Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by WANG HONG and Version 3 by Sirius Huang.
Polyester is a kind of polymer composed of ester bond-linked polybasic acids and polyol. This type of polymer has a wide range of applications in various industries, such as automotive, furniture, coatings, packaging, and biomedical. The traditional process of synthesizing polyester mainly uses metal catalyst polymerization under high-temperature. This condition may have problems with metal residue and undesired side reactions. As an alternative, enzyme-catalyzed polymerization is evolving rapidly due to the metal-free residue, satisfactory biocompatibility, and mild reaction conditions.
- polyester
- enzyme
- enzyme-catalyzed
- polymerization
1. Introduction
Polyester is a common polymer compound characterized by the existence of ester linkages in the chain’s primary structure, obtained by the gradual polycondensation of hydroxyl-containing compounds and carboxyl-containing compounds [1][2][1,2], which ranks fourth in nature after polysaccharides, proteins, and DNA. In recent years, a series of new polymeric materials have been developed rapidly due to their non-toxic and stable characteristics, for instance, polyester. Polyester is mainly divided into two categories of thermoplastic saturated polyester and thermosetting unsaturated polyester. Traditional saturated polyesters, which include polybutylene terephthalate (PBT) and polyethylene terephthalate (PET) that have high mechanical strength, abrasion resistance, corrosion resistance, and temperature resistance, as well as other outstanding properties, have been widely used as synthetic fibers and engineering plastics. With the “white pollution” intensified, polylactic acid (PLA), polybutylene succinate (PBS), and other bioplastics have been designed and manufactured [3][4][3,4]. These bioplastics have good biodegradability, biocompatibility, and non-toxicity characteristic, and they can be an alternative material to replace traditional plastics in medical and healthcare industry [5][6][5,6]. Meanwhile, thermosetting unsaturated polyester consists of an unsaturated group in addition to ester linkages in the main polymer chain, which is responsible for crosslinking process when a free radical initiator and crosslinker are added. Thermosetting polyester is an insoluble polymer with high rigidity, resilience, corrosion resistance, and weatherability, which has a wide range of applications in various industries as coatings and adhesive materials. At present, both types of polyester are mainly synthesized using the chemical polymerization method.
2. Current Polyester Synthesis Method and a Possible Alternative
The process of producing polyester via the chemical synthesis route requires a high temperature (above 180 °C) which may lead to excessive oxidation or even significantly damage the stability of product performance. Such high reaction temperatures would produce undesirable side reactions when using monomers with low thermal or chemical stability. As a result, this polymerization method restricts the types of polymers that can be formed [7]. Conventional methods for synthesizing aliphatic polyesters usually use alkoxy aluminum, zinc-like, and tin-like metal-containing oxides or organic compounds as catalysts [8][9][10][8,9,10]. This toxic metal catalyst normally might negatively affect the biosafety and biocompatibility of the end product if it cannot be removed after the process of making the polymer. Additionally, a significant quantity of organic solvents in the production process may cause environmental pollution (solution polymerization) if the management of the organic solvent is inappropriate. Generally, the hydrocarbon solvent is used to manufacture polyester, such as xylene or benzene, to avoid the viscosity buildup during the polymerization and to remove water produced from the esterification process via azeotropic distillation.
Polyesters can also be synthesized by enzyme catalysis. The Nobel Prize Winner Sumner proved in 1947 that enzymes are proteins with catalytic properties [11]. Some studies also discovered that enzymes have the advantages of high catalytic efficiency, mild reaction conditions, nontoxicity, and reusability [7][12][7,12]. In recent years, owing to the development of functional organic polymer materials and the apparent advantages of enzyme catalysts, enzyme-catalyzed synthesis of organic macromolecules has been actively researched, where enzyme-catalyzed polymerization is an essential route for polyester production [13].
There are many studies have been previously conducted to produce polyester using an enzymatic process due to several advantages, such as (i) enzymes are derivable from renewable resources; (ii) mild reaction conditions where thermal degradation of the raw materials or product can be avoided; (iii) no metal catalyst residue which makes the product achieve better biocompatibility; (iv) stereoselective, regioselective, and chemoselective, usually only a single substrate or a class of structurally similar substrates can participate in the reaction, retaining the active function of other groups of the monomer, avoiding unwanted side reactions [14]; and (v) biodegradable polyester can be synthesized using enzymes as a catalyst. As a comparison with chemical methods, enzyme-catalyzed polymerization of polyesters is a green process, which may be considered an alternative route for polyester synthesis. This process is seen as promising to resolve some issues caused by polyester synthesis, such as the disposal of unwanted side reactions as well as the metal catalyst residues. Additionally, the process may have added-value if the synthesis involves renewable resources and materials.
However, enzyme-catalyzed synthesis may have disadvantages such as high cost, large enzyme consumption, and a lesser number of high molecular weight polymers that can be produced [15]. With the development of the enzyme-catalyzed synthesis of polyesters, the challenges are believed to be addressed when more attempts are conducted. The current research on enzyme-catalyzed polymerization of polyesters mainly focuses on the types of enzymes and polymerization methods. This artexticle focuses on the main routes of enzyme-catalyzed polyester synthesis and the increasingly attractive lipase catalysts used for polymerization, summarizes the progress of enzyme-catalyzed polyester synthesis in different solvent systems, and discusses the development trend to provide a comprehensive reference for further research.
3. Enzyme-Catalyzed Synthesis of Polyesters
There are several types of reaction involved in the enzyme-catalyzed polyester mechanism, including enzyme-catalyzed ring-opening polymerization (eROP), enzyme-catalyzed polycondensation, and their combination.
3.1. Enzyme-Catalyzed Ring-Opening Polymerization
The eROP has been widely studied since the new type was first reported in 1993 [16][17][16,17]; some simple eROP are shown schematically in Figure 1. Cyclic esters (lactones) can be easily polymerized via eROP, and no alcohol or water is produced during the reaction. Thus, no by-product removal techniques need to be considered, and a high molecular weight of the product could be yielded [18][19][20][21][18,19,20,21]. Lars van der Mee et al. [22] achieved the polymerization of lactones with 6- to 13- and the 16-membered ring using Novozym 435 as catalyze via eROP. The number average molecular weight (Mn) of the synthesized polyesters ranged from 6600 g/mol to 23,600 g/mol. It was also found that Novozym 435 has a similar affinity for all lactones, and the polymerization rate of catalytic macrocyclic lactones is greater than that of small cyclic lactones. In addition, the eROP of large ring-size lactones can be catalyzed by lipases, which is difficult to achieve by metal catalysis methods [23]. Manzini et al. [24] successfully polymerized more than a dozen giant macrolides (minimum 12-membered ring, maximum 84-membered ring) via Candida antarctica lipase B (CALB)-catalyzed eROP at 70 °C, and products with higher molecular weights can be obtained with longer polymerization times. Furthermore, eROP allows for the copolymerization of two or more different cyclic monomers. Srivastava et al. [25] synthesized a high molecular weight copolymer of 1,5-dioxepan-2-one (DXO) and ε-caprolactone (CL) through Lipase CA-catalyzed eROP at 60 °C. Scanning electron microscopy (SEM) analysis showed that the scaffolds of the copolymer were highly porous and contained interconnected pores. The porous structure of the samples considerably altered the tensile and viscoelastic characteristics of the scaffolds which are free from toxic metallic residue. As known, polylactones are widely applied in the pharmaceutical, biomedical, and packaging sectors because of their excellent biodegradability and biocompatibility [26][27][28][26,27,28]. With the growing demand for environmentally friendly, safe, non-toxic, and subsequently, functionalized polylactones, the eROP of lactones is gaining attraction as an alternative method to traditional synthesis methods [29][30][31][32][29,30,31,32].
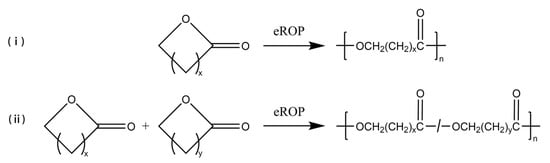
Figure 1. The eROP of (i) lactones, (ii) different lactones. x and y represent the number of carbon atoms in straight-chain or branched-chain, x, y > 0.
3.2. Enzyme-Catalyzed Polycondensation
In the early 1980s, the first enzyme-catalyzed polycondensation was reported [33][34][33,34], and ever since, lipases have become recognized as significant tools for polymer material production. Enzyme-catalyzed polycondensation plays a crucial role in polymerization as the monomers could have a wide range of selectivity, and the products could also have diversity [7], as schematized in Figure 2. Hydroxy acids, as simple monomers containing one hydroxyl group and one carboxyl group, can be polymerized to polyhydroxyalkanoates (PHAs) by enzyme-catalyzed polycondensation. Ohara et al. [35] studied the enzymatic polycondensation of lactic acid using Novozym 435, and the high-yield oligomeric PLA was obtained. The enzymatic polycondensation of diols and diacids is commonly studied, for example, Mahapatro A et al. [36] used CALB to catalyze the polycondensation of adipic acid and octanediol for 24 h at 70 °C, and the Mn of the product could reach 17,800 g/mol. Meanwhile, Mahapatro et al. [37] compared the enzyme-catalyzed polycondensation of various diols and various diacids in a study. The results showed that the long-chain sebacic acid with octanedioic acid could be reacted faster as compared to others. Meanwhile, adipic acid and octanedioic acid could produce a polymer with the highest molecular weight among them. Daniel et al. [38] synthesized linear polyesters of hexadecanedioic acid and octanediol using the Novozym 435 enzymatic polycondensation method to produce polyester films, which were found to have multivacant surfaces, exhibiting potential value in medical or pharmaceutical fields. The enzymatic polycondensation reaction also produces water or alcohols, and the lower boiling point alcohols are easy to be removed from the reaction system than water, thus driving the equilibrium toward catalysis, and a higher molecular weight product could be obtained [39]. Therefore, in order to make the enzymatic polycondensation reaction easier, diacid derivatives are often used as monomers [40][41][42][43][40,41,42,43]. Azim et al. [44] utilized lipase to catalyze the polycondensation of diethyl succinate and butanediol to produce PBS with a weight average molecular weight (Mw) of 38,000 g/mol after a two-step reaction at temperatures of 80 °C and 95 °C, respectively. Jiang et al. [45] utilized diethyl succinate and dimethyl itaconate as the diacid derivative monomers with 1,4-butanediol to synthesize poly(butylene succinate-co-itaconate) (PBSI) via Novozym 435 catalyzed enzymatic polycondensation under vacuum condition to eliminate the alcohol generated by the reaction. PBSI with an Mn reaching 13,288 g/mol with 90% yield was obtained, and the unsaturated group was well preserved. On the other hand, hyperbranched polyol polyester could be produced from polyol using the enzyme-catalyzed polycondensation method [46][47][48][46,47,48]. Hyperbranched polyol polyesters are elastomers mainly used to replace soft tissues, and glycerol polyesters are among the most studied polyesters [49][50][49,50]. Kulshrestha et al. [51] studied the enzyme-catalyzed polycondensation of glycerol, adipic acid, and 1,8-octanediol at different durations, ranging from 5 min to 42 h. The results indicated that a significant interaction between monomers was observed after 45 min. The results indicated that the growth of branched glycerol polyesters occurs after linear growth. Taresco et al. [52] studied the Novozym 435 catalyzed polycondensation of glycerol and divinyl adipate to produce polyglycerol adipate (PGA) at different temperatures. The results showed that the degree of branching of PGA increased from 5% to 30% when the temperature was increased from 50 °C to 70 °C. According to Giovanni B et al. [53] the degree of branching of poly (glycerol sebacate) (PGS) can be adjusted to 56% when the substrate ratio of sebacic acid/glycerol was increased to 1.5/1.0 in the CALB catalyzed enzymatic polycondensation reaction. Therefore, the degree of branching of PGS can be controlled by adjusting the substrate ratio to meet the needs of medical applications. Enzymatic polycondensation offers a greener way to synthesize polyesters from many bio-based monomers, which could reduce environmental and health impacts, and therefore, enzymatic polycondensation has received considerable attention in recent years [3][4][39][54][3,4,39,54].
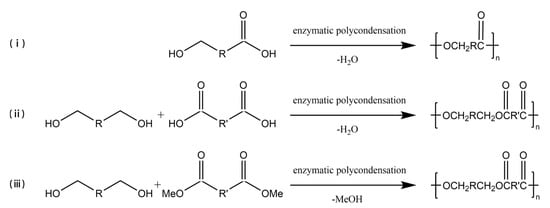
Figure 2.
The enzyme-catalyzed polycondensation of (
i
) hydroxyacids, (
ii
) diols and diacids, and (
iii
) diols and diacid derivatives.
3.3. Enzyme-Catalyzed Copolymerization
Enzyme-catalyzed copolymerization can be employed to prepare multi-block copolymers (Figure 3), which can change the properties of copolyesters in a wide range to obtain polymer materials for different applications. Kobayashi et al. [55] had a study to polymerize carboxylic anhydride monomer and ethylene glycol via enzyme-catalyzed copolymerization, and a high yield of polymer was obtained. In later studies, carboxylic anhydride monomers were expanded to include anhydrides of various diacids [56][57][56,57]. Jiang et al. [58] successfully used Novozym 435 to catalyze the enzyme-catalyzed copolymerization of ω-pentadecalactone (PDL), diethyl succinate, and 1,4-butanediol to synthesize a new ternary polyester. The Mn of the copolymer could be reached 24,400 g/mol, and the polymer was stable up to 300 °C with less than 0.1% weight loss.
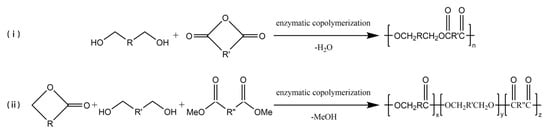
Figure 3.
The enzyme-catalyzed copolymerization of (
i
) diols and carboxylic anhydride (
ii
) lactones, diols, and diacid derivatives.
