1. Introduction
Nanotechnology represents a revolutionary path for technological development in regards to the management of materials on a nanometric scale (one billion times smaller than one meter)
[16][1]. The use of nanotechnology in natural products has gained prominence in many studies since it has resulted in effective alternative products for a number of purposes. Natural assets such as essential oils are metabolites that are of interest to various industries, but they have peculiar characteristics such as the volatility of their chemical constituents, which is why they appear as active ingredients of various encapsulated systems. Once their bioactive properties have been proven, it is necessary to use technological tools to circumvent the problem of high volatility and, at the same time, make the most of their bioactive power.
The technique of encapsulation of essential oils has been widely applied as a nanotechnological tool to protect these assets from the external environment, as well as modulate their release according to specific needs
[17,18,19][2][3][4]. Encapsulation of bioactive compounds represents a viable and efficient alternative. In addition, this technique can increase the physical stability of substances, protect them from interactions with the environment, decrease their volatility, increase their bioactivity, reduce toxicity and even allow the release of assets over time in specific media
[20,21][5][6].
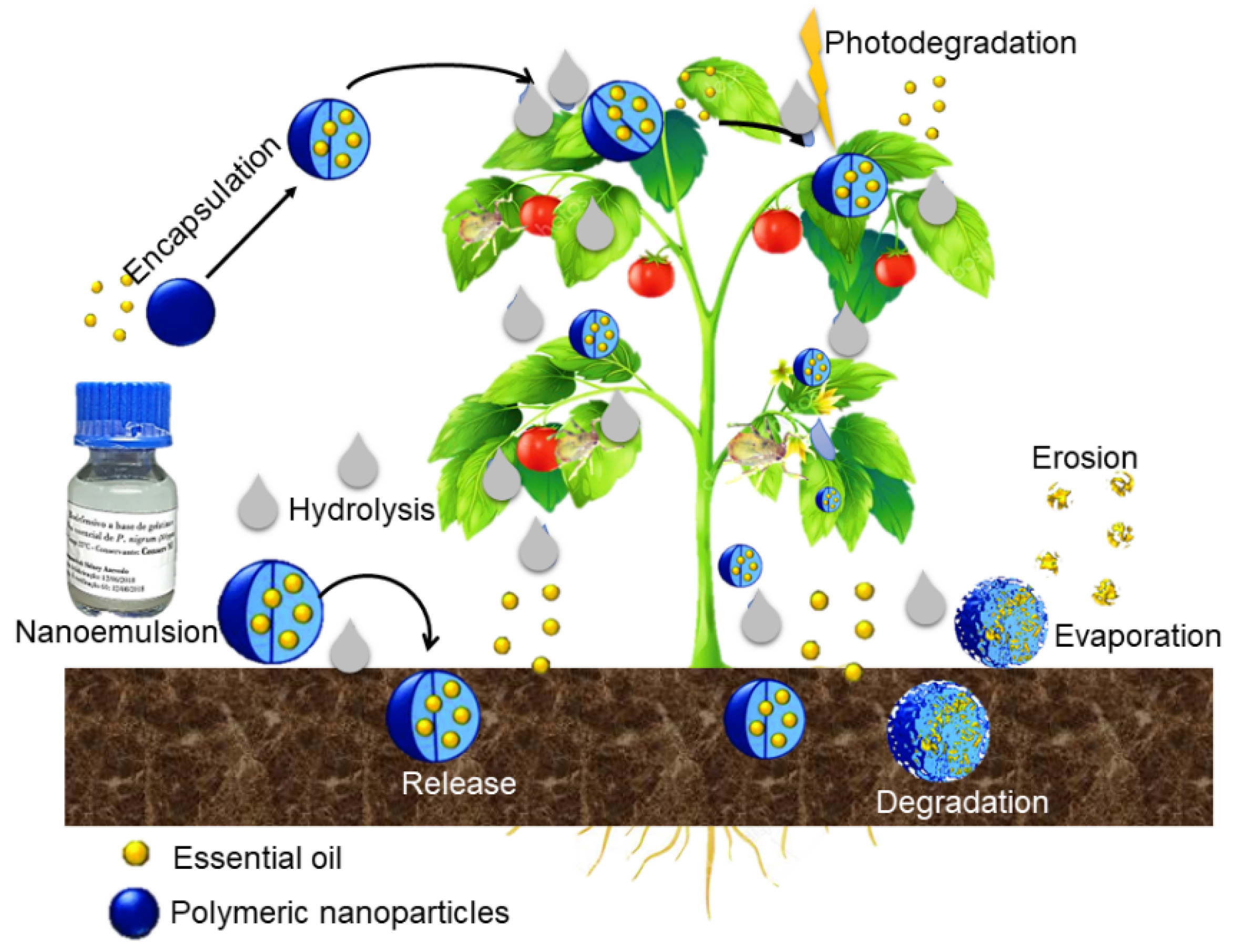
The controlled-release mechanism consists of displacing the assets present in the nanoparticles (NPs) to the application medium. This displacement is gradual and is related to the concentration that is released over time, bringing benefits such as reduced evaporation of volatile assets, easy handling, reduction in phytotoxicity and environmental pollutants, which results in advantages for both the ecosystem and human health
[22,23][7][8].
The investigation of the bioactive release profile in polymeric NPs provides important information about the mechanisms that guide this action. There are several possible mechanisms of bioactive release: release due to erosion or degradation of polymers; self-diffusion through pores; release through the erosion of the surface of the polymer and pulsed delivery initiated by the application of a magnetic field
[24,25][9][10]. Studies of release mechanisms in nanoparticles conducted by Yasmin et al.
[25][10] showed that the asset can be diffused through the polymer wall, and release occurs by diffusion or erosion of the matrix. Moreover, the asset can be released through the slow degradation of the polymer wall, or by the cleavage of the matrix through the action of enzymes. Controlled release can be schematized illustratively, as shown in
Figure 1.
Figure 1. Scheme of the controlled release of essential oil and the degradation of polymeric nanoparticles. The nanosystem comprises the active ingredient encapsulated by a biodegradable polymer. Controlled release occurs due to the degradation of the polymer. When the polymer interacts with the environment in which the release takes place, there is a breakdown of the wall material, which can be due to thermal degradation or photodegradation, and then the release of the active ingredient occurs.
Several types of materials have been used as carriers of these active ingredients, principally natural and synthetic polymers
[18,20,21,26][3][5][6][11]. Carriers, also known as wall materials, are the materials responsible for protecting the asset. During the development of encapsulating nano- or microparticles, they act as a protective membrane of the asset. In the case of colloidal systems, the carriers protect the asset from the aqueous medium. However, it is necessary to have a profound knowledge of the characteristics of the carriers in terms of biodegradability, capacity for surface functionalization, conjugation, complexation, encapsulation capacity and chemical affinity with the active substance
[16,27][1][12].
Nanoencapsulated systems cover applications such as those that are biodefensive (being directly used in the control of pests and disease vectors)
[20[5][13],
28], in medicine (especially in the selective delivery of drugs)
[29[14][15][16][17][18],
30,31,32,33], in cosmetics (in the protection of substances prone to oxidation and in the delivery of active substances in deeper layers of the skin)
[34][19], in food technology (protecting highly antioxidant substances and vitamins)
[35[20][21],
36], and in the most diverse applications in which it is necessary to guide the active ingredient to the site of action, as well as control its release rate. This type of technology allows one to maintain the characteristics and properties of active compounds, such as their protective capabilities, stabilization and prolonged release.
The encapsulation of natural assets, such as essential oils, in general, has been developed by a number of researchers in order to improve chemical stability, increase the activity of these substances and reduce volatilization, thus improving their biological potential.
Xavier et al.
[26][11] encapsulated the essential oil from
Cinnamodendron dinisii using zein as a carrier, applied in a chitosan matrix to produce an active nanocomposite film packaging for food preservation. The chitosan films obtained and functionalized with the nanoparticles demonstrated antioxidant and antimicrobial activity and were efficient in preserving ground beef. Campelo et al.
[27][12] developed a new pharmaceutical form of semi-solid dosage based on essential oil from cloves and polysaccharides for the treatment of vaginal candidiasis. The nanoemulsions showed excellent colloidal stability and adequate pH for this specific application.
Corrado et al.
[37][22] encapsulated essential oil from oregano in nanoparticles based on polyhydroxybutyrate and poly-3-hydroxybutyrate-co-hydroxyhexanoate using a solvent evaporation technique and achieved an encapsulation efficiency of greater than 60%. The nanoparticles obtained showed a regular distribution with a size range of 150–210 nm. When comparing the effectiveness of pure EO with encapsulated EO, it was possible to observe that encapsulated EO has greater bioactivity against microorganisms such as
Micrococcus luteus. The authors emphasize the importance of nanoencapsulation of volatile bioactive compounds in biodegradable polymer matrices and conclude that the results pave the way for the effective exploitation of nanosystems developed for active packaging.
Azevedo et al.
[28][13] developed an environmentally-friendly nanoparticle system for the encapsulation of the essential oil from
Piper nigrum using gelatin and poly(ε-caprolactone) (PCL) as carriers. By evaluating the encapsulation efficiency, electrical conductivity, turbidity, pH and organoleptic properties (color and odor) after the addition of different preservatives, the authors studied the stability of the formulation generated. In this research, a particle size of between 114 ± 3 nm and 519 ± 13 nm and encapsulation efficiency of 98 ± 2% were obtained. Due to the pronounced bioactivity of the encapsulated essential oil, they concluded that the developed system has potential as a stable alternative product and as a controlling agent.
2. Nanoparticles
Nanoparticles can be formed from composites and are developed through the association of two materials that together give rise to a third material with properties superior to the separate forming components
[16][1]. They can be called carriers and consist of multilayers that are superimposed according to the desired application under the study. Its formation depends on a number of factors, the main one being the chemical and physical interaction between the layers and the encapsulated material so that the stability of the system is achieved
[31,38][16][23].
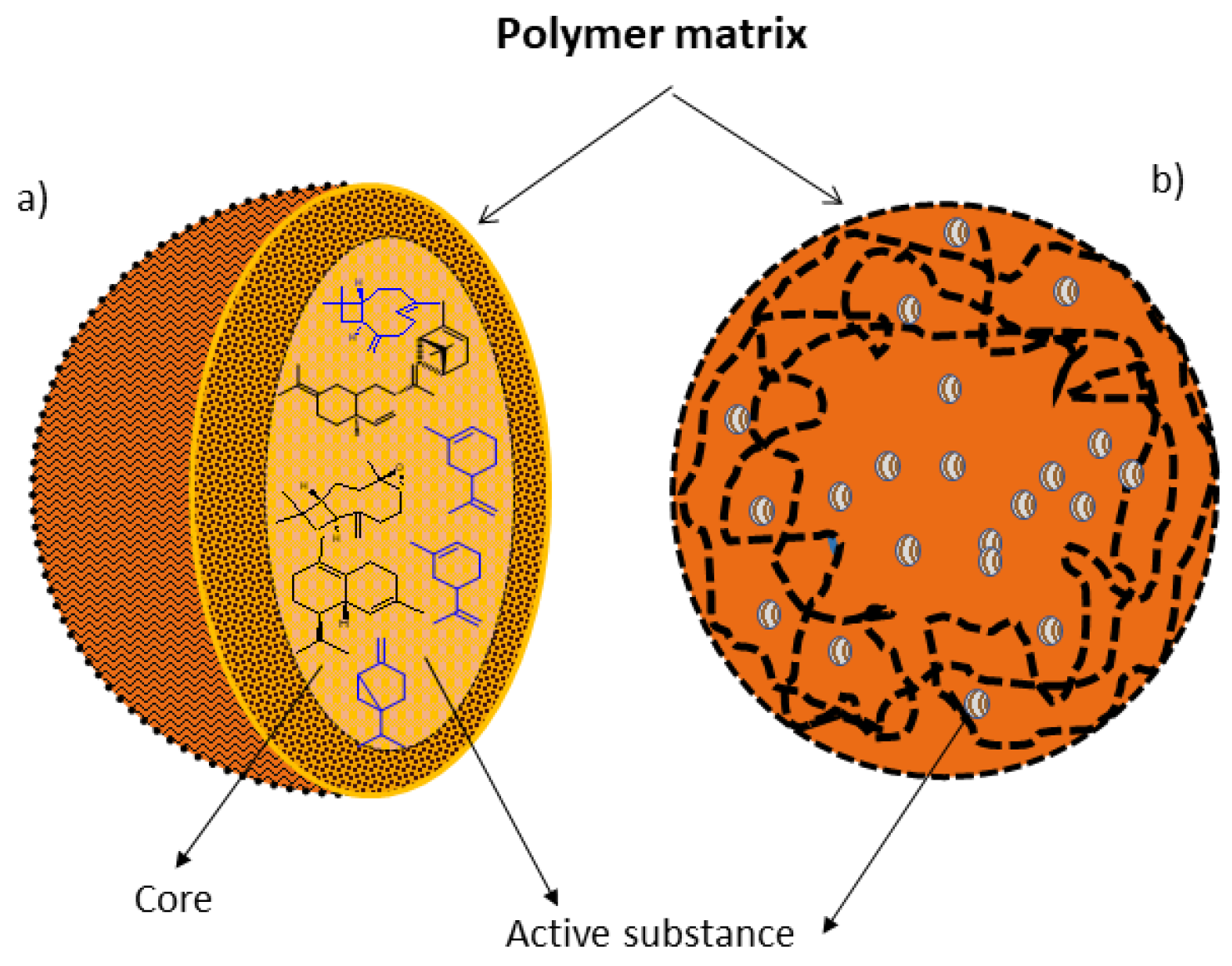
Nanoparticles, when they have a structured inner layer, are called nanocapsules and, when they have a continuous matrix, they are called nanospheres
[29][14] (
Figure 2). Nanocapsules are particles consisting of a polymeric wall containing a cavity inside, by which the active ingredient is adsorbed, though this may also be present in the polymeric wall; while nanospheres are formed by a polymeric matrix in which chemical components can be retained or adsorbed
[30][15].
Figure 2. Polymer nanoparticles. (a) Nanocapsule: active ingredients dissolved in the matrix core and (b) Nanospheres: active ingredients dissolved throughout the polymer matrix. In the nanocapsules, the active substance is in the nucleus and is surrounded by a polymeric membrane. In the nanosphere, the active substance is dispersed in the polymeric matrix, and therefore it does not have a defined nucleus.
The development of polymer nanosystems is rapidly expanding and plays a key role in various areas, from pollution control to environmental technology, from electronics to photonics, from medicine to biotechnology, from materials to sensors, and so on. Several reports in the literature emphasize the growing interest in this area. This trend is based on the unique properties of polymer nanosystems, which meet numerous applications and market needs
[31,32][16][17].
The combination of biopolymer materials represents an important alternative for encapsulating essential oils. However, understanding the nanoparticle development project is crucial for obtaining stable formulations, adequate controlled-release and surface functionalization for further conjugation with bioactive molecules or ligands. The development of these encapsulating nanoparticles involves a series of parameters that are related to the specific purpose of action of the encapsulated substances and also to the controlled release mechanisms, which can often be related to the type of carrier used
[33,34,35,36][18][19][20][21]. Its formation depends on certain factors, the main one being the chemical and/or physical interaction between the layers and the encapsulated active substance.
Encapsulated systems are efficient strategies for transporting the active substance to its site of action through the choice of a carrier and the appropriate route, with the main objectives being protecting its content from environmental factors (light, moisture, oxygen and interactions with other compounds), in addition to controlled release and release under stimuli (such as changes in pH, physical disruption, swelling, dissolution, etc.). In addition, encapsulation can also mask the unpleasant taste and/or odor and increase the acting time of the active compound, thus prolonging its effect. In this case, the type of nanoparticle and the place where the active substance will be exposed (adsorbed on the surface or not) will depend on the desired final characteristics, such as application, size, size distribution, degree of biodegradability and compatibility of the polymer with the active substance
[18][3].
3. Development of Nanoparticles
Several methodologies exist for the development of encapsulation of polymeric nanoparticles, which in their formulations generally employ materials such as polymers (synthetic or natural), surfactants, bioactive compounds, organic solvents and essential oils, depending on the formulation. For the preparation of nanoparticles with the purpose of carrying natural active ingredients, the physicochemical properties of the polymer must be taken into account. Polymers and their degradation products must be biocompatible and biodegradable, and cause no harm or impact to the environment
[38,39][23][24].
According to Mora-Huerta et al.
[33][18], the following classic methods of obtaining nanoparticles are generally used: nanoprecipitation, emulsion-diffusion, double emulsification, emulsion coacervation, polymer coating and layer-by-layer.
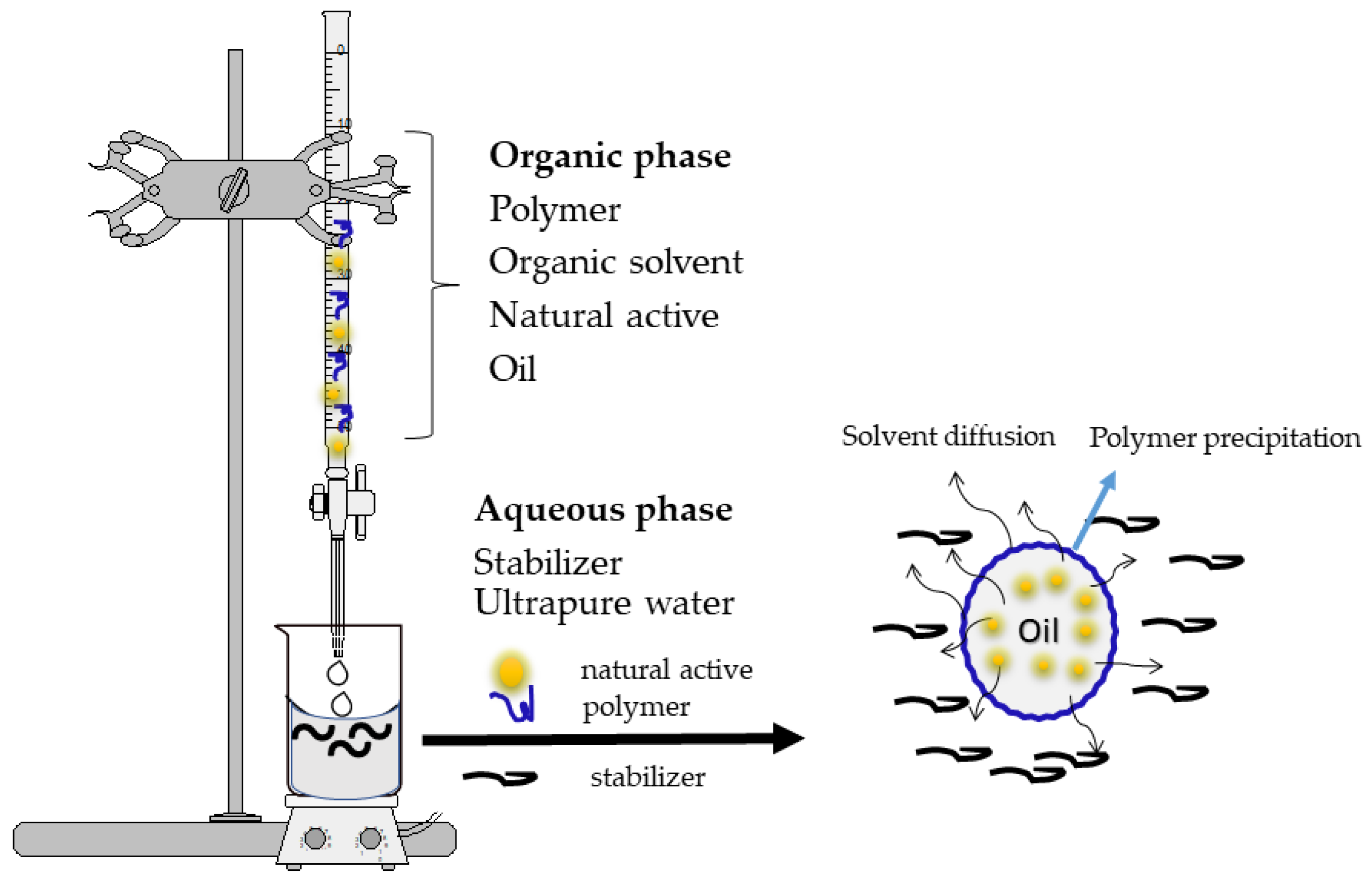
The nanoprecipitation method (
Figure 3) is also called solvent displacement or interfacial deposition. This method requires two phases, one organic and one aqueous. The organic phase essentially consists of a solution or a mixture of solvents (ethanol, acetone, hexane, dichloromethane, etc.), of a macromolecule with a carrier role (synthetic, semisynthetic or natural polymer), the active substance, oil and a lipophilic surfactant. However, the aqueous phase consists of a mixture of surfactant in an aqueous medium. In this method, the nanoparticles are obtained as a colloidal suspension that is formed when the organic phase is slowly added to the aqueous phase with moderate agitation. The main variables of the procedure are those associated with the conditions of addition of the organic phase to the aqueous phase, such as the organic phase injection rate and the aqueous phase agitation rate
[33,34,40,41][18][19][25][26].
Figure 3. Formation of polymeric nanoparticles using the nanoprecipitation method. The organic phase consists of a solution—or a mixture—of a polymer (carrier), the organic solvent, the active substance (natural active ingredient), oil and a lipophilic surfactant. The aqueous phase consists of a mixture of surfactant (stabilizer) and ultrapure water. Using a burette and a magnetic stirrer, the organic phase is slowly added to the aqueous phase.
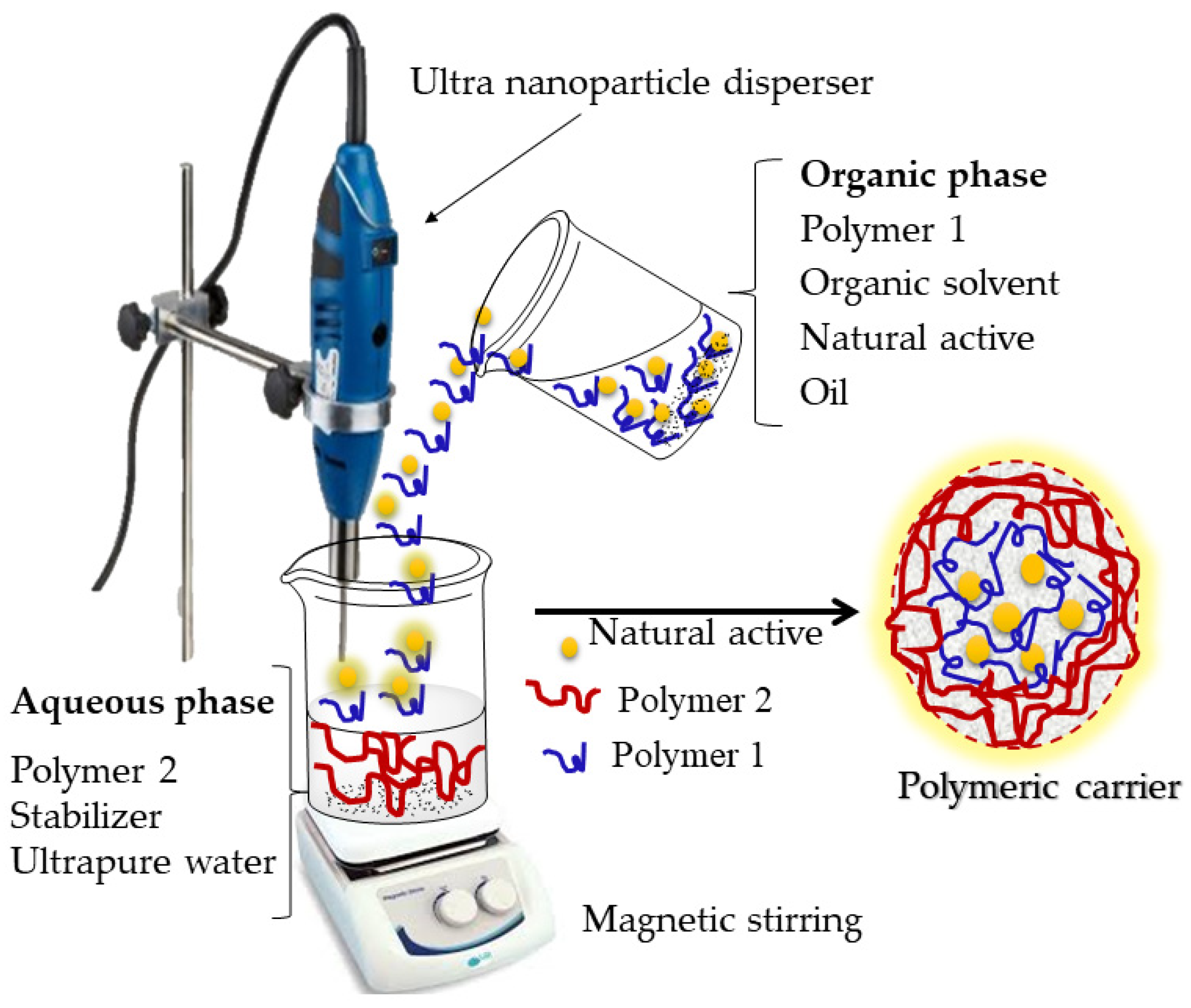
The preparation of nanoparticles using the emulsion–diffusion method (Figure 4) allows the nanoencapsulation of lipophilic and hydrophilic active substances.
Figure 4. Formation of carrier systems using the emulsion–diffusion method. The organic phase contains the polymer, the organic solvent, the natural active ingredient, and the oil. The aqueous phase contains a different polymer, a stabilizer and the ultrapure water. The particles are formed by polymer precipitation along with the encapsulation of the natural active ingredient.
The procedure requires three phases: organic, aqueous and dilution. When the goal is the nanoencapsulation of a lipophilic active substance, the organic phase contains the polymer, the active substance, the oil and an organic solvent that is partially miscible with water. The organic phase is emulsified under vigorous stirring in the aqueous phase and, after primary emulsion formation, the organic solvent is diffused to the external aqueous phase by adding excess water (dilution), which leads to polymer precipitation and nanoparticle formation
[33,39][18][24].
The nanocapsule formation mechanism suggested by Quintanar-Guerrero et al.
[42][27] is based on the theory that each emulsion droplet produces several nanocapsules, and that these are formed by the combination of polymer precipitation and interfacial phenomena during solvent diffusion
[43][28].
The double emulsification method consists of the formation of two emulsions that are usually prepared using two surfactants: a hydrophobic one intended to stabilize the water/oil interface of the internal emulsion, and a hydrophilic one to stabilize the external interface of the oil droplets for water/oil/water emulsions. The primary emulsion is formed with the use of ultrasound, and the hydrophobic surfactant stabilizes the water/oil interface of the internal phase. The secondary emulsion can also be formed with ultrasound, and the dispersion of the nanoparticles is stabilized by the addition of another surfactant (hydrophilic)
[33,39,40][18][24][25].
The emulsion coacervation method involves the formation of an oil/water emulsion, in which the organic phase is composed of the solvent and the bioactive compound, and the aqueous phase is composed of the polymer, a stabilizing agent and water. The emulsion can be formed by the use of ultrasound or mechanical stirring. Then, the coacervation process is carried out by adding electrolytes, adding a water-immiscible solvent or dehydrating agent, or changing the temperature. Finally, the coacervation process is supplemented with additional measures for the formation of lattices, which makes it possible to obtain the nanoparticles. The formation of nanoparticles occurs during the coacervation phase, in which there is precipitation of the polymer from the continuous emulsion phase to form a film that agglomerates into nanoparticles
[31,33,39,40][16][18][24][25].
The polymer-coating method is used for the deposition of a thin polymer layer on the surface of the nanoparticle that was previously formed by the adsorption of the polymer on uncoated nanoparticles when incubated with a polymer solution under stirring. Likewise, this polymeric layer can be added during the final phase of the methods mentioned above
[33,39][18][24].
The layer-by-layer method favors the acquisition of vesicular particles, which are also called polyelectrolyte capsules. The mechanism of formation is based on irreversible electrostatic attraction that leads to the adsorption of polyelectrolytes in the formed layers. A polymer layer is adsorbed by incubation in the polymer solution, which decreases the solubility of the polymer by dropwise addition of solvent. This procedure is then repeated with a second polymer and several polymer layers are deposited sequentially
[33][18].