TWIK-related acid-sensitive K+ (TASK) channels, including TASK-1, TASK-3, and TASK-5, are important members of the two-pore domain potassium (K2P) channel family. TASK-5 is not functionally expressed in the recombinant system. TASK channels are very sensitive to changes in extracellular pH and are active during all membrane potential periods. They are similar to other K2P channels in that they can create and use background-leaked potassium currents to stabilize resting membrane conductance and repolarize the action potential of excitable cells. TASK channels are expressed in both the nervous system and peripheral tissues, including excitable and non-excitable cells, and are widely engaged in pathophysiological phenomena, such as respiratory stimulation, pulmonary hypertension, arrhythmia, aldosterone secretion, cancers, anesthesia, neurological disorders, glucose homeostasis, and visual sensitivity. Therefore, they are important targets for innovative drug development.
- TASK channels
- biophysical properties
- gating profiles
- biological roles
- targeted compounds
1. Introduction
2. The Structure, Localization, and Electrophysiological Properties of TASK Channels
2.1. Structure
As the closest homologue, the crystal structure of the human TASK-1 (hTASK-1) channel has currently been resolved at a resolution of 3.0 Å using X-ray crystallography [101][17]. Similar to other members of the K2P family, TASK channels have two-fold symmetry, with each subunit containing four transmembrane α-helices (M1–M4), an inner pore region located between M1 and M2, an inner pore region located between M3 and M4, two pore helices (PH1 and PH2) suspended like cradles in their respective pores, and two selectivity filters (SF1 and SF2) shaped by four pore loops that interact with each other to determine the ionic selectivity of the channels [102][18] (Figure 1). The structures of TASK-1 channel are from protein data bank (PDB) and are generated by PyMOL 2.5.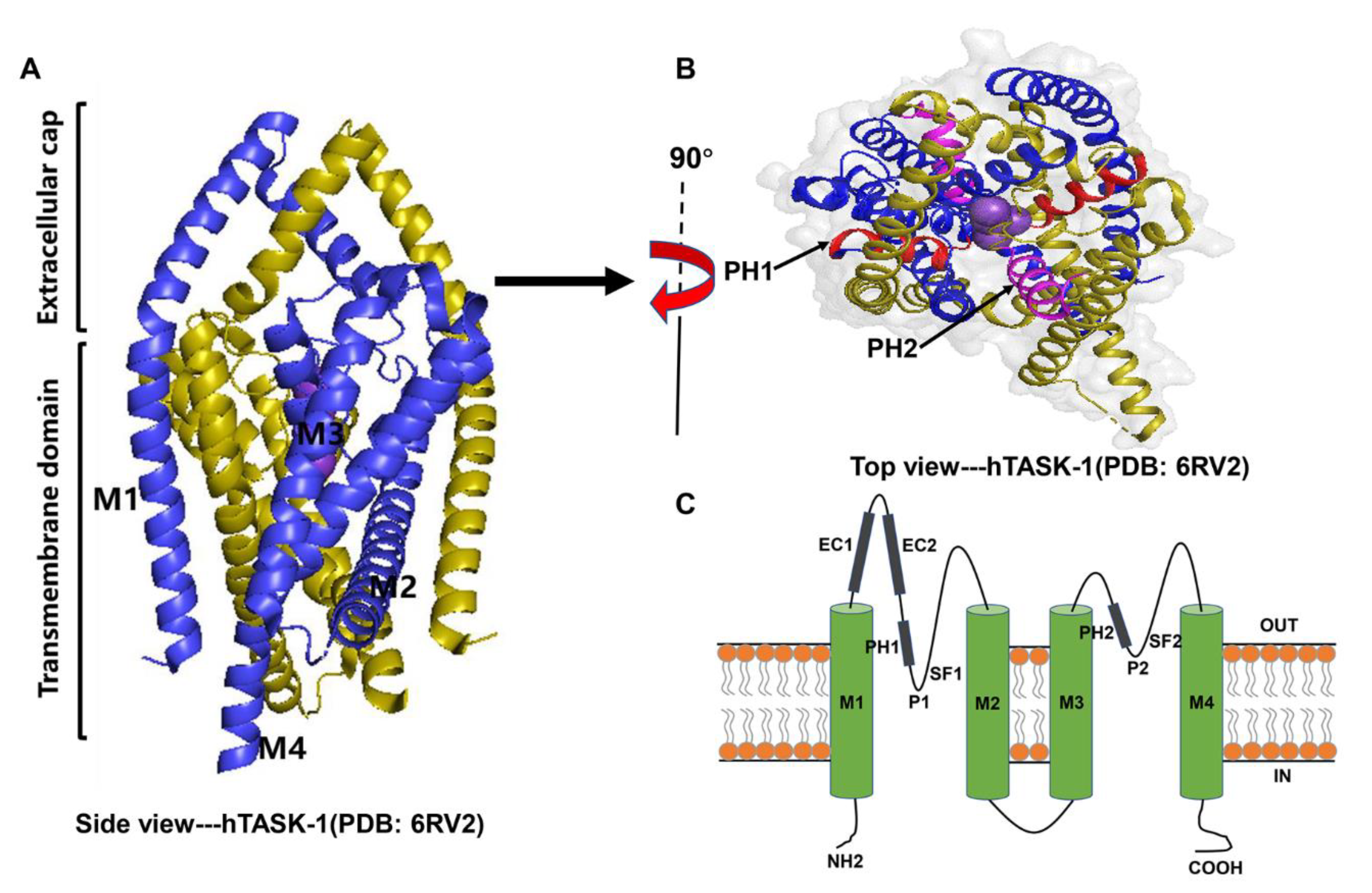
2.2. Expression and Localization
The TASK-1 channel is abundantly expressed in the human central nervous system (CNS) and in peripheral tissues. In the human CNS, the highest expression levels of TASK-1 are in the cerebellum, thalamus, and pituitary gland, whereas the lowest expression level is in the corpus callosum. In human peripheral tissues, TASK-1 expression level is the highest in the pancreas, placenta, lungs and pulmonary arteries [17,18][19][20]. It is lower in the prostate, stomach, small intestine, and heart and lowest in the liver, spleen, skeletal muscles, and testes [17][19]. In rats and mice, the highest levels of TASK-1 mRNA in the CNS were observed in the cerebellum and somatic motoneurons, while the lowest levels were observed in the septum and striatum [16,110][16][21]. In humans, the TASK-3 channel is predominantly found in the CNS and is expressed in almost all regions of the brain, with the highest expression in the cerebellum and higher expression in the cerebral cortex, thalamus, nucleus accumbens, hippocampus, and hypothalamus. Its expression is lowest in the spinal cord, caudate nucleus, and corpus callosum. In addition, small amounts are expressed in peripheral tissues, such as the stomach, testes, skeletal muscles, uterus, kidney, spleen, pancreas, prostate, and small intestine. Very low levels are expressed in the heart, liver, and lungs [17][19]. In rat tissues, TASK-3 mRNA is widely expressed in the brain (with the highest expression levels found in the cerebellum), kidney, liver, lungs, colon, stomach, spleen, testes, and skeletal muscles. Its expression level is lowest in the heart and small intestine [71,110,111][21][22][23]. In humans, the TASK-5 channel is highly expressed in the pancreas and is also relatively abundantly expressed in the liver, kidney, lungs, ovary, testes, and heart [13].2.3. Electrophysiological Properties
K2P channels are active across the entire physiological membrane potential range and generate background leaked potassium currents [112][24]. TASK channels are important members of the K2P channel family and have a current–voltage (I–V) relationship curve similar to the Goldman–Hodgkin–Katz equation at extra- and intra-cellularly different K+ concentrations, indicating that the generation of TASK channel currents correlates with K+ concentrations on both sides of the cell membrane. This also suggests that these channels show a high level of voltage-dependent gating despite the absence of a traditional voltage-activating domain because they have an ionic check valve located at the SF [113][25]. TASK channel currents are outwardly rectified and insensitive to potassium channel blockers, such as Ba2+, Cs+, TEA, and 4-AP [114][26]. TASK-1 and TASK-3 have different sensitivities to ruthenium red (RR or RuR), zinc, and anandamide. For instance, RR and zinc selectively block the TASK-3 channel but not the TASK-1 channel [115[27][28],116], whereas anandamide has a more potent blocking effect on TASK-1 than the TASK-3 channel [117][29]. In addition, TASK-1 and TASK-3 channel proteins can form functional heterodimers and are insensitive to mechanical forces [71,118][22][30]. The second messenger diacylglycerol can also inhibit TASK channels by activating G protein-coupled receptors [119,120][31][32]. TASK channels are sensitive to changes in extracellular pH but not intracellular pH, and can be blocked by external protons [121][33]. The histidine residue at position 98, which is near SF1, and the aspartic acid residue at position 204, which is near SF2, are the key sites for sensing extracellular pH variations [104,122][34][35]. Extracellular membrane acidification significantly inhibits TASK channels, whereas alkalization weakly activates them (Figure 2). Variations in extracellular pH also affect the ion selectivity of TASK channels, with TASK-1, TASK-3, and TWIK-1 channels becoming less permeable to potassium ions and more permeable to sodium ions when the extracellular solution becomes acidified [123,124,125][36][37][38].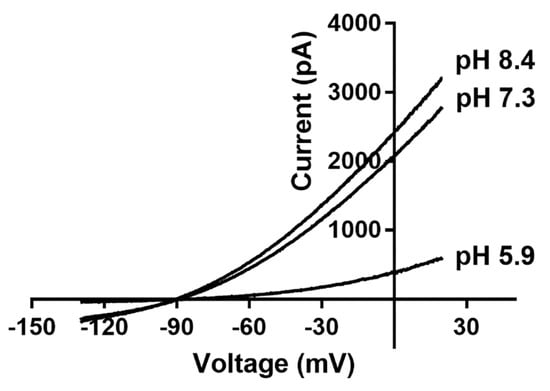
3. The Biological Roles of TASK Channels
3.1. Breathing Rhythm
3.2. Pulmonary Artery Hypertension
3.3. Cardiac Arrhythmia
3.4. Aldosterone Secretion
3.5. Pain
3.6. Anesthetics
3.6.1. Volatile Anesthetics
3.7. Cancers
3.8. Neurological Activities and/or Disorders
3.8.1. Sleep
3.9. Other Roles
References
- Sepúlveda, F.V.; Pablo Cid, L.; Teulon, J.; Niemeyer, M.I. Molecular aspects of structure, gating, and physiology of pH-sensitive background K2P and Kir K+-transport channels. Physiol. Rev. 2015, 95, 179–217.
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. CMLS 2015, 72, 3677–3693.
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ channels: Function-structural overview. Compr. Physiol. 2012, 2, 2087–2149.
- Kim, D.M.; Nimigean, C.M. Voltage-Gated Potassium Channels: A Structural Examination of Selectivity and Gating. Cold Spring Harb. Perspect. Biol. 2016, 8, a029231.
- Trombetta-Lima, M.; Krabbendam, I.E.; Dolga, A.M. Calcium-activated potassium channels: Implications for aging and age-related neurodegeneration. Int. J. Biochem. Cell Biol. 2020, 123, 105748.
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366.
- Lesage, F.; Lazdunski, M. Molecular and functional properties of two-pore-domain potassium channels. Am. J. Physiol. Renal Physiol. 2000, 279, F793–F801.
- Goldstein, S.A.; Bockenhauer, D.; O’Kelly, I.; Zilberberg, N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat. Rev. Neurosci. 2001, 2, 175–184.
- Huang, L.; Xu, G.; Jiang, R.; Luo, Y.; Zuo, Y.; Liu, J. Development of Non-opioid Analgesics Targeting Two-pore Domain Potassium Channels. Curr. Neuropharmacol. 2022, 20, 16–26.
- Lesage, F.; Guillemare, E.; Fink, M.; Duprat, F.; Lazdunski, M.; Romey, G.; Barhanin, J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996, 15, 1004–1011.
- Duprat, F.; Lesage, F.; Fink, M.; Reyes, R.; Heurteaux, C.; Lazdunski, M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997, 16, 5464–5471.
- Rajan, S.; Wischmeyer, E.; Xin Liu, G.; Preisig-Müller, R.; Daut, J.; Karschin, A.; Derst, C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J. Biol. Chem. 2000, 275, 16650–16657.
- Ashmole, I.; Goodwin, P.A.; Stanfield, P.R. TASK-5, a novel member of the tandem pore K+ channel family. Pflugers Arch. 2001, 442, 828–833.
- Brohawn, S.G.; del Mármol, J.; MacKinnon, R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 2012, 335, 436–441.
- Cotten, J.F. TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth. Analg. 2013, 116, 810–816.
- Talley, E.M.; Lei, Q.; Sirois, J.E.; Bayliss, D.A. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 2000, 25, 399–410.
- Rödström, K.E.J.; Kiper, A.K.; Zhang, W.; Rinné, S.; Pike, A.C.W.; Goldstein, M.; Conrad, L.J.; Delbeck, M.; Hahn, M.G.; Meier, H.; et al. A lower X-gate in TASK channels traps inhibitors within the vestibule. Nature 2020, 582, 443–447.
- Enyedi, P.; Czirják, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605.
- Medhurst, A.D.; Rennie, G.; Chapman, C.G.; Meadows, H.; Duckworth, M.D.; Kelsell, R.E.; Gloger, I.I.; Pangalos, M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res. Mol. Brain Res. 2001, 86, 101–114.
- Olschewski, A.; Veale, E.L.; Nagy, B.M.; Nagaraj, C.; Kwapiszewska, G.; Antigny, F.; Lambert, M.; Humbert, M.; Czirják, G.; Enyedi, P.; et al. TASK-1 (KCNK3) channels in the lung: From cell biology to clinical implications. Eur. Respir. J. 2017, 50, 1700754.
- Talley, E.M.; Solorzano, G.; Lei, Q.; Kim, D.; Bayliss, D.A. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci. 2001, 21, 7491–7505.
- Kim, Y.; Bang, H.; Kim, D. TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem. 2000, 275, 9340–9347.
- Rusznák, Z.; Pocsai, K.; Kovács, I.; Pór, A.; Pál, B.; Bíró, T.; Szücs, G. Differential distribution of TASK-1, TASK-2 and TASK-3 immunoreactivities in the rat and human cerebellum. Cell. Mol. Life Sci. 2004, 61, 1532–1542.
- Zilberberg, N.; Ilan, N.; Goldstein, S.A. KCNKØ: Opening and closing the 2-P-domain potassium leak channel entails “C-type” gating of the outer pore. Neuron 2001, 32, 635–648.
- Schewe, M.; Nematian-Ardestani, E.; Sun, H.; Musinszki, M.; Cordeiro, S.; Bucci, G.; de Groot, B.L.; Tucker, S.J.; Rapedius, M.; Baukrowitz, T. A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K+ Channels. Cell 2016, 164, 937–949.
- O’Connell, A.D.; Morton, M.J.; Sivaprasadarao, A.; Hunter, M. Selectivity and interactions of Ba2+ and Cs+ with wild-type and mutant TASK1 K+ channels expressed in Xenopus oocytes. J. Physiol. 2005, 562 Pt 3, 687–696.
- Wang, X.; Guan, R.; Zhao, X.; Zhu, D.; Song, N.; Shen, L. TASK1 and TASK3 Are Coexpressed With ASIC1 in the Ventrolateral Medulla and Contribute to Central Chemoreception in Rats. Front. Cell. Neurosci. 2018, 12, 285.
- Clarke, C.E.; Veale, E.L.; Green, P.J.; Meadows, H.J.; Mathie, A. Selective block of the human 2-P domain potassium channel, TASK-3, and the native leak potassium current, IKSO, by zinc. J. Physiol. 2004, 560 Pt 1, 51–62.
- Maingret, F.; Patel, A.J.; Lazdunski, M.; Honoré, E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001, 20, 47–54.
- Czirják, G.; Enyedi, P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J. Biol. Chem. 2002, 277, 5426–5432.
- Wilke, B.U.; Lindner, M.; Greifenberg, L.; Albus, A.; Kronimus, Y.; Bünemann, M.; Leitner, M.G.; Oliver, D. Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat. Commun. 2014, 5, 5540.
- Bista, P.; Pawlowski, M.; Cerina, M.; Ehling, P.; Leist, M.; Meuth, P.; Aissaoui, A.; Borsotto, M.; Heurteaux, C.; Decher, N.; et al. Differential phospholipase C-dependent modulation of TASK and TREK two-pore domain K+ channels in rat thalamocortical relay neurons. J. Physiol. 2015, 593, 127–144.
- Lesage, F.; Barhanin, J. Molecular physiology of pH-sensitive background K2P channels. Physiology (Bethesda) 2011, 26, 424–437.
- González, W.; Zúñiga, L.; Cid, L.P.; Arévalo, B.; Niemeyer, M.I.; Sepúlveda, F.V. An extracellular ion pathway plays a central role in the cooperative gating of a K2P K+ channel by extracellular pH. J. Biol. Chem. 2013, 288, 5984–5991.
- Yuill, K.; Ashmole, I.; Stanfield, P.R. The selectivity filter of the tandem pore potassium channel TASK-1 and its pH-sensitivity and ionic selectivity. Pflugers Arch. 2004, 448, 63–69.
- Chen, H.; Chatelain, F.C.; Lesage, F. Altered and dynamic ion selectivity of K+ channels in cell development and excitability. Trends Pharmacol. Sci. 2014, 35, 461–469.
- Ma, L.; Zhang, X.; Zhou, M.; Chen, H. Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J. Biol. Chem. 2012, 287, 37145–37153.
- Chatelain, F.C.; Bichet, D.; Douguet, D.; Feliciangeli, S.; Bendahhou, S.; Reichold, M.; Warth, R.; Barhanin, J.; Lesage, F. TWIK1, a unique background channel with variable ion selectivity. Proc. Natl. Acad. Sci. USA 2012, 109, 5499–5504.
- Koizumi, H.; Smerin, S.E.; Yamanishi, T.; Moorjani, B.R.; Zhang, R.; Smith, J.C. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J. Neurosci. 2010, 30, 4273–4284.
- Bayliss, D.A.; Barhanin, J.; Gestreau, C.; Guyenet, P.G. The role of pH-sensitive TASK channels in central respiratory chemoreception. Pflugers Arch. 2015, 467, 917–929.
- Buckler, K.J. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflügers Arch.-Eur. J. Physiol. 2015, 467, 1013–1025.
- Cunningham, K.P.; MacIntyre, D.E.; Mathie, A.; Veale, E.L. Effects of the ventilatory stimulant, doxapram on human TASK-3 (KCNK9, K2P9.1) channels and TASK-1 (KCNK3, K2P3.1) channels. Acta Physiol. 2020, 228, e13361.
- Kim, D.; Cavanaugh, E.J.; Kim, I.; Carroll, J.L. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J. Physiol. 2009, 587 Pt 12, 2963–2975.
- Trapp, S.; Aller, M.I.; Wisden, W.; Gourine, A.V. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J. Neurosci. 2008, 28, 8844–8850.
- Mulkey, D.K.; Talley, E.M.; Stornetta, R.L.; Siegel, A.R.; West, G.H.; Chen, X.; Sen, N.; Mistry, A.M.; Guyenet, P.G.; Bayliss, D.A. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J. Neurosci. 2007, 27, 14049–14058.
- Li, B.; Rietmeijer, R.A.; Brohawn, S.G. Structural basis for pH gating of the two-pore domain K+ channel TASK2. Nature 2020, 586, 457–462.
- Guyenet, P.G.; Bayliss, D.A.; Stornetta, R.L.; Ludwig, M.G.; Kumar, N.N.; Shi, Y.; Burke, P.G.; Kanbar, R.; Basting, T.M.; Holloway, B.B.; et al. Proton detection and breathing regulation by the retrotrapezoid nucleus. J. Physiol. 2016, 594, 1529–1551.
- Hayabuchi, Y. The Action of Smooth Muscle Cell Potassium Channels in the Pathology of Pulmonary Arterial Hypertension. Pediatr. Cardiol. 2017, 38, 1–14.
- Le Ribeuz, H.; Capuano, V.; Girerd, B.; Humbert, M.; Montani, D.; Antigny, F. Implication of Potassium Channels in the Pathophysiology of Pulmonary Arterial Hypertension. Biomolecules 2020, 10, 1261.
- Southgate, L.; Machado, R.D.; Gräf, S.; Morrell, N.W. Molecular genetic framework underlying pulmonary arterial hypertension. Nat. Rev. Cardiol. 2019, 17, 85–95.
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fundam. Clin. Pharmacol. 2019, 33, 504–523.
- Tennant, B.P.; Cui, Y.; Tinker, A.; Clapp, L.H. Functional expression of inward rectifier potassium channels in cultured human pulmonary smooth muscle cells: Evidence for a major role of Kir2.4 subunits. J. Membr. Biol. 2006, 213, 19–29.
- Olschewski, A.; Li, Y.; Tang, B.; Hanze, J.; Eul, B.; Bohle, R.M.; Wilhelm, J.; Morty, R.E.; Brau, M.E.; Weir, E.K.; et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ. Res. 2006, 98, 1072–1080.
- Wiedmann, F.; Frey, N.; Schmidt, C. Two-Pore-Domain Potassium (K2P-) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes. Cells 2021, 10, 2914.
- Rinné, S.; Kiper, A.K.; Schlichthörl, G.; Dittmann, S.; Netter, M.F.; Limberg, S.H.; Silbernagel, N.; Zuzarte, M.; Moosdorf, R.; Wulf, H.; et al. TASK-1 and TASK-3 may form heterodimers in human atrial cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 81, 71–80.
- Staudacher, K.; Staudacher, I.; Ficker, E.; Seyler, C.; Gierten, J.; Kisselbach, J.; Rahm, A.K.; Trappe, K.; Schweizer, P.A.; Becker, R.; et al. Carvedilol targets human K2P 3.1 (TASK1) K+ leak channels. Br. J. Pharmacol. 2011, 163, 1099–1110.
- Besana, A.; Barbuti, A.; Tateyama, M.A.; Symes, A.J.; Robinson, R.B.; Feinmark, S.J. Activation of protein kinase C epsilon inhibits the two-pore domain K+ channel, TASK-1, inducing repolarization abnormalities in cardiac ventricular myocytes. J. Biol. Chem. 2004, 279, 33154–33160.
- Gurney, A.; Manoury, B. Two-pore potassium channels in the cardiovascular system. Eur. Biophys. J. 2009, 38, 305–318.
- Liang, B.; Soka, M.; Christensen, A.H.; Olesen, M.S.; Larsen, A.P.; Knop, F.K.; Wang, F.; Nielsen, J.B.; Andersen, M.N.; Humphreys, D.; et al. Genetic variation in the two-pore domain potassium channel, TASK-1, may contribute to an atrial substrate for arrhythmogenesis. J. Mol. Cell. Cardiol. 2014, 67, 69–76.
- Donner, B.C.; Schullenberg, M.; Geduldig, N.; Hüning, A.; Mersmann, J.; Zacharowski, K.; Kovacevic, A.; Decking, U.; Aller, M.I.; Schmidt, K.G. Functional role of TASK-1 in the heart: Studies in TASK-1-deficient mice show prolonged cardiac repolarization and reduced heart rate variability. Basic Res. Cardiol. 2011, 106, 75–87.
- Putzke, C.; Wemhöner, K.; Sachse, F.B.; Rinné, S.; Schlichthörl, G.; Li, X.T.; Jaé, L.; Eckhardt, I.; Wischmeyer, E.; Wulf, H.; et al. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc. Res. 2007, 75, 59–68.
- Gierten, J.; Ficker, E.; Bloehs, R.; Schweizer, P.A.; Zitron, E.; Scholz, E.; Karle, C.; Katus, H.A.; Thomas, D. The human cardiac K2P3.1 (TASK-1) potassium leak channel is a molecular target for the class III antiarrhythmic drug amiodarone. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 261–270.
- Kraft, M.; Büscher, A.; Wiedmann, F.; L’Hoste, Y.; Haefeli, W.E.; Frey, N.; Katus, H.A.; Schmidt, C. Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option. Front. Pharmacol. 2021, 12, 638445.
- Davies, L.A.; Hu, C.; Guagliardo, N.A.; Sen, N.; Chen, X.; Talley, E.M.; Carey, R.M.; Bayliss, D.A.; Barrett, P.Q. TASK channel deletion in mice causes primary hyperaldosteronism. Proc. Natl. Acad. Sci. USA 2008, 105, 2203–2208.
- Manichaikul, A.; Rich, S.S.; Allison, M.A.; Guagliardo, N.A.; Bayliss, D.A.; Carey, R.M.; Barrett, P.Q. KCNK3 Variants Are Associated With Hyperaldosteronism and Hypertension. Hypertension 2016, 68, 356–364.
- Guagliardo, N.A.; Yao, J.; Stipes, E.J.; Cechova, S.; Le, T.H.; Bayliss, D.A.; Breault, D.T.; Barrett, P.Q. Adrenal Tissue-Specific Deletion of TASK Channels Causes Aldosterone-Driven Angiotensin II-Independent Hypertension. Hypertension 2019, 73, 407–414.
- Penton, D.; Bandulik, S.; Schweda, F.; Haubs, S.; Tauber, P.; Reichold, M.; Cong, L.D.; El Wakil, A.; Budde, T.; Lesage, F.; et al. Task3 potassium channel gene invalidation causes low renin and salt-sensitive arterial hypertension. Endocrinology 2012, 153, 4740–4748.
- Chen, A.X.; Nishimoto, K.; Nanba, K.; Rainey, W.E. Potassium channels related to primary aldosteronism: Expression similarities and differences between human and rat adrenals. Mol. Cell. Endocrinol. 2015, 417, 141–148.
- Barrett, P.Q.; Guagliardo, N.A.; Bayliss, D.A. Ion Channel Function and Electrical Excitability in the Zona Glomerulosa: A Network Perspective on Aldosterone Regulation. Annu. Rev. Physiol. 2021, 83, 451–475.
- Meadows, H.J.; Randall, A.D. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology 2001, 40, 551–559.
- Reeh, P.W.; Kress, M. Molecular physiology of proton transduction in nociceptors. Curr. Opin. Pharmacol. 2001, 1, 45–51.
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177.
- Plant, L.D. A Role for K2P Channels in the Operation of Somatosensory Nociceptors. Front. Mol. Neurosci. 2012, 5, 21.
- Sun, W.H.; Chen, C.C. Roles of Proton-Sensing Receptors in the Transition from Acute to Chronic Pain. J. Dent. Res. 2016, 95, 135–142.
- Li, X.Y.; Toyoda, H. Role of leak potassium channels in pain signaling. Brain Res. Bull. 2015, 119 Pt A, 73–79.
- Gada, K.; Plant, L.D. Two-pore domain potassium channels: Emerging targets for novel analgesic drugs: IUPHAR Review 26. Br. J. Pharmacol. 2019, 176, 256–266.
- Ren, W.J.; Ulrich, H.; Semyanov, A.; Illes, P.; Tang, Y. TASK-3: New Target for Pain-Relief. Neurosci. Bull. 2020, 36, 951–954.
- Marsh, B.; Acosta, C.; Djouhri, L.; Lawson, S.N. Leak K⁺ channel mRNAs in dorsal root ganglia: Relation to inflammation and spontaneous pain behaviour. Mol. Cell. Neurosci. 2012, 49, 375–386.
- Pollema-Mays, S.L.; Centeno, M.V.; Ashford, C.J.; Apkarian, A.V.; Martina, M. Expression of background potassium channels in rat DRG is cell-specific and down-regulated in a neuropathic pain model. Mol. Cell. Neurosci. 2013, 57, 1–9.
- Morenilla-Palao, C.; Luis, E.; Fernández-Peña, C.; Quintero, E.; Weaver, J.L.; Bayliss, D.A.; Viana, F. Ion channel profile of TRPM8 cold receptors reveals a role of TASK-3 potassium channels in thermosensation. Cell Rep. 2014, 8, 1571–1582.
- Liao, P.; Qiu, Y.; Mo, Y.; Fu, J.; Song, Z.; Huang, L.; Bai, S.; Wang, Y.; Zhu, J.J.; Tian, F.; et al. Selective activation of TWIK-related acid-sensitive K+ 3 subunit-containing channels is analgesic in rodent models. Sci. Transl. Med. 2019, 11, eaaw8434.
- García, G.; Noriega-Navarro, R.; Martínez-Rojas, V.A.; Gutiérrez-Lara, E.J.; Oviedo, N.; Murbartián, J. Spinal TASK-1 and TASK-3 modulate inflammatory and neuropathic pain. Eur. J. Pharmacol. 2019, 862, 172631.
- Patel, A.J.; Honoré, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999, 2, 422–426.
- Sirois, J.E.; Lynch, C., 3rd; Bayliss, D.A. Convergent and reciprocal modulation of a leak K+ current and I(h) by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones. J. Physiol. 2002, 541 Pt 3, 717–729.
- Meuth, S.G.; Budde, T.; Kanyshkova, T.; Broicher, T.; Munsch, T.; Pape, H.C. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J. Neurosci. 2003, 23, 6460–6469.
- Lazarenko, R.M.; Willcox, S.C.; Shu, S.; Berg, A.P.; Jevtovic-Todorovic, V.; Talley, E.M.; Chen, X.; Bayliss, D.A. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J. Neurosci. 2010, 30, 7691–7704.
- Berg, A.P.; Talley, E.M.; Manger, J.P.; Bayliss, D.A. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J. Neurosci. 2004, 24, 6693–6702.
- Conway, K.E.; Cotten, J.F. Covalent modification of a volatile anesthetic regulatory site activates TASK-3 (KCNK9) tandem-pore potassium channels. Mol. Pharmacol. 2012, 81, 393–400.
- Talley, E.M.; Bayliss, D.A. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: Volatile anesthetics and neurotransmitters share a molecular site of action. J. Biol. Chem. 2002, 277, 17733–17742.
- Buljubasic, N.; Rusch, N.J.; Marijic, J.; Kampine, J.P.; Bosnjak, Z.J. Effects of halothane and isoflurane on calcium and potassium channel currents in canine coronary arterial cells. Anesthesiology 1992, 76, 990–998.
- Mu, D.; Chen, L.; Zhang, X.; See, L.H.; Koch, C.M.; Yen, C.; Tong, J.J.; Spiegel, L.; Nguyen, K.C.; Servoss, A.; et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell 2003, 3, 297–302.
- Pei, L.; Wiser, O.; Slavin, A.; Mu, D.; Powers, S.; Jan, L.Y.; Hoey, T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc. Natl. Acad. Sci. USA 2003, 100, 7803–7807.
- Zúñiga, R.; Valenzuela, C.; Concha, G.; Brown, N.; Zúñiga, L. TASK-3 Downregulation Triggers Cellular Senescence and Growth Inhibition in Breast Cancer Cell Lines. Int. J. Mol. Sci 2018, 19, 1033.
- Zúñiga, R.; Concha, G.; Cayo, A.; Cikutović-Molina, R.; Arevalo, B.; González, W.; Catalán, M.A.; Zúñiga, L. Withaferin A suppresses breast cancer cell proliferation by inhibition of the two-pore domain potassium (K2P9) channel TASK-3. Biomed. Pharmacother. 2020, 129, 110383.
- Innamaa, A.; Jackson, L.; Asher, V.; Van Shalkwyk, G.; Warren, A.; Hay, D.; Bali, A.; Sowter, H.; Khan, R. Expression and prognostic significance of the oncogenic K2P potassium channel KCNK9 (TASK-3) in ovarian carcinoma. Anticancer Res. 2013, 33, 1401–1408.
- Rusznák, Z.; Bakondi, G.; Kosztka, L.; Pocsai, K.; Dienes, B.; Fodor, J.; Telek, A.; Gönczi, M.; Szucs, G.; Csernoch, L. Mitochondrial expression of the two-pore domain TASK-3 channels in malignantly transformed and non-malignant human cells. Virchows Arch. 2008, 452, 415–426.
- Nagy, D.; Gönczi, M.; Dienes, B.; Szöőr, Á.; Fodor, J.; Nagy, Z.; Tóth, A.; Fodor, T.; Bai, P.; Szücs, G.; et al. Silencing the KCNK9 potassium channel (TASK-3) gene disturbs mitochondrial function, causes mitochondrial depolarization, and induces apoptosis of human melanoma cells. Arch. Dermatol. Res. 2014, 306, 885–902.
- Bachmann, M.; Rossa, A.; Antoniazzi, G.; Biasutto, L.; Carrer, A.; Campagnaro, M.; Leanza, L.; Gonczi, M.; Csernoch, L.; Paradisi, C.; et al. Synthesis and cellular effects of a mitochondria-targeted inhibitor of the two-pore potassium channel TASK-3. Pharmacol. Res. 2021, 164, 105326.
- Wrzosek, A.; Gałecka, S.; Żochowska, M.; Olszewska, A.; Kulawiak, B. Alternative Targets for Modulators of Mitochondrial Potassium Channels. Molecules 2022, 27, 299.
- Yao, J.; McHedlishvili, D.; McIntire, W.E.; Guagliardo, N.A.; Erisir, A.; Coburn, C.A.; Santarelli, V.P.; Bayliss, D.A.; Barrett, P.Q. Functional TASK-3-Like Channels in Mitochondria of Aldosterone-Producing Zona Glomerulosa Cells. Hypertension 2017, 70, 347–356.
- Cikutović-Molina, R.; Herrada, A.A.; González, W.; Brown, N.; Zúñiga, L. TASK-3 Gene Knockdown Dampens Invasion and Migration and Promotes Apoptosis in KATO III and MKN-45 Human Gastric Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 6077.
- Sun, H.; Luo, L.; Lal, B.; Ma, X.; Chen, L.; Hann, C.L.; Fulton, A.M.; Leahy, D.J.; Laterra, J.; Li, M. A monoclonal antibody against KCNK9 K+ channel extracellular domain inhibits tumour growth and metastasis. Nat. Commun. 2016, 7, 10339.
- Bedoya, M.; Rinné, S.; Kiper, A.K.; Decher, N.; González, W.; Ramírez, D. TASK Channels Pharmacology: New Challenges in Drug Design. J. Med. Chem. 2019, 62, 10044–10058.
- Bista, P.; Cerina, M.; Ehling, P.; Leist, M.; Pape, H.C.; Meuth, S.G.; Budde, T. The role of two-pore-domain background K+ (K2P) channels in the thalamus. Pflugers Arch. 2015, 467, 895–905.
- Meuth, S.G.; Aller, M.I.; Munsch, T.; Schuhmacher, T.; Seidenbecher, T.; Meuth, P.; Kleinschnitz, C.; Pape, H.C.; Wiendl, H.; Wisden, W.; et al. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol. Pharmacol. 2006, 69, 1468–1476.
- Linden, A.M.; Sandu, C.; Aller, M.I.; Vekovischeva, O.Y.; Rosenberg, P.H.; Wisden, W.; Korpi, E.R. TASK-3 knockout mice exhibit exaggerated nocturnal activity, impairments in cognitive functions, and reduced sensitivity to inhalation anesthetics. J. Pharmacol. Exp. Ther. 2007, 323, 924–934.
- Pang, D.S.; Robledo, C.J.; Carr, D.R.; Gent, T.C.; Vyssotski, A.L.; Caley, A.; Zecharia, A.Y.; Wisden, W.; Brickley, S.G.; Franks, N.P. An unexpected role for TASK-3 potassium channels in network oscillations with implications for sleep mechanisms and anesthetic action. Proc. Natl. Acad. Sci. USA 2009, 106, 17546–17551.
- Gotter, A.L.; Santarelli, V.P.; Doran, S.M.; Tannenbaum, P.L.; Kraus, R.L.; Rosahl, T.W.; Meziane, H.; Montial, M.; Reiss, D.R.; Wessner, K.; et al. TASK-3 as a potential antidepressant target. Brain Res. 2011, 1416, 69–79.
- Dadi, P.K.; Vierra, N.C.; Jacobson, D.A. Pancreatic β-cell-specific ablation of TASK-1 channels augments glucose-stimulated calcium entry and insulin secretion, improving glucose tolerance. Endocrinology 2014, 155, 3757–3768.
- Dadi, P.K.; Luo, B.; Vierra, N.C.; Jacobson, D.A. TASK-1 Potassium Channels Limit Pancreatic α-Cell Calcium Influx and Glucagon Secretion. Mol. Endocrinol. 2015, 29, 777–787.
- Wen, X.; Liao, P.; Luo, Y.; Yang, L.; Yang, H.; Liu, L.; Jiang, R. Tandem pore domain acid-sensitive K channel 3 (TASK-3) regulates visual sensitivity in healthy and aging retina. Sci. Adv. 2022, 8, eabn8785.
