Cyclic dinucleotides(CDNs) are a class of bacterial and mammalian second messengers with potent immunomodulatory and immunostimulatory properties. CDNs mediate a potent systemic as well as a mucosal vaccine response and induces a balanced memory Th1/Th2/Th17 and CD8+ T cell response. CDNs do not cause acute toxicity in mice and have been reported safe in humans from the recent clinical trials (ClinicalTrials.gov: NCT02675439, NCT03010176, NCT03172936, NCT03937141, and NCT0414414). As therapeutic cancer vaccine adjuvants, CDNs induce potent anti-tumor immunity,
including cytotoxic T cells and NK cell activation that achieve durable regression in multiple mice models of tumor. In this entry, we review the status of CDN vaccine adjuvant research, including their superior adjuvant activities, in vivo mode of action, and confounding factors that affect their efficacy in humans.
- vaccine adjuvant
- cyclic dinucleotide
- lung dendritic cell
- immunology
- STING
- cancer immunotherapy
1. Introduction:
Vaccination is one of the notable achievements of modern medical science. With a successful demonstration of the prevention of smallpox in people infected with cowpox, Edward Jenner laid the foundation of vaccinology. Vaccine
development has come a long way since then and significantly reduced disease burden worldwide. With the introduction of recombinant technology, recombinant and sub-unit antigens with improved reactogenicity and safety profile have emerged. However, these new generations of vaccine antigens often require an adjuvant to improve the magnitude and quality of the vaccine response. Since it's discovery in the 1930s, alum/aluminiuim hydroxide has been the choice as an adjuvant in commercially available vaccines [1]. As a vaccine adjuvant, alum significantly boosts antibody production but is limited to Th2 response rendering it ineffective against intracellular pathogens that require Th1 and CD8+ T cell response. Despite intensive research on new vaccine adjuvants, very few adjuvants have been licensed for human application.
2. CDNs as aA uUniversal vVaccine aAdjuvant:
Cyclic dinucleotides (CDNs) including cyclic di- AMP (CDA); cyclic di-GMP (CDG) or cyclic GMP-AMP (cGAMP) are a class of bacterial and mammalian second messengers with potent immunomodulatory functions[2][3][4][5]. CDN adjuvanted protein subunit vaccines generated mucosal immunity and protected mice from respiratory bacterial and viral infections such as influenza [6][7][8], Mycobacterium tuberculosis [9], anthrax [10], Klebsiella pneumoniae [11] and Streptococcus pneumoniae [2][3], Acinetobacter baumannii [12], and methicillin-resistant Staphylococcus aureus[13]. CDNs also induce long-lasting CD8+ T cell immunity in mice [14][15].
2.1. CDNs as vVaccine aAdjuvants aAgainst iInfectious dDiseases:
The mucosal surface is a natural point of entry for many respiratory pathogens such as influenza, Streptococcus pneumoniae, Mycobacterium tuberculosis, Staphylococcus aureus, B. anthracis, coronavirus, rotavirus, etc. Moreover, immunization at one mucosal surface, particularly intranasal vaccination in mice, monkeys, and humans has generated not only a local IgA response, but also enhanced the IgA response in salivary glands, upper and lower respiratory tracts, and even in distant genital tracts, and the small and large intestines[16][17][18][19][20]. Furthermore, rectal immunization of mice with a non-living peptide-based vaccine effectively induced a systemic CTL response[21]. However, a major obstacle in the development of a mucosal vaccine is the availability of a potent mucosal adjuvant, which not only generates a local mucosal IgA response but also enhances mucosal Th and CTL responses along with a systemic reaction. The most common mucosal immunostimulatory molecule used for vaccine studies are the bacterial enterotoxins, cholera toxin, and E. coli heat-labile toxin. Mechanistically, these toxins form a pentameric subunit, which binds to the gangliosides (preferentially GM1), thereby facilitating the uptake. Recent studies, however, raised safety concerns. Studies found that the enterotoxins are accumulated in the olfactory epithelium nerves and the olfactory bulb that induce inflammatory responses in meninges, the olfactory nerve, and glomerular layers of the olfactory bulb promoting neuronal damage [22][23][24]. Moreover, when immunized intranasally, CDNs induce potent mucosal vaccine responses comparable to the cholera toxin that is the most potent experimental mucosal adjuvant so far[3]. In 2007, Ebensen et al. first demonstrated CDNs as a mucosal adjuvant[7]. They showed that CDG/β- Gal i.n. immunized mice not only mounted a systemic IgG response but also generated an enhanced β-Gal specific IgA response after 42 days in lung BALF and vaginal lavage [7]. Intranasal immunization of CDG/β-Gal
also enhanced the serum IgG2a and IgG1 production by 640- and 320-fold as compared to only β-Gal immunized mice [7]. The systemic cellular response was also enhanced by CDG/β-Gal immunization, as was observed with the production of interferon (IFN)-γ (2000-fold), IL-5, and IL-2 in the ex vivo recall assay compared to only β-Gal immunized mice [7]. CDNs also induce mucosal Th17[5][25]. The mucosal adjuvant potential of CDNs was further strengthened when Madhun et al. showed that i.n. administration of a plant-based H5N1 influenza antigen induced high frequencies of multifunctional Th1 cells [6]. In comparison, the same vaccine when it immunized i.m. did not generate a Th1 response, which is critical for viral infections [6].
2.2. CDNs as aAn aAdjuvant in cCancer tTherapy:
Cancer-related death is the second leading cause of death. Cancer development is accompanied by an immunosuppressive microenvironment and T cell dysfunction and exhaustion [26][27]. CDNs have a potent anti-tumor activity. Demaria et al. showed that intratumoral injection of cGAMP enhanced the anti-tumor CD8+ T cell response inhibiting the growth of injected tumors in mice models of melanoma and colon cancer[28]. Intratumoral injection of cGAMP in B16F10 lung metastasis induced a systemic CD8+ T cell response to restrict the growth of distant tumors [29]. Nicolai et al. showed that the CDA activated natural killer (NK) cells-dependent and CD8+ T cell-independent mechanism for tumor rejection originated from different tissue types[30]. CDA induced tumor regression even in Rag2-/- mice but was strongly depleted in Rag2-/- Il2rg-/- mice lacking NK cells, B, and T cells [30]. Moreover, suppressing the NK cell activity by NK1.1 antibody resulted in rapid tumor growth of MC-38-B2m-/- (colorectal), B16-F10-B2m-/- (melanoma), CT26-B2m-/- (colorectal), C1498-B2m-/- (leukemic), and RMA-B2m-/- (lymphoma) tumour models[30]. The study was clinically relevant as human tumors such as Hodgkin’s lymphoma often exhibit a partial loss of MHC I thereby unable to activate a CTL response. Activating NK cell-mediated anti-tumor immunity then becomes essential. A major hurdle in cancer immunotherapy is the "cold" tumors, which lack inherent immunogenicity reflecting in the inability of checkpoint inhibitors to attenuate tumor growth. A study by Francica et al. showed that the CDN mediated tumor necrosis factor (TNF)-α production by innate immune cells are responsible for acute tumor clearance, and blocking TNF-α inhibits tumor necrosis and clearance
against "cold" tumors[31].
2.3. CDNs iInduce pPotent, sSafe, and bBalanced vVaccine rResponse:
The safety profile of the vaccine adjuvant is crucial while administration avoids unwanted side effects. Histopathology studies and cytokine profiling suggested that the CDG adjuvanted vaccine also induced the production of anti-inflammatory cytokine-like IL-10, thereby maintaining a balanced pro- and anti-inflammatory cytokine profile[32]. CDG also induced the production of IL-22, thereby facilitating lung epithelium repair along with effectively clearing S. pneumoniae from the organs[32]. Thus, CDNs have shown promising and safe mucosal vaccine activities in animal models.
3. Particle mMediated dDelivery of CDNs:
One hurdle in CDN delivery is the negative charge of the phosphate molecules that may prevent them from crossing the plasma membrane to activate STING in the cytosol. However, with the advent of nanotechnology, nanoparticle, and microparticle mediated delivery vehicle such as liposomes, emulsions, virus-like particles, biodegradable polymers, immune-stimulating complexes (ISCOM) have received tremendous attention. These new delivery methods provide a sustained release of the cargo from the matrix and improve bioavailability and dosing frequency[33][34]. Thus, nanoparticle or microparticle encapsulated CDNs have been fabricated and studied as an alternative delivery approach of CDNs in vivo.
3.1. Encapsulated CDN aAdjuvants for iInfectious dDiseases:
In 2015, Hanson M. C. et al. showed that CDG encapsulated in a PEGylated liposome enhanced its retention in the lymph node by 15-fold, which otherwise disseminated into the blood following s.c. immunization [35]. Incorporation of HIV GP antigen membrane-proximal external protein (MPER) with a CDG encapsulated liposome enhanced 5.3-fold antigen-specific Tfh cell formation as compared to CDG or liposome only immunized mice[35]. There were improved germinal center B cell production and 11-fold increased antigen-specific IgG titers[35]. Lastly, the humoral responses were more durable than vaccines administered with the TLR agonist MPLA [35]. Acetylated-dextran encapsulating cGAMP microparticles enhanced type I IFNs responses by nearly 1000-fold in vitro and 50-fold in vivo [36]. cGAMP microparticles (i.m.) increased antigen-specific antibody titers by~100-fold, enhanced Th1 responses, and expanded germinal center B cells[36].
Most recently, Wang J. et al. designed a biomimetic pulmonary surfactant encapsulating the cGAMP (PS-GAMP) Perth H3N2 vaccine[8]. The PS-GAMP adjuvanted flu vaccine (i.n.) provided heterotypic cross-protection against the Michigan15 H1N1 strain 30 days post-immunization in ferrets[8]. These biomimetic liposomes activated STING pathways in both alveolar macrophages and alveolar epithelial cells and enhanced the recruitment of CD11b+ DC and the differentiation of CD8+ T cell and humoral response [8]. It generated heterotypic protection against H3N2, H5N1, H7N9 viruses as early as two days after a single dose of immunization and promoted the formation of a durable CD8+ Trm in mice[8].
3.2. Encapsulated CDN aAdjuvants for cCancer iImmunotherapy:
Schulz et al. demonstrated that administering a 100 ng of cGAMP/dextran microparticle (i.t.) was sufficient to reduce the tumor size as compared to the soluble cGAMP, which had to be immunized at a 10× higher concentration to obtain the same effect[37]. Miyabe et al. synthesized a pH-sensitive synthetic lipid YSK05 for cytosolic delivery of CDG as a result of the high fusogenic property of the lipid vesicle at acidic pH [38]. In a mouse model infected with EG7-OVA cells, s.c. immunization of liposome/CDG (300 ng) completely inhibited tumor growth while the soluble CDG could not restrict tumor growth [38].
Intravenous injection of liposome/cGAMP in tumor-bearing mice led to over 200-fold increase of lung IFNβ [39]. Liposome/cGAMP treated mice showed a significantly reduced median tumor nodule length in the lung when compared to PBS‐treated controls [39]. In comparison, free cGAMP treated mice failed to reduce the tumor length. Furthermore, liposome/cGAMP formulation generated tumor-specific memory and provided nearly total protection against re-challenge even after 60 days of treatment [39].
Intratumoral injection of immunostimulants is effective in the local site. However, it often fails to generate a systemic response and is ineffective on distant tumor sites. Using a phosphatidylserine coated liposome loaded with cGAMP (NP-cGAMP) in combination with radiotherapy (IR), Liu Y. et al. detected significantly elevated lung IFNβ, and TNFα, IFNγ, IL-6, IL-12p40 in a lung metastasis mouse mode[40]. The combinatorial therapy inhibited metastases in both the IR- and non-IR-treated lungs and caused complete regression of lung metastases in some mice. This combinatorial therapy also promoted an anti-tumor memory response [40].
In summary, CDNs can be administered directly via i.n., s.c., or encapsulated in nano/microparticles via i.m., s.c., i.n., i.t., or i.v. routes. Encapsulated CDNs lower the dose (>10-fold less) needed to induce effective vaccine responses in vivo. Furthermore, encapsulated CDNs offer the advantage of long term storage upon lyophilization, which is desirable in developing countries.
4. Mode of aAction of CDN Adjuvants:
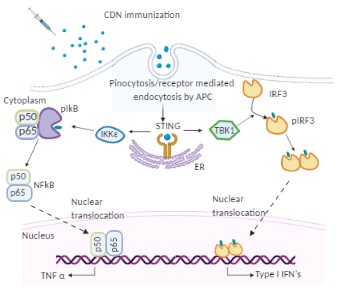
Figure 1. The molecular mode of action of cyclic dinucleotides (CDNs) in dendritic cells (DCs). Immunized CDNs are taken up by pinocytosis or phagocytosis by dendritic cells in vivo. In the cytoplasm, CDNs bind to STING dimers located on the endoplasmic reticulum (ER) membrane, which undergoes conformational changes and activation. The STING (Stimulator of interferon genes) activation recruits kinases TANK binding kinase 1 (TBK 1) or IκB kinase (Iκκε). TBK 1 phosphorylates interferon regulatory factor 3 (IRF3), which dimerizes and translocates to the nucleus to activate type I IFNs. Iκκε phosphorylates nuclear factor-κB (NF-κB) inhibitor IκBα leading to dissociation of IκBα from NF-κB and translocation of the later to the nucleus to activate pro-inflammatory cytokines such as TNF-α.
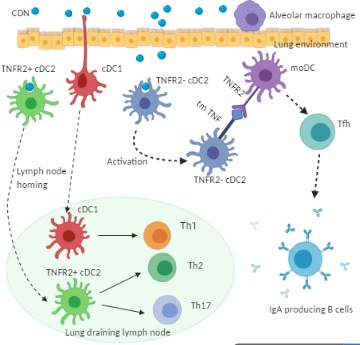
Figure 2. Cellular mechanism of CDN adjuvanticity by lung DCs. Intranasal immunization of CDN promotes its uptake by functionally distinct lung DC subsets: cDC1, TNFR2+ cDC2, and TNFR2- cDC2. Upon the CDN uptake, cDC1 and TNFR2+ cDC2 mature and migrate towards the lung draining lymph node where they direct naïve T cells towards Th1, Th2, and Th17 effector cells. The TNFR2- cDC2 population, on the CDN uptake, is activated but does not migrate. Instead, the TNFR2- cDC2 produces transmembrane TNF, which engages TNFR2 on monocyte-derived DCs (moDCs) to trigger lung moDCs activation. Activated lung moDCs induce Tfh, GC formation, and IgA production in the lung.
5. Confounding fFactors in CDN aAdjuvanticity in hHumans:
Murine studies have consistently shown promising results for CDN adjuvants in infectious diseases and cancer therapeutics. However, CDN human clinical trials have failed to yield the desired effect. Merck's synthetic CDN MK-1454 (NCT03010176) administered intratumorally failed to generate any response against metastatic solid tumors patients (head and neck squamous cell carcinoma, triple-negative breast cancer, and anaplastic thyroid carcinoma). Aduro Biotech's synthetic CDN, ADU-S100, also had disappointing clinical responses (weak response in only 5% of the treated patients) (NCT02675439). Two confounding factors may explain the ineffectiveness of CDNs in humans.
5.1. The hHeterogeneity of the hHuman STING gGene:
The CDN adjuvanticity depends on STING in vivo [41]. Unlike murine STING, the human STING gene is highly heterogeneous and shows population stratification [42][43][44]. Human STING genes have five alleles that have a population frequency above 1%. They are R232 (WT), H232, HAQ (R71H-G230A-R293Q), AQ (G230A-R293Q), and Q293. The HAQ-STING is extremely popular
in East Asians but very rare in Africans[42]. In contrast, AQ and Q293 are only found in Africans[45]. In a recent clinical trial, Sebastian M. et al. showed that HAQ carriers had low anti-PPS antibodies production in response to Pneumovax® 23 immunization (ClinicalTrials.gov: NCT02471014). The result is in line with the previous data from a HAQ knock-in mouse mode [42][46]. Lastly, Kennedy R. B. et al. demonstrated that PBMC from H232/H232 individuals had an impaired STING-mediated innate immunity (~90% decrease of IFNα induction) to poxviruses. A large human population carries these STING alleles[42]. For example, the HAQ/HAQ, HAQ/WT, HAQ/H232, and H232/H232 humans account for ~65% of East Asians and ~30% of Caucasians[42][43][47][44]. Thus, large human populations carry STING alleles that impact CDN vaccine responses.
5.2. The iImpact of aAge in CDN aAdjuvanticity:
The median age of patients in one CDN clinical trial was 61 (ClinicalTrials.gov: NCT02675439). How age affects CDN efficacy was not well-addressed. In early 2019, Wannemuehler and M et al. showed that CDN induced serum antibody production in 20-month-old female BALB/c mice[48]. They did not examine memory T cells responses or distinguish high- and low-affinity antibodies production [48]. They immunized mice (s.c.) with 20 µg CDN [48], a rather high dose of CDN for mice. In 2020, Gogoi H et al. reported that CDG (5 µg, i.n.)-induced high-affinity, durable antibodies, and Th1/Th17 responses were severely reduced in one-year-old (~equivalent age of 42.5-year-old in humans) and two-year-old (~equivalent age of 70-year-old in humans) C57BL/6J mice [109]. Aging decreases the response to vaccination [49][50][51][52]. Thus, similar to most vaccines, CDN vaccine adjuvanticity is also negatively impacted by age.
6. Conclusion and fFuture of CDN vVaccine aAdjuvants:
CDN adjuvants induce balanced, durable humoral, cellular mucosal immune responses, and potent anti-tumor immunity that is highly desirable for vaccine protection from a broad spectrum of pathogens and cancers. STING-targeting CDNs, thus, will continue to garner attention from the community. To advance CDNs as a human vaccine and cancer adjuvants, more rigorous research to understand their mode of action in vivo are needed. For example, examine the therapeutic efficacy of CDNs in vivo using mice that have the equivalent age of human cancer patients and develop CDN compositions that may enhance their adjuvant activities in aged mice.
References
- Philippa Marrack; Amy S. McKee; Michael W. Munks; Towards an understanding of the adjuvant action of aluminium. Nature Reviews Immunology 2009, 9, 287-293, 10.1038/nri2510.
- Abiodun D. Ogunniyi; James C. Paton; Alun C. Kirby; Jonathan A. McCullers; Jan Cook; Mamoru Hyodo; Yoshihiro Hayakawa; David K. R. Karaolis; c-di-GMP is an effective immunomodulator and vaccine adjuvant against pneumococcal infection. Vaccine 2008, 26, 4676-4685, 10.1016/j.vaccine.2008.06.099.
- HongBin Yan; Rhonda KuoLee; Kha Tram; Hongyu Qiu; Jianbing Zhang; Girishchandra B Patel; Wangxue Chen; 3′,5′-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochemical and Biophysical Research Communications 2009, 387, 581-584, 10.1016/j.bbrc.2009.07.061.
- Peter M. Gray; Gail Forrest; Thomas Wisniewski; Gene Porter; Daniel C. Freed; Julie A. DeMartino; Dennis M. Zaller; Zhiqiang Guo; Joseph Leone; Tong-Ming Fu; et al.Kalpit A. Vora Evidence for cyclic diguanylate as a vaccine adjuvant with novel immunostimulatory activities. Cellular Immunology 2012, 278, 113-119, 10.1016/j.cellimm.2012.07.006.
- Thomas Ebensen; Rimma Libanova; Kai Schulze; Tetyana Yevsa; Michael Morr; Carlos Guzmán; Bis-(3′,5′)-cyclic dimeric adenosine monophosphate: Strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 2011, 29, 5210-5220, 10.1016/j.vaccine.2011.05.026.
- Abdullah S Madhun; Lars R. Haaheim; Jane Kristin Nøstbakken; Thomas Ebensen; Jessica Chichester; Vidadi Yusibov; Carlos A. Guzmán; Rebecca J. Cox; Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 2011, 29, 4973-4982, 10.1016/j.vaccine.2011.04.094.
- Thomas Ebensen; Kai Schulze; Peggy Riese; Claudia Link; Michael Morr; Carlos A. Guzmán; The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 2007, 25, 1464-1469, 10.1016/j.vaccine.2006.10.033.
- Ji Wang; Peiyu Li; Yang Yu; Yuhong Fu; Hongye Jiang; Min Lu; Zhiping Sun; Shibo Jiang; Lu Lu; Mei X. Wu; et al. Pulmonary surfactant–biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810, 10.1126/science.aau0810.
- Erik S. Van Dis; Kimberly M. Sogi; Chris S. Rae; Kelsey E. Sivick; Natalie H. Surh; Meredith L. Leong; David B. Kanne; Ken Metchette; Justin J. Leong; Jacob R. Bruml; et al.Vivian ChenKartoosh HeydariNathalie CadieuxTom EvansSarah M. McWhirterThomas W. DubenskyDaniel A. PortnoySarah Stanley STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Reports 2018, 23, 1435-1447, 10.1016/j.celrep.2018.04.003.
- Tara Martin; Junbae Jee; Eunsoo Kim; Haley E. Steiner; Estelle Cormet-Boyaka; Prosper N. Boyaka; Sublingual targeting of STING with 3'3'-cGAMP promotes systemic and mucosal immunity against anthrax toxins.. Vaccine 2017, 35, 2511-2519, 10.1016/j.vaccine.2017.02.064.
- David K. R. Karaolis; Michael W. Newstead; Xianying Zeng; Mamoru Hyodo; Yoshihiro Hayakawa; Urvhashi Bhan; Hallie Liang; Theodore J. Standiford; Cyclic Di-GMP Stimulates Protective Innate Immunity in Bacterial Pneumonia. Infection and Immunity 2007, 75, 4942-4950, 10.1128/iai.01762-06.
- Lisa Zhao; Rhonda KuoLee; Greg Harris; Kha Tram; HongBin Yan; Wangxue Chen; c-di-GMP protects against intranasal Acinetobacter baumannii infection in mice by chemokine induction and enhanced neutrophil recruitment. International Immunopharmacology 2011, 11, 1378-1383, 10.1016/j.intimp.2011.03.024.
- Dong-Liang Hu; Kouji Narita; Mamoru Hyodo; Yoshihiro Hayakawa; Akio Nakane; David K. R. Karaolis; c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine 2009, 27, 4867-4873, 10.1016/j.vaccine.2009.04.053.
- Leticia Corrales; Laura Hix Glickman; Sarah M. McWhirter; David B. Kanne; Kelsey E. Sivick; George E. Katibah; Seng-Ryong Woo; Edward Lemmens; Tamara Banda; Justin J. Leong; et al.Ken MetchetteThomas W. DubenskyThomas F Gajewski Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Reports 2015, 11, 1018-1030, 10.1016/j.celrep.2015.04.031.
- Juan Fu; David B. Kanne; Meredith Leong; Laura Hix Glickman; Sarah M. McWhirter; Edward Lemmens; Ken Mechette; Justin J. Leong; Peter Lauer; Weiqun Liu; et al.Kelsey E. SivickQ. ZengKevin C. SoaresLei ZhengDaniel A. PortnoyJoshua J. WoodwardDrew M. PardollThomas W DubenskyYoung J. Kim STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Science Translational Medicine 2015, 7, 283ra52-283ra52, 10.1126/scitranslmed.aaa4306.
- P A Kozlowski; S Cu-Uvin; M R Neutra; T P Flanigan; Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women.. Infection and Immunity 1996, 65, 1387-1394, 10.1128/iai.65.4.1387-1394.1997.
- Pamela A. Kozlowski; Selvi B. Williams; Rebecca M. Lynch; Timothy P. Flanigan; Rosalyn R. Patterson; Susan Cu-Uvin; Marian R. Neutra; Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle.. The Journal of Immunology 2002, 169, 566-574, 10.4049/jimmunol.169.1.566.
- Herman F. Staats; Sean P. Montgomery; Thomas J. Palker; Intranasal Immunization Is Superior to Vaginal, Gastric, or Rectal Immunization for the Induction of Systemic and Mucosal Anti-HIV Antibody Responses. AIDS Research and Human Retroviruses 1997, 13, 945-952, 10.1089/aid.1997.13.945.
- Anna Rudin; Gerdt C. Riise; Jan Holmgren; Antibody Responses in the Lower Respiratory Tract and Male Urogenital Tract in Humans after Nasal and Oral Vaccination with Cholera Toxin B Subunit. Infection and Immunity 1999, 67, 2884-2890, 10.1128/iai.67.6.2884-2890.1999.
- Michael A Egan; Siew Yen Chong; Michael Hagen; Shakuntala Megati; Eva B Schadeck; Priscilla Piacente; Ben-Jiang Ma; David C Montefiori; Barton F Haynes; Zimra R Israel; et al.John H EldridgeHerman F. Staats A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine 2004, 22, 3774-3788, 10.1016/j.vaccine.2004.03.011.
- Igor M. Belyakov; Michael A. Derby; Jeffrey D. Ahlers; Brian L. Kelsall; Patricia Earl; Bernard Moss; Warren Strober; Jay A. Berzofsky; Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proceedings of the National Academy of Sciences 1998, 95, 1709-1714, 10.1073/pnas.95.4.1709.
- Frederik W. Van Ginkel; Raymond J. Jackson; Yoshikazu Yuki; Jerry R. McGhee; Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues.. The Journal of Immunology 2000, 165, 4778-4782, 10.4049/jimmunol.165.9.4778.
- Margot Mutsch; Weigong Zhou; Philip Rhodes; Matthias Bopp; Robert T. Chen; Thomas Linder; Christian Spyr; Robert Steffen; Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell's Palsy in Switzerland. New England Journal of Medicine 2004, 350, 896-903, 10.1056/nejmoa030595.
- Robert B. Couch; Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy.. New England Journal of Medicine 2004, 350, 860-1, 10.1056/NEJMp048006.
- Rimma Libanova; Thomas Ebensen; Kai Schulze; Daniela Bruhn; Miriam Nörder; Tetyana Yevsa; Michael Morr; Carlos A. Guzmán; The member of the cyclic di-nucleotide family bis-(3′, 5′)-cyclic dimeric inosine monophosphate exerts potent activity as mucosal adjuvant. Vaccine 2010, 28, 2249-2258, 10.1016/j.vaccine.2009.12.045.
- Y Jiang; Yongsheng Li; B Zhu; T-cell exhaustion in the tumor microenvironment.. Cell Death & Disease 2015, 6, e1792-e1792, 10.1038/cddis.2015.162.
- Daniela S. Thommen; Ton N. Schumacher; T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547-562, 10.1016/j.ccell.2018.03.012.
- Olivier DeMaria; Aude De Gassart; Sanja Coso; Nicolas Gestermann; Jeremy Di Domizio; Lukas Flatz; Olivier Gaide; Olivier Michielin; Patrick Hwu; Tatiana V. Petrova; et al.Fabio MartinonRobert L. ModlinDaniel E. SpeiserMichel Gilliet STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proceedings of the National Academy of Sciences 2015, 112, 15408-15413, 10.1073/pnas.1512832112.
- Takayuki Ohkuri; Akemi Kosaka; Kei Ishibashi; Takumi Kumai; Yui Hirata; Kenzo Ohara; Toshihiro Nagato; Kensuke Oikawa; Naoko Aoki; Yasuaki Harabuchi; et al.Esteban CelisHiroya Kobayashi Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunology, Immunotherapy 2017, 66, 705-716, 10.1007/s00262-017-1975-1.
- Christopher J. Nicolai; Natalie Wolf; I-Chang Chang; Georgia Kirn; Assaf Marcus; Chudi O. Ndubaku; Sarah M. McWhirter; David H Raulet; NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists.. Science Immunology 2020, 5, eaaz2738, 10.1126/sciimmunol.aaz2738.
- Brian James Francica; Ali Ghasemzadeh; Anthony L. Desbien; Debebe Theodros; Kelsey E. Sivick; Gabrielle L. Reiner; Laura Hix Glickman; Ariel E. Marciscano; Andrew B. Sharabi; Meredith L. Leong; et al.Sarah M. McWhirterThomas W. DubenskyDrew M. PardollCharles G. Drake TNFα and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors. Cancer Immunology Research 2018, 6, 422-433, 10.1158/2326-6066.cir-17-0263.
- Steven M Blaauboer; Samira Mansouri; Heidi R Tucker; Hatti L Wang; Vincent D Gabrielle; Lei Jin; The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. eLife 2015, 4, e06670, 10.7554/elife.06670.
- M H El-Shabouri; Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A.. International Journal of Pharmaceutics 2002, 249, 101-108, 10.1016/s0378-5173(02)00461-1.
- Liandong Hu; Xing Tang; Fude Cui; Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. Journal of Pharmacy and Pharmacology 2004, 56, 1527-1535, 10.1211/0022357044959.
- Melissa C. Hanson; Monica P. Crespo; Wuhbet Abraham; Kelly D. Moynihan; Gregory L. Szeto; Stephanie H. Chen; Mariane B. Melo; Stefanie Mueller; Darrell J. Irvine; Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. Journal of Clinical Investigation 2015, 125, 2532-2546, 10.1172/jci79915.
- Robert D. Junkins; Matthew D. Gallovic; Brandon M. Johnson; Michael A. Collier; Rebekah Watkins-Schulz; Ning Cheng; Clément N. David; Charles E. McGee; Gregory D. Sempowski; Ivo Shterev; et al.Karen McKinnonEric M. BachelderKristy AinslieJenny Pan-Yun Ting A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination.. Journal of Controlled Release 2017, 270, 1-13, 10.1016/j.jconrel.2017.11.030.
- Rebekah Watkins Schulz; Pamela Tiet; Matthew D. Gallovic; Robert D. Junkins; Cole J Batty; Eric M. Bachelder; Kristy Ainslie; Jenny Pan-Yun Ting; A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8+ T cell-mediated anti-tumor immunity.. Biomaterials 2019, 205, 94-105, 10.1016/j.biomaterials.2019.03.011.
- Hiroko Miyabe; Mamoru Hyodo; Takashi Nakamura; Yusuke Sato; Yoshihiro Hayakawa; Hideyoshi Harashima; A new adjuvant delivery system ‘cyclic di-GMP/YSK05 liposome’ for cancer immunotherapy. Journal of Controlled Release 2014, 184, 20-27, 10.1016/j.jconrel.2014.04.004.
- Sandeep T. Koshy; Alexander Cheung; Luo Gu; Amanda Graveline; David J. Mooney; Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Advanced Biosystems 2017, 1, 1600013, 10.1002/adbi.201600013.
- Yang Liu; William N. Crowe; Lulu Wang; Yong Lu; W. Jeffrey Petty; Amyn A. Habib; Dawen Zhao; An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases.. Nature Communications 2019, 10, 5108-15, 10.1038/s41467-019-13094-5.
- Steven M. Blaauboer; Vincent D. Gabrielle; Lei Jin; MPYS/STING-Mediated TNF-α, Not Type I IFN, Is Essential for the Mucosal Adjuvant Activity of (3′–5′)-Cyclic-Di-Guanosine-Monophosphate In Vivo. The Journal of Immunology 2013, 192, 492-502, 10.4049/jimmunol.1301812.
- Seema Patel; Steven M. Blaauboer; Heidi R. Tucker; Samira Mansouri; Juan S. Ruiz-Moreno; Lutz Hamann; Ralf R. Schumann; Bastian Opitz; Lei Jin; The common R71H-G230A-R293Q (HAQ) human TMEM173 is a null allele. The Journal of Immunology 2016, 198, 776-787, 10.4049/jimmunol.1601585.
- Lei Jin; Liang-Guo Xu; Ivana V. Yang; Elizabeth J. Davidson; David A. Schwartz; Mark M. Wurfel; John C. Cambier; Identification and characterization of a loss-of-function human MPYS variant. Genes & Immunity 2011, 12, 263-269, 10.1038/gene.2010.75.
- Seema Patel; Lei Jin; TMEM173 variants and potential importance to human biology and disease.. Genes & Immunity 2018, 20, 82-89, 10.1038/s41435-018-0029-9.
- Seema Patel; Steven M. Blaauboer; Heidi R. Tucker; Samira Mansouri; Juan S. Ruiz-Moreno; Lutz Hamann; Ralf R. Schumann; Bastian Opitz; Lei Jin; Response to Comment on "The Common R71H-G230A-R293Q Human TMEM173 Is a Null Allele".. The Journal of Immunology 2017, 198, 4185-4188, 10.4049/jimmunol.1700322.
- Seema Patel; Steven M. Blaauboer; Heidi R. Tucker; Samira Mansouri; Juan S. Ruiz-Moreno; Lutz Hamann; Ralf R. Schumann; Bastian Opitz; Lei Jin; Response to Comment on "The Common R71H-G230A-R293Q Human TMEM173 Is a Null Allele".. The Journal of Immunology 2017, 198, 4185-4188, 10.4049/jimmunol.1700322.
- Guanghui Yi; Volker P. Brendel; Chang Shu; Pingwei Li; Satheesh Palanathan; C. Cheng Kao; Single Nucleotide Polymorphisms of Human STING Can Affect Innate Immune Response to Cyclic Dinucleotides. PLoS ONE 2013, 8, e77846, 10.1371/journal.pone.0077846.
- Ross J. Darling; Sujata Senapati; Sean M. Kelly; Marian L. Kohut; Balaji Narasimhan; Michael J. Wannemuehler; STING pathway stimulation results in a differentially activated innate immune phenotype associated with low nitric oxide and enhanced antibody titers in young and aged mice. Vaccine 2019, 37, 2721-2730, 10.1016/j.vaccine.2019.04.004.
- Diana Boraschi; Paola Italiani; Immunosenescence and vaccine failure in the elderly: Strategies for improving response. Immunology Letters 2014, 162, 346-353, 10.1016/j.imlet.2014.06.006.
- Sheri M. Eaton; Eve M. Burns; Kimberly Kusser; Troy D. Randall; Laura Haynes; Age-related Defects in CD4 T Cell Cognate Helper Function Lead to Reductions in Humoral Responses. The Journal of Experimental Medicine 2004, 200, 1613-1622, 10.1084/jem.20041395.
- Alexander C Maue; Laura Haynes; CD4+ T Cells and Immunosenescence – A Mini-Review. Gerontology 2008, 55, 491-495, 10.1159/000214842.
- Julie S Lefebvre; Laura Haynes; Aging of the CD4 T Cell Compartment. Open Longevity Science (Formerly 'The Open Aging Journal') 2011, 6, 83-91, 10.2174/1876326X01206010083.