Neurodegenerative diseases (NDs) commonly present misfolded and aggregated proteins. Considerable research has been performed to unearth the molecular processes underpinning this pathological aggregation and develop therapeutic strategies targeting NDs. Fibrillary deposits of α-synuclein (α-Syn), a highly conserved and thermostable protein, are a critical feature in the development of NDs such as Alzheimer’s disease (AD), Lewy body disease (LBD), Parkinson’s disease (PD), and multiple system atrophy (MSA). Inhibition of α-Syn aggregation can thus serve as a potential approach for therapeutic intervention. TRecently, the degradation of target proteins by small molecules has emerged as a new therapeutic modality, gaining the hotspot in pharmaceutical research. Additionally, interest is growing in the use of food-derived bioactive compounds as intervention agents against NDs via functional foods and dietary supplements. According to reports, dietary bioactive phospholipids may have cognition-enhancing and neuroprotective effects, owing to their abilities to influence cognition and mental health in vivo and in vitro.
1. Overview
Neurodegeneration has been identified as the pathophysiological hallmark in most brain-related disorders. Many neurodegenerative diseases (NDs) involve the misfolding and aggregation of specific proteins into abnormal, toxic species
[1][2][1,2]. Early diagnosis is essential for treatment planning and ensuring that the right support can be provided to patients and their families. However, therapeutic strategies for NDs present unique challenges for drug development. Although no curative treatment is available for NDs, the range of therapeutic and supportive options are expanding. The most common NDs are Alzheimer’s disease (AD), Lewy body disease (LBD), Parkinson’s disease (PD), and multiple system atrophy (MSA)
[3]. The observation of amyloid-like protein aggregation in the brains of patients with NDs was first described in the 1960s, and thus far, three-dimensional structures of several of these aggregates have been elucidated
[4][5][4,5]. These structural data have been utilized for the design of inhibitors of amyloid-like aggregation proteins
[6][7][8][6,7,8]. Since decades, the presence of amyloids in NDs has been associated exclusively with pathologies
[9][10][9,10]. Additionally, strong evidence indicates that the activation of inflammatory processes is a hallmark of NDs. Elevated levels of pro-inflammatory cytokines and chemokines, as well as activated microglia and astrocytes, are found in the brains of patients with AD, even at very early stages of the disease
[11]. Ageing affects homeostatic processes that protect against protein misfolding and is associated with an increase in oxidative stress, neuroinflammation, and mitochondrial-lysosomal dysfunction, which have been shown to directly activate microglia cells and astrocytes
[12][13][14][15][12,13,14,15]. It has been argued that extracellular amyloid-β peptide (Aβ) in AD activates microglia and astrocytes, which in turn release tumor necrosis factor alpha (TNF-α) and other cytokines. TNF-α signaling enhances Aβ production and Aβ-induced neuroinflammation
[16]. Neuroinflammation, oxidative stress, and mitochondrial dysfunction have all been linked to the progression of NDs
[11][17][18][11,17,18]. The neuroprotective effects of resveratrol reportedly stem from its ability to inhibit microglial activation and regulate neuroinflammation
[19][20][21][19,20,21].
The Our
esearchers' recent study demonstrated that porcine liver decomposition product (PLDP) could improve cognitive function in elderly adults by providing a rich source of phospholipids (PLs) and lysophospholipids (LPLs)
[22]. Notably, the main constituent lipids of synaptic vesicles include cholesterol and phospholipids
[23]. Although the concentration of PLDP-derived lipids in LPLs is not sufficient to yield cognitive benefits, LPLs may confer anti-neuroinflammatory effects
[24][25][24,25]. Previous research suggests that LPLs have served not only as structural components of biological membranes but also as biologically active molecules
[26]. LPLs influence a plethora of processes, including neurogenesis in the central nervous system (CNS)
[27][28][29][27,28,29]. Growing interest in the involvement of extracellular LPLs in the pathology of NDs increasingly indicates that these small molecules may have therapeutic potential for NDs.
2. Effects of LPLs on Cognitive Decline
2.1. Dietary Sources of LPLs
Because of their superior emulsification properties, LPLs have numerous applications in the food, cosmetic, and pharmaceutical industries
[30][107]. However, their properties depend strongly on the fatty acid component present and the specific polar head bound to the glycerol backbone
[31][108]. LPLs, as promising feed additives, have been widely used to supplement farm animals diets to improve growth performance, feed efficiency, and dietary fat absorption
[32][109]. LPL supplementation can increase the apparent total tract digestibility of animals
[33][110]. PLs are widely available in the intestinal lumen after eating, and their hydrolysis is catalyzed by phospholipase A2 (PLA2). LPLs (particularly LPC), the digestive products of PLs, have direct roles in mediating chylomicron assembly and secretion
[34][111]. LPC is present in the plasma circulation at relatively high levels and includes species containing both saturated and unsaturated fatty acids
[35][112]. Interestingly, the intake of PL-enriched diets during postnatal brain development was found to increase the number of striatal circuits
[36][113]. In PD, dopaminergic neurons are progressively degenerated, leading to striatal dopamine depletion and movement deficits. α-Syn is intimately involved in the pathogenesis of PD, and has been implicated in the regulation of dopamine synthesis, release, and reuptake
[37][114]. It is possible that dietary PLs have several benefits in health, including improvements in cognition across the lifespan.
2.2. Cognitive Function and LPLs
Mild cognitive impairment (MCI) is characterized by impairment of certain cognitive functions, as defined by Petersen et al.
[38][115], and a diagnosis of mild neurocognitive disorder essentially comprises MCI
[39][116]. It can occur in a subtle form, such as MCI, in the early stages of PD in up to 25% of newly diagnosed patients
[40][41][42][117,118,119]. MCI is a distinct stage of cognitive loss that falls between the expected cognitive decline of physiological aging and the more serious loss of mental abilities associated with dementia. It is characterized by impairments in memory, language, thinking, or judgment that are severe enough to interfere with daily life
[43][120]. Individuals with MCI have an increased risk of developing dementia caused by AD or other neurological conditions
[44][121]. Growing evidence supports the hypothesis that dietary factors may play a role in healthy aging, including exertion of a protective effect against age-related cognitive decline
[45][46][122,123]. The concept of functional foods was first introduced in Japan, a country with a long history of using foods for their health benefits
[47][124]. The market for functional food ingredients covers the sale of functional food ingredients containing bioactive compounds and ingredients used in manufacturing functional food products
[48][125]. The ingredients in functional foods provide health benefits, and some of them include supplements or other additives. Functional foods and bioactive compounds can strongly intensify the therapeutic efficacy of certain drugs by influencing different pathways
[49][126]. A new understanding of these interactions is emerging owing to intense research on nutraceuticals in diseases, including NDs
[50][127]. For example, Yuyama et al. showed that glucosylceramides (GlcCer) from konjac extract are linked to the attenuation of amyloid-like protein in the CNS
[51][128]. In the brains of GlcCer-treated mice, decreased levels of several inflammatory cytokines were accompanied by the recovery of impaired synaptic densities
[52][129]. Future studies need to evaluate GlcCer and examine their relationship with α-Syn in the brain to determine the possible use of plasma GlcCer as a prognostic biomarker of disease progression. Several dietary components including PLs, plasmalogens, ω3 fatty acid, carotenoids, vitamins, and phenolic compounds have been identified to affect cognitive functions
[53][130]. Many studies indicate that neuroinflammation plays a fundamental role in the progression of the neuropathological changes that characterize NDs
[54][55][56][131,132,133]; microglia are the main cellular effectors of this process
[57][134]. Indeed, neuroinflammation is initiated by microglia, which are resident immune cells of the CNS. Once activated, microglia can synthesize and release several neurotrophic factors and antioxidants to withstand the pathological progression of NDs
[58][135].
2.3. PLDP and LPLs
In previous studies, over 50% of patients with PD without dementia demonstrated cognitive alterations and 20% primarily exhibited memory deficits
[59][136].
ThWe
researchers previously reported that PLDP induces a significant increase in the Hasegawa’s dementia scale-revised (HDS-R) score and the Wechsler memory scale (WMS) score in a randomized, double-blind, placebo-controlled study in healthy humans
[22]. PLDP was recently approved as a food with functional claims (FFC) in Japan. FFC was introduced in April 2015 for increasing the availability of products that are clearly labeled with certain health functions
[60][137]. Oral administration of PLDP has been shown to enhance visual memory and delayed recall in healthy adults
[22]. Notably, the oral route is the most common and preferred method of drug administration for several reasons, such as non-invasiveness, patient compliance, and convenience of drug administration
[61][138]. These results suggest that PLDP may help improve brain function. Interestingly, PLDP is a rich source of PLs, and composition analysis of PLDP revealed that the most abundant PLs and LPLs belonged to the phosphatidylcholine (PC) and phosphatidylethanolamine (PE) classes
[25][62][25,139]. LPLs influence signaling, proliferation, neural activity, and inflammation to mediate various important processes, including the pathogenesis of cerebral ischemia, vascular dementia, and AD
[25][63][64][65][25,140,141,142]. However, no direct evidence indicates that any PLDP component improves cognitive function, and the effect of PLDP components on cognitive function remains unclear.
The Our
esearchers' recent study identified novel cooperative actions of LPLs that inhibit IL-6 expression and the accumulation of intracellular ROS in microglia after lipopolysaccharide (LPS)-induced neuroinflammation
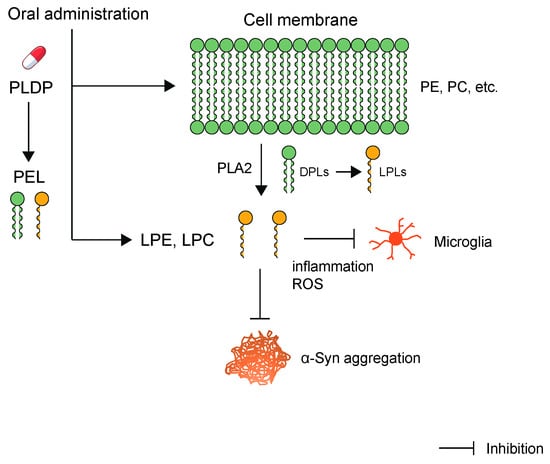
[25]. This activation was significantly inhibited by lysophosphatidylcholine (LPC) and lysophosphatidylethanolamine (LPE), suggesting that these molecules exert significant protective effects against LPS-induced inflammation (
Figure 1). This finding is important considering that microglia-mediated neuroinflammation is regarded as a pathological mechanism in many NDs, such as AD and LBD, and is a pivotal event accelerating cognitive or functional decline. Furthermore, dietary LPC and docosahexaenoic acid (DHA) have been reported to efficiently increase DHA levels in the brain, improving brain function in adult mice
[66][143]. This report describes a novel nutraceutical approach for preventing and treating neurological diseases (such as AD) associated with DHA deficiency.
Figure 1. PLDP is a rich source of phospholipids. PLDP extracted lipids (PEL) was extracted from PLDP using the Bligh and Dyer method. This PEL is a rich source of LPLs, including LPC and LPE. LPC and LPE exerted significant protective effects against LPS-induced inflammation and oxidative stress in microglial cells. Various isoforms of PLA2 enzyme hydrolyze PC and PE at the sn-2 position to form LPLs, including LPC and LPE, respectively. α-Syn is bound to LPLs, which are known to be contained in PEL, strongly inhibit α-Syn aggregation.