]
- Shaw, O.M.; Pool, B.; Dalbeth, N.; Harper, J.L. The effect of diet-induced obesity on the inflammatory phenotype of non-adiposeresident macrophages in an in vivo model of gout. Rheumatology 2014, 53, 1901–1905. [CrossRef]
- Shaw, O.M.; Pool, B.; Dalbeth, N.; Harper, J.L. The effect of diet-induced obesity on the inflammatory phenotype of non-adiposeresident macrophages in an in vivo model of gout. Rheumatology 2014, 53, 1901–1905. [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of gout. Ann. Intern. Med. 2005, 143, 499–516. [CrossRef]
- Shaw, O.M.; Pool, B.; Dalbeth, N.; Harper, J.L. The effect of diet-induced obesity on the inflammatory phenotype of non-adiposeresident macrophages in an in vivo model of gout. Rheumatology 2014, 53, 1901–1905. [CrossRef]
