Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Anastasia Fornari and Version 2 by Sirius Huang.
Cellulose is the most abundant renewable source on Earth. Due to several of their characteristics, such as their renewability, sustainability, and eco-friendliness, nanocellulose-based materials are arousing growing interest from researchers in various fields of study and applications.
- nanocellulose
- CNC
- CNF
- BNC
1. Introduction
Cellulose is the most abundant renewable source on Earth; it can be found in plants, algae, microorganisms, such as some bacteria, and other natural sources. In fact, its annual production is approximately 1.5 × 1012 tons [1][2]. Chemically, it is composed of a linear polysaccharide produced from ringed glucose monomers linked together through β-(1,4) glycosidic bonding, and its microscopic morphology corresponds to alternating regions of crystalline and amorphous zones, which, together, form the characteristic macroscopic fibrous structure of cellulose (Figure 1) [3][4].
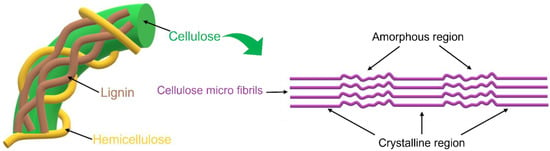
Figure 1.
Amorphous and crystalline regions of cellulose micro fibrils.
The ordered crystalline domains are more resistant to chemical, mechanical, and enzymatic treatments and thus have a higher resistance to degradation compared to the amorphous ones; these crystalline regions are held together by hydrogen bonds that make cellulose stable, but decrease its solubility in water and other solvents [3][5].
The hierarchical structure of cellulose is described in Figure 2.
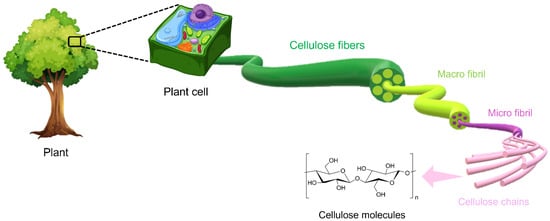
Figure 2. The hierarchical structure of cellulose. Reproduced under terms of the CC-BY license [6]. Copyright © 2020, The Authors, published by Front. Chem.
This polymer, seen as an inexhaustible source of raw materials and, consequently, all of the nanocellulose materials obtained from it, has gained increasing interest thanks to its attractive characteristics, such as its ease of availability, high surface area, good mechanical properties, renewability, and biocompatibility [6][7].
To define cellulose nanomaterials, the nomenclature established by the Technical Association of the Pulp and Paper Industry (TAPPI) of the Nanotechnology Division must be considered, and, from the standardization TAPPI WI 3021, it is possible to define nanocellulose as the crystallite or cellulose fiber possessing at least one dimension in the nanoscale range (1–100 nm) [6].
The class of nanocellulose materials includes all those derived from cellulose with several shapes, dimensions, chemical surface, and properties. Therefore, from the separation of cellulose fibers, different types of nanoscale cellulose can be obtained; in particular, through the function of their sources, the degree of crystallinity, and the extraction and production method, if a bottom-up or top-down technique is used, it is possible to distinguish three categories of nanocellulose: cellulose nanocrystals (CNCs), nano-fibrillated cellulose (CNF), and bacterial nanocellulose (BNC). CNCs consist of cylindrical, elongated, inflexible, and rod-like nanoparticles with dimensions of 4–70 nm in width and 100–6000 nm in length and a crystallinity index of around 54–88 %; they are usually obtained by hydrolysis. Nano-fibrillated cellulose (CNF), commonly obtained by mechanical treatment, with a braided network structure, is composed of longer and flexible fibers with dimensions of 20–100 nm in width and >10,000 nm in length and a low crystallinity index (<50 %). Finally, bacterial nanocellulose (BNC), also known as microbial nanocellulose, is the most promising and cost-effective biomaterial, especially for the biomedical industry, with its highest crystallinity index > 88 % [1]. The BNC consists of ultrafine nanofibers that are 20–100 nm in diameter and micrometers lengths that form an identifying 3D network [1][6][8]. All of these categories of nanocellulose are related to each other because they can all be obtained from the same natural sources, and each can form stable structures in a liquid medium or matrix via hydrogen bonds. In fact, nanocellulose contains a great number of hydroxyl groups (-OH) that can form hydrogen bonds either between different chains (intermolecular bonds) or in the same chain (intramolecular bonds), making this material highly hydrophilic and allowing us to modify it via different chemical and physical strategies [9]. Its hydrophilicity can sometimes be a drawback, especially in the case of applications where humidity can be a problem [10]. Consequently, chemical modifications on hydroxyl groups are often carried out in order to decrease the hygroscopicity of nanocellulose [11].
However, these nanomaterials can also cause compatibility problems with the matrix that incorporates them, such as hydrophobic polymers in the case of nanocomposite materials [12]; therefore, their dispersibility must be guaranteed. For CNCs, this problem does not exist, because it has special self-assembling behavior that makes it highly interesting for the study and the development of advanced materials [4].
2. Cellulose Nanocrystals (CNCs)
The term cellulose nanocrystals, or even crystalline nanocellulose (CNC), describes the rod-like shaped nanoparticles obtained from different sources, such as cotton, wood pulp, plant residue, etc., and this is the most common type of nanocellulose [13].
These nanostructures are produced with an extraction process consisting of two successive steps: the initial pre-treatment of the raw material and the following hydrolysis into CNCs. In the first step, raw materials are treated to remove the impurities and all other chemical constituents present in cellulose sources, such as hemicellulose and lignin; to achieve this, alkaline and bleaching treatments are carried out [14]. Then, hydrolysis is carried out to obtain the nanocellulose crystals; this step is based on a strong acid process that uses especially sulfuric acid (H2SO4), hydrochloric acid (HCl), or phosphoric acid (H3PO4), [4]. The acid hydrolysis is useful for removing, under controlled conditions, the disordered and amorphous regions of the cellulose fibers, leaving the crystalline domains, which then take on the characteristic crystalline shape, with a crystalline index of 54–88% [6][15]. Sulfuric acid hydrolysis is the most efficient and widely used extraction method and, during the isolation treatment, it allows for a better dispersion of cellulose crystals in polar solvents due to the introduction of sulfate half ester groups on the cellulose chain, which, with a negatively charged surface coating, enhance intermolecular repulsive interactions [10].
Cellulose nanocrystals have many interesting properties, such as a good stability, wide surface area, higher tensile strength (10 GPa), high elastic modulus (140–150 GPa), and several optical qualities.
Their dimensions depend on the nature of the starting material, but they are certainly in the nanometer range. In particular, the length and width differ depending on temperature, time, and purity of the cellulose source [5][16]. For example, CNCs obtained from plants have a diameter of 3–5 nm and length of up to 100–300 nm, whereas the CNCs collected from tunicates have a diameter of 10–20 nm and length of up to 500–2000 nm [16]. The morphological characteristics of CNCs are studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) or dynamic light scattering (DLS) [13].
3. Cellulose Nanofibrils (CNFs)
Cellulose nanofibrils, also called nano-fibrillated cellulose (CNF), are primarily obtained from wood with a mechanical treatment of wood pulp, but they can also be extracted from sugar beets, potato tubers, hemp, and flax [17].
Their characteristic structure is formed by an entangled network of long and flexible fibers, with dimensions of 20–100 nm in width and >10,000 nm in length [6].
This type of nanocellulose presents the same amount of amorphous and crystalline regions; the mechanical treatments are the most common methods used to produce it, such as mechanical defibrillation or disintegration caused by mechanical forces. The main disadvantage of these methods is the significant energy consumption. For this reason, and mainly to prevent the aggregation of the micro and nanofibrils in the final product, the mechanical treatments are usually combined with enzymatic and/or chemical treatment, such as the catalytic 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation method, which allows us to obtain nanofibrils by mechanical disintegration, generating CNFs with a uniform size. This approach, moreover, does not change the crystallographic forms of the cellulosic fibers while increasing the surface charge due to the carboxyl groups introduced by TEMPO, which is an interesting advantage for the production of nanocellulose composite materials [18][19][20]. Nevertheless, the mechanical production methods of this type of nanocellulose remain the most advantageous; in fact, they do not require additives, do not modify the surface charge compared to the starting material, and give excellent yields [21].
CNFs have many interesting properties, such as their mechanical strength and rigidity, that make them an alternative to artificial fillers used for the reinforcement of plastic composite materials; moreover, the low thermal expansion and limited oxygen transmission rate permit their application in electronic devices, food packaging, and printing. Furthermore, the easy functionalization of CNFs also allows for their use in hydrophobic matrices with which they would have little affinity [10].
4. Bacterial Nanocellulose (BNC)
Nanocellulose produced by bacteria is called microbial cellulose or bacterial nanocellulose (BNC), and its purity is higher than other kinds of nanocellulose [22][23]. This type of nanostructured cellulose is produced by a bottom-up method consisting of assembling low-molecular-weight sugars by means of microorganisms; the single fibers that are achieved have a thickness of a few nanometers. Usually, two culture methods are used to produce BNC: static and stirred techniques; through the function of the chosen technique, it is possible to modify different characteristics, such as the surface morphology and physical and mechanical properties of the BNC and, therefore, to use it for several applications [2]. The main properties of BNC are its high crystallinity (80–90%), high purity, high flexibility, high water content (approximately 99% in the hydrogel structures inside the network of nanocellulose), and high mechanical and thermal resistance.
Moreover, due to its biocompatibility, bio-functionality, and nontoxicity, bacterial nanocellulose is a very promising material in the fields of medical implants and biotechnology, as well as for the applications of engineering tissues reconstruction or regenerative medicine. However, the principal challenge to be faced is the scale-up of industrial BNC production, which, today, is difficult to achieve due to the low productivity, highly expensive culture media, and high fermentation costs [13].
One way to overcome these problems can be the use of industrial wastes as extraction sources, which have a low cost and are readily available, and can also be processed with clean technology approaches, thus promoting not only a reduction in disposal costs of waste for the industries that supply the raw materials, but also the protection of the environment [24].
In conclusion, Table 1 shows the comparison between the different types of nanocellulose.
Table 1.
Comparison between the different types of nanocellulose
[8]
.
| Cellulose Nanocrystals (CNCs) | Cellulose Nanofibrils (CNFs) | Bacterial Nanocellulose (BNC) | |
|---|---|---|---|
| Images |  Transmission electron microscope (100 nm) |
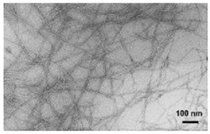 Transmission electron microscope (100 nm) |
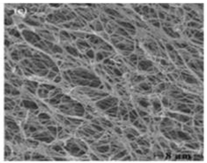 Scanning microscope (8 µm) |
| Synonyms | Crystallites, whiskers, nanowhiskers, cellulose nanocrystals, rod-like cellulose microcrystals | Microfibrillated cellulose, nanofibrils, microfibrils, cellulose, nanofibers | Biocellulose, bacterial cellulose, microbial cellulose |
| Common source | Wood, cotton, hemp, flax, rice straw, wheat straw, ramie, avicel, mcc, tunicin, algae, bacterial cellulose, pea hull fibers, branch-bark of mulberry, black spruce and eucalyptus, tunicate, valonia, kraft wood, pine and spruce, palm oil, pineapple leaf fibers, grass, swede root, chardonnay grape skins, coconut fibers, softwood wood flout, kenaf fibers, rice husk, sabdariffa fibers, wood fibers, corncob. | Pea hull, kenaf, hardwood and softwood pulp, cotton linter, cotton, cassava bagasse, sugarcane bagasse, cotton, algae (valonia), tunicate cellulose, bacterial cellulose, sugar beet pulp, wheat straw, date palm tree (rachis/leaflets), coconut husk fibers, recycled pulp, acacia pulp, banana, capimdourado, mulberry, flax, hemp, luffacylindrica, mengkuang leaves, curauna. | Low molecular weight sugar, alcohols, several bacteria species such as gluconacetobacter, agrobacterium, pseudomonas, rhizobium, and sarcin. |
| Formation process | Chemical or enzymatic treatment prior to delamination or wood pulp by mechanical pressure, acid hydrolysis | Acid hydrolysis, integration of mechanical shearing process via high pressure homogenization and enzymatic hydrolysis, TEMPO-mediated oxidation | Bacterial synthesis and cultivation in aqueous culture media present by glucose, oxygen and phosphate. |
| Average size | Diameter: 5–80 nm Length: 100–250 nm |
Diameter: 5–70 nm Length: 100–250 nm (from plant); 100 nm to several micrometer (from cellulose of tunicates, algae, bacteria) |
Diameter: 20–100 nm Length: 1.0–5.0 µm Different types of nanofibers networks |
| Tensile Strength | 2–6 GPa | 2–4 GPa | 200–300 MPa |
| Young’s modulus | 50–143 GPa | 15–150 GPa | 15–35 Pa |
| Crystallinity Index | 54–88% | <50% | >88% |
References
- Owoyokun, T.; Pérez Berumen, C.M.; Martínez Luévanos, A.; Cantú, L.; Ceniceros, A.C.L. Cellulose Nanocrystals: Obtaining and Sources of a Promising Bionanomaterial for Advanced Applications. Biointerface Res. Appl. Chem. 2020, 11, 11797–11816.
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent Advances in Nanocellulose-Based Different Biomaterials: Types, Properties, and Emerging Applications. J. Mater. Res. Technol. 2021, 14, 2601–2623.
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828.
- Huang, J.; Dufresne, A.; Lin, N. Nanocellulose from Fundamentals to Advanced Materials, 10th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; ISBN 978-3-527-34269-3.
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials Chemistry and the Futurist Eco-Friendly Applications of Nanocellulose: Status and Prospect. J. Saudi Chem. Soc. 2018, 22, 949–978.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392.
- Mishra, D.; Shanker, K.; Khare, P. Nanocellulose-Mediated Fabrication of Sustainable Future Materials. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Elsevier: Amsterdam, The Netherlands, 2020; pp. 217–236. ISBN 978-0-12-816789-2.
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N. Nanocellulose Reinforced Starch Polymer Composite: A Review of Preparation, Properties and Application. In Proceedings of the 5th International Conference on Applied Sciences and Engineering (ICASEA, 2018), Capthorne Hotel, Cameron Highlands, Malaysia, 7 April 2018; p. 17.
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage; Springer: Dordrecht, The Netherlands, 2015; ISBN 978-94-017-9302-5.
- Kumar, R.; Rai, B.; Gahlyan, S.; Kumar, G. A Comprehensive Review on Production, Surface Modification and Characterization of Nanocellulose Derived from Biomass and Its Commercial Applications. Express Polym. Lett. 2021, 15, 104–120.
- Wei, L.; Agarwal, U.P.; Hirth, K.C.; Matuana, L.M.; Sabo, R.C.; Stark, N.M. Chemical Modification of Nanocellulose with Canola Oil Fatty Acid Methyl Ester. Carbohydr. Polym. 2017, 169, 108–116.
- Kumar, R.; Kumari, S.; Rai, B.; Das, R.; Kumar, G. Effect of Nano-Cellulosic Fiber on Mechanical and Barrier Properties of Polylactic Acid (PLA) Green Nanocomposite Film. Mater. Res. Express 2019, 6, 125108.
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes 2021, 9, 1594.
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89.
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2020, 2, 1–8.
- Mali, P.; Sherje, A.P. Cellulose Nanocrystals: Fundamentals and Biomedical Applications. Carbohydr. Polym. 2022, 275, 118668.
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231.
- Farooq, A.; Jiang, S.; Farooq, A.; Naeem, M.A.; Ahmad, A.; Liu, L. Structure and Properties of High Quality Natural Cellulose Nano Fibrils from a Novel Material Ficus Natalensis Barkcloth. J. Ind. Text. 2019, 152808371988753.
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.D.L.T.M.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411.
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630.
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and Crop Wastes: A New Source for Nanocellulose Biorefinery. Ind. Crops Prod. 2016, 93, 26–38.
- Abdullah, N.A.; Rani, M.S.A.; Mohammad, M.; Sainorudin, M.H.; Asim, N.; Yaakob, Z.; Razali, H.; Emdadi, Z. Nanocellulose from Agricultural Waste as an Emerging Material for Nanotechnology Applications—An Overview. Polimery 2021, 66, 157–168.
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive Review on Nanocellulose: Recent Developments, Challenges and Future Prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884.
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of Bacterial Cellulose from Industrial Wastes: A Review. Cellulose 2019, 26, 2895–2911.
More
