Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Asarudheen Abdudeen and Version 2 by Vivi Li.
The additive manufacturing (AM) process is used for joining materials to make objects from 3D model data, usually layer upon layer, contrary to subtractive manufacturing methods. This technology plays a significant role in fabricating orthopedic implants, especially parts of hip implants (HI), such as femoral head, stem, neck, polyethylene linear, acetabular shell, and so on, using biomaterials. These biodegradable resources are those that can be utilized as tissue substitutes since they are accepted by live tissues.
- AM
- biomaterials
- biocompatibility
- topology optimization
- titanium alloys
1. Introduction
In ancient times, when the joints of the bones were damaged, it was very difficult to replace them. Either treatment had to be performed till the end of life, or any supporting belt was put on. The problem is encountered when some of the elements between the bones are damaged or broken. The invention of biomaterials has created major changes in the medical field [1][2][3][4][1,2,3,4]. Biocompatible materials are generally used to replace or support damaged biological tissues [5][6][7][5,6,7]. These biomaterials are used to produce bone fragments and broken parts [8][9][10][11][12][13][14][8,9,10,11,12,13,14]. They can be applied to hard tissues in orthopedics or orthodontics or to support soft tissues, such as blood vessel or artificial valves in the heart [15].
Nowadays, AM plays an important role in the medical field [16][17][18][19][20][21][22][23][24][16,17,18,19,20,21,22,23,24], especially in the production of some internal organs using artificial tissues that are accepted by the natural living tissues of human beings. AM consists of design modeling and production. The 3D model for the production can be designed by the CAD software or a model obtained through CT scan or MRI. We know our body will reject all the unnecessary substance. Therefore, materials that are not harmful and acceptable by living organisms are used. Biomaterials can enhance the quality and longevity of human life [25][26][27][28][29][25,26,27,28,29]. The science and technology associated with the field of biomaterial has now led to a multi-million-dollar business. Biomaterials are used in different parts of the human body as replacement implants in shoulders, hips, knees, and elbows [30][31][32][33][34][30,31,32,33,34]. However, the number of replacements are high in the cases of the spinal area, hip, and knee [25].
Approximately 230 million major surgical procedures are performed on a daily basis. Most of these procedures involve repair, reconstruction, or replacement of one or more damaged bones or joints and also tissues and organs. The combination of the medical field and AM has emerged as an alternative technique to manufacture substitute joints and their components to restore, improve, and maintain the function of joints. In orthopedic implants, several biomaterials are used for the construction of joints and components [35][36][37][35,36,37]. Biocompatible metals and alloys, such as Co-Cr alloys, stainless steel, Ti, and its alloys, are the main materials used as implants [38]. Biodegradable Mg-based alloys are also used for making metal implants [39].
Several review papers related to biomaterials have been published in recent years, such as references [38][40][41][42][43][44][38,40,41,42,43,44], in which most of them have concentrated on AM techniques used for the fabrication of different biomaterials [38][42][44][38,42,44]. Some of them have studied their thermal effects and corrosion rate [40][43][40,43], and a few studies have been carried out on surface modification for bioimplants [41]. Furthermore, some other articles have presented a discussion of specific objectives; for example, Attar et al. [45] used metals that are accepted by living tissues. They reported that metals such as surgical-grade stainless steel (316L), Co-Cr alloys, and pure commercial Ti or Ti alloys do not affect negatively the human body and will have good mechanical properties, high strength-to-weight ratio, and excellent corrosion resistance. Mostly these metals are used for joint replacements, dental roots, and orthopedic fixations, for stents and orthopedic implants. Prakasam M et al. and Minagar S et al. [46][47][46,47] studied the main advantage of metal implants. It was revealed that they have brilliant mechanical properties, which make them the most commonly used implant materials in bone surgical repair. Meanwhile, the main disadvantage of metals in their use as biomaterials is their lack of tissue adherence and excess deterioration [48][49][50][51][52][48,49,50,51,52]. This will result in either a second surgery to remove the implant or keeping the implant in the body with the risk of toxicity due to accumulation of metal ions due to rusting.
Other than metals, ceramic materials are biocompatible and have very high resistance to corrosion and compression with low electrical and thermal conductivities [53][54][55][53,54,55]. Habibovic P et al. [56] reported that ceramic biomaterials are mostly suited for medical implants; they have very low toxicity and help to achieve higher efficiency in forming new bone tissues. They reported that ceramics can replicate human tissues or can be absorbed back into the body to encourage the restoration of healthy tissues. They are composed of bioactive glass, glass ceramics, calcium silicates, hydroxyapatite, alumina, zirconia radiation glasses, and resorbable calcium phosphates.
Bose S et al. [57] mentioned that the most common material used in the 3D printing industry are polymers because of their diversity. They revealed that polymers are widely used more than ceramics and metallic biomaterials. The ease of manufacturing to produce various shapes, ease of secondary processing stages, reasonable cost, and availability with desired physical and mechanical properties are the major advantages of polymers when compared with metallic and ceramic biomaterials [58][59][60][61][62][63][58,59,60,61,62,63]. Devi P et al. [64] highlighted the main reasons behind the wide usage of polymers. They are biocompatibility, biodegradability, sterilizability, adequate mechanical and physical properties, and excellent manufacturability because of their low melting points. Karageorgiou et al. [65] revealed that many natural polymers benefit from being biocompatible and biodegradable. They stated that polymers can modify their chemical structure due to their flexibility. Jones L et al. [66] found that a variety of polymers have been used in medical care, including preventive medicine, clinical inspections, and surgical treatment. They mentioned that the commonly used polymer in orthopedic surgery is polymethyl methacrylate.
Despite many existing review papers, to the best of the authors’ knowledge, there is no study that has reviewed the best material specifically for making implants, its manufacturing process, benefits, defects, and comparison with other biomaterials.
2. Biomaterials for Biological Tissue Replacements
Different biomaterials are integrated with tissue replacements. This area summarizes commonly used materials for making biological implants. The most commonly used material will be projected after the following subsections.2.1. Metals
Metals are the main materials that are used in the replacements of bones or used as a support for broken bones [67][68][69][70][71][72][73][74][75][67,68,69,70,71,72,73,74,75]. Metals that are accepted by living tissues are only used in this case. Using these metals does not affect negatively the human body and will result in good mechanical properties, high strength-to-weight ratio, and remarkable resistance to corrosion [76]. These required metal parts are manufactured by traditional processing technologies, including casting, machining, and powder metallurgy—all of these processes need more time and energy and have a high metal consumption rate [45]. However, AM techniques are replacing these conventional methods nowadays [77]. Mostly the metals are used for biomedical replacement surgeries [78]. Among these metals, Ti alloy is considered to be the best material for orthopedic implants [79]. In the case of fracture fixations, the fixation devices used to immobilize the bone are wires, screws, plates, and intramedullary nails. The primary benefit of metal implants is their superior mechanical qualities, which make them the most popular implant type for bone surgical repair [46][47][46,47]. The main disadvantage of metals in being used as a biomaterial is their lack of tissue adherence and low rate of disintegration [48][49][50][51][52][48,49,50,51,52]. This will result either in a second surgery to remove the implant or keeping the implant in the body with the risk of toxicity due to the accumulation of metal ions due to corrosion.2.2. Ceramics
Generally, ceramic materials are biocompatible and have very high resistance to corrosion and compression with low electrical and thermal conductivities [53][54][55][53,54,55]. Ceramic biomaterials are mostly suited for medical implants; they have very low toxicity, and they help in the higher efficiency of forming new born tissues [56]. These have the ability to perform like human tissues or to absorb into the body and promote the regeneration of natural tissues. They include radiotherapy glasses, bioactive glass, hydroxyapatite, glass ceramic calcium silicates, alumina and zirconia, and resorbable calcium phosphates [80]. Mostly ceramics are used for dental implants and some orthopedic implants. The ceramics used in bone replacement and healing are categorized into three types based on the body’s reaction. The first is the bioinert ceramic, which is almost inert in the body and forms a thin nonadherent fibrous layer at the connection. The bioactive ceramic falls under the second type and can firmly attach to bones. Finally, the third type is the bioresorbable ceramic, which progressively deteriorates over time, eventually being replaced by natural bone [56]. The use of ceramics in medical applications is limited due to their poor tensile strength [81].2.3. Polymers
Polymers are the most common material in the 3D printing industry because of their diversity [57]. Polymeric biomaterials are considered to be one of the best materials for medical end use. Polymers are widely used more than ceramic biomaterials [82]. The required mechanical and physical properties, the simplicity of secondary processing stages, the cost, and the ease of manufacturing to manufacture diverse shapes are the major advantages of polymers when compared with metallic and ceramic biomaterials [58][59][60][61][62][63][58,59,60,61,62,63]. The main reasons behind the wide usage of polymers are biocompatibility, biodegradability, sterilizability, adequate mechanical and physical properties, and low melting point, which make them easy to fabricate [64]. Many natural polymers have the advantage of biodegradability and biocompatibility [65] Polymers can modify their chemical structure due to their flexibility. Biodegradable and biocompatible polymers are generally called biopolymers [83][84][85][83,84,85]. In medical care, a variety of polymers have been used, including preventive medicine, clinical inspections, and surgical treatment [64]. A commonly used polymer in orthopedic surgery is polymethyl methacrylate [66]. Even though polymers have lot of positives, the use of polymers is confined due to its low load handling capacity and low tissue affinity [86]. According to the aforementioned forms of publications, all three types of materials can be employed based on the implant type, location inside the body, size, design, and mechanical demands that they must withstand. The most widely accepted and used biomaterials are metallic since early days. Studies are underway to make ceramics and polymeric materials common substitutes for metals. Hence, this rentryview focuses on metallic biomaterials used for making implants. The next part of the entryreview discusses the AM methods involved in the manufacturing of metallic biomaterials.3. AM Techniques for Metallic Biomaterials
AM is simply defined as the addition of material in a layer-by-layer fashion. This technology uses data from a computer-aided design (CAD) software or from any 3D object scanners to direct a hardware to deposit the material, layer upon layer, in precise geometric shapes. Currently, there are many types of AM technologies for making metallic biomaterials. The relevant studies classified according to the AM processes used in the manufacturing of biomaterials are as follows:3.1. Direct Energy Deposition Technique (DED)
Bose S et al. [57] explain DED as a process in which a direct source of energy is used to simultaneously deposit, melt, and solidify the material that is fed as either a powder or as a filament. Electron beam or lasers are mainly used as the direct energy source. Yousuf S et al. [87] say that the continuous feeding of starting material is needed in the form of powder or filament for the process. Then the feeding material becomes melted by the energy source and becomes deposited in a layerwise pattern. The heat conduction in direct energy deposit processes is more effective because the heat, material, and shield gas are transferred directly from the nozzle to the build area. The laser direct deposition technique is the most popular one that falls under this category [88]. DED features a nozzle that moves around multiple axes. Davoodi E et al. mention that the DED process can be used for fabricating a wide range of metallic biomaterials. Moreover, this technique has proven its capability of manufacturing porous biomedical implants [89]. Furthermore, multimaterial structures produced by DED have benefits and can be constructed using existing technology and process management [90]. DED is faster and less expensive as compared with powder bed fusion when creating midsize metal parts [91]. Even though DED is a method having various advantages, it has certain disadvantages, such as support structures being necessary for overhangs, limitations in producing complex shapes, and lower accuracy than powder bed fusion AM processes [92]. The working of DED is shown in Figure 1.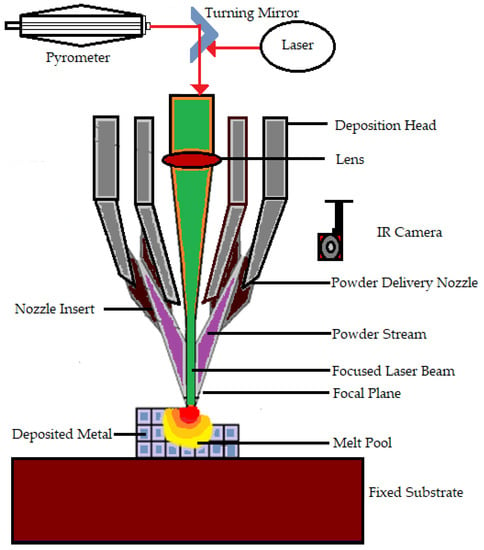
Figure 1.
Working of the direct energy deposition technique.
3.2. Material Extrusion (ME)
Susmita Bose et al. [93] define material extrusion as an AM technique that builds three-dimensional models using a continuous filament made of thermoplastic or composite material. The process has a large number of variables that influence the quality of the final model, but when these variables are tightly controlled, they have great potential and viability. Fuse deposition modeling is a common material extrusion process [94]. Ortega Z et al. [95] mention that the material is selectively distributed through a nozzle or orifice using a sliced CAD part model. The filament material is fed to a nozzle using a driving gear in the polymer material extrusion AM. Then, to build the complex 3D structure, the filament is deposited onto the build platform layer by layer. The nozzle temperature must be high to melt the filament material (e.g., 200–230 °C for ABS and 180–200 °C for PLA polymers). To prevent component warping and delamination, heat lamps are utilized to control the temperature in the area surrounding the build platform. This technique is comparatively less expensive and easier to run and has more biomedical applications, including biological cells, ceramics, and polymers [96]. Adding more parts to a single build did not reduce the processing time per part [93]. Most material extrusion systems use a spool of plastic filament as the feedstock. Even though material extrusion is a successful method for manufacturing metallic biomaterials Cheuh Y et al. [97] found that material extrusion produces metal components with a lower density than laser powder bed fusion. Figure 2 shows the representation of material extrusion process.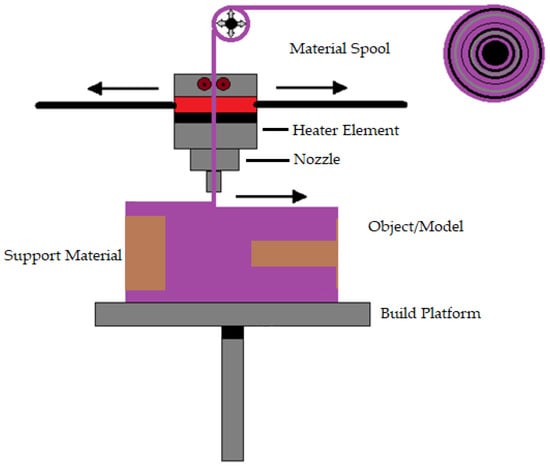
Figure 2.
Representation of material extrusion.
3.3. Powder Bed Fusion (PBF)
In PBF, very fine powders are used as thin layers and are evenly and densely stacked on a platform. The particles in each layer are fused using a laser beam or a binder [98]. PBF approaches allow for excellent dimensional precision at large relative densities [99] often with high repeatability and surface roughness [100]. Additionally, the process has high porosity when the powder is forced with a binder [101]. PBF uses thermal energy to fuse certain areas on the powder bed. PBF is one of the first generation of commercialized AM processes [102]. The printing processes of PBF and binder jetting are similar. N Derimow et al. [103] say that an oxidation-free environment is needed for PBF to prevent oxidation of the feedstock powder. A hopper or a reservoir below the side of the bed provides fresh material supply. PBF’s advantages include the fact that it has less material wastage and less cost, improves production development times, enables rapid prototyping and low-volume production, is capable of building functionally graded parts, performs efficient recycling of unmelted powder, and provides elimination of the need for machining fixtures [57]. In addition, even though the high powder density across the build geometry can act as a self-support [87], the PBF approach frequently requires structural support to combat shape distortions of the manufactured sample. In the case of titanium, PBF can create materials with high strength, high detail, and load bearing [104]. On the other hand, using tiny, spherical metallic powders enables high packing densities, enhances component consolidation, and reduces flaws [105]. Behzad Fotovvat et al. [106] focuses on the PBF–AM technique of direct metal laser sintering for Ti-6Al-4V ELI material’s dimensional precision. The impact of size, geometry, and position on the build platform was studied using a variety of geometric pieces with different thicknesses and sizes. They came to the conclusion that accuracy is not affected by where the components are placed on the build platform and that this method typically produces high-resolution results. They concluded that inaccuracy is not a function of the location of the parts on the build platform, and such process usually shows results with high resolution. Meanwhile, from the findings of Samira et al. [107] when compared with laser metal deposition, laser PBF provides the best level of accuracy for dimensions, perpendicularity, and position tolerance. The PBF process is shown in Figure 3.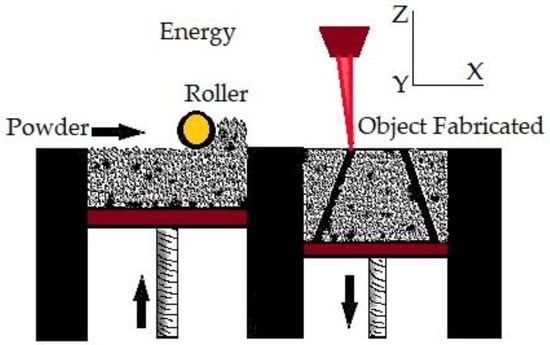
Figure 3.
Powder bed fusion.
3.4. Vat Polymerization (VP)
The first commercially available AM technology is vat polymerization. It produces parts through selectively curing liquid photoreactive polymers [108]. Vat polymerization is also known as stereolithography (SLA). This process has high accuracy in building parts, superior part quality, and quick construction pace [57]. This technology is known for its smooth surface finish and highly detailed parts, average build speed, and a wide range of materials [38]. The SLA process is based on layer-by-layer photosensitive resin polymerization using UV light [109]. Increasing the number of parts reduces the time per part in vat polymerization. Support structures are essential in the case of vat polymerization [93]. Biomaterials in large sizes can be manufactured by vat polymerization using different materials, such as metal, ceramics, and polymers. Figure 4 shows the vat polymerization technique.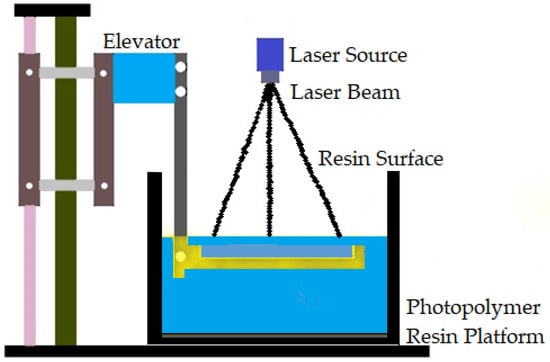
Figure 4.
Vat polymerization technique.
3.5. Binder Jetting (BJ)
Binder jetting (BJ) is a powder-based AM process that employs a liquid binder, instead of an electron beam or laser source, for locally binding the powders and creating the intricate ceramic, polymeric, and metallic structures [112]. Shick T M et al. [113] mentioned that to form a designed structure layer by layer in the BJ technique, the liquid binding agent is spread to a selected area on the power bed based on the part geometry. The binder acts as an adhesive to bond the powder layers. In a normal BJ system, there are three axes, namely x, y, and z. The x and y axes are responsible for the horizontal position, and the z axis refers to the depth for x and y [57]. For a successful build, the binder jetting needs an understanding of many process variables. BJ is able to be conducted on dry powders as well as on wet powders. Fine powder BJ can give good surface finishing and low surface roughness [114]. There are two types of binders: organic and inorganic. Binder jetting is capable of printing a variety of materials, including metals, sands, and ceramics [115]. A schematic representation of binder jetting is shown in Figure 5.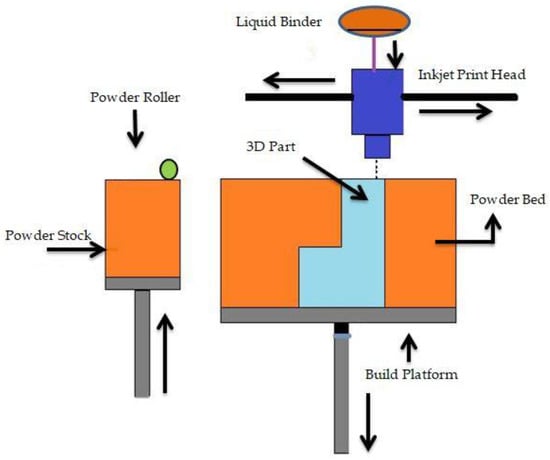
Figure 5.
Schematic representation of binder jetting.
3.6. Material Jetting (MJ)
Material jetting is one of the fastest and more accurate 3D printing technologies. It uses liquid photopolymer droplets to build parts. MJ creates objects in a similar method to a 2D inkjet printer [24]. MJ builds layer upon a layer until the part is finished. This process is also quite similar to SLA since it uses a UV light source to cure the resin [16]. MJ is the only AM technology that can combine different print materials within the same 3D-printed model in the same print job. The key feature of MJ is that objects appear perfectly realistic. Even compared with other 3D printing methods that use photopolymers, MJ hands down as print heads is capable of forming objects more accurately [116]. The photopolymer liquid allows the 3D printer to form smoother parts, which appear shinier when support material is not used. This process can be used for full color printing [117]. Objects created through this process are rigid, flexible, opaque, and translucent. MJ is also used in medical hospitals and universities to 3D-print full-color anatomical models that look like real body parts. This makes it possible for surgeons to plan and be ready for an operation more quickly than they could with a 2D picture [93][96][93,96]. F Klocke et al. [118] found that although the accuracy of the laser-assisted MJ method is equivalent to that of the EBM process, future accuracy increases at high building rates due to enhanced process parameters and automated tool path generation are still very likely.3.7. Sheet Lamination (SL)
SL processes include ultrasonic AM and laminated object manufacturing [119]. The ultrasonic AM process uses sheets or ribbons of metal, which are bound together using ultrasonic welding [120]. The process does require additional CNC machining and removal of inbound metal, often during the process. Laminated object manufacturing uses a similar layer-by-layer approach but uses paper as material and adhesive instead of welding [93]. Laminated objects are often used for aesthetic and visual models and are not suitable for structural use [121]. UAM uses metals and includes aluminums, copper, stainless steel, and titanium [122]. The process has low temperature and allows for internal geometries to be created. The process can bond different materials and requires relatively little energy, as the metal is not melted [123]. M Salmi et al. [119] mention that sheet lamination is only used for medical models or phantoms. They also state that sheet lamination devices are rare, and the vendors have receivership status. Different types of AM technologies and their medical applications are listed in Table 1.Table 1.
AM technologies and their medical applications.
| SI No. | AM Techniques | Medical Application | References |
|---|---|---|---|
| 1. | Electron beam melting (EBM) | Fabrication of implant | [124][125][124[126],125,126] |
| 2. | Direct metal laser sintering (DMLS) | Manufacturing of prostheses or implants | [127][128][129][127,128,129] |
| 3. | Selective laser sintering (SLS) | Customized implants for training, human anatomy | [88][130][131][88,130,131] |
| 4. | Stereolithography (SLA) | Prosthetics, anatomical model | [132][133][132,133] |
| 5. | Multijet printing | Orthopedics, dental | [134][135][134,135] |
| 6. | Color jet printing (CJP) | Implants of heart | [136][137][136,137] |
| 7. | Inkjet 3D printing | Surgical planning, medical Education |
[137][138][137,138] |
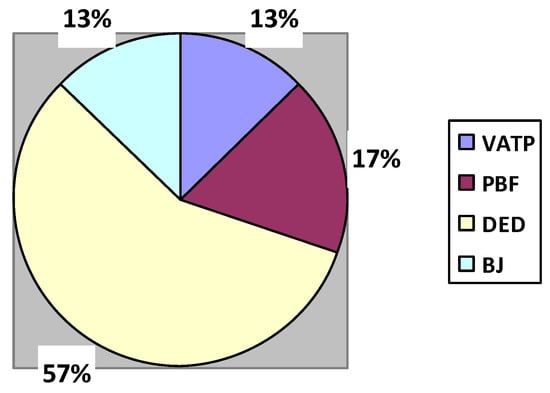
Figure 6.
Percentage contribution of AM methods in the medical industry.
