Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Camila Xu.
Stannous tungstate (SnWO4), which is a potential catalyst due to its narrow band gap, suitable band position, and nontoxicity.
- stannous tungstate
- photocatalysis
- photoelectrocatalysis
1. Introduction
With the rapid development of technology, energy has become a necessity for social operation, and the human consumption of non-renewable traditional energy (from sources such as oil and coal) is decreasing. The energy crisis has become an urgent problem that needs to be solved. Therefore, researchers are enthusiastically looking for alternative renewable clean energy sources. Among the many potential energy sources, solar energy has attracted significant attention due to its large reserves and reproducibility. Although solar energy exhibits a variety of excellent properties, its dispersion, instability and intermittency have led to its low utilization. Therefore, exploring efficient ways to better use solar energy is necessary for the generation of alternative power. Photocatalytic and photoelectrochemical methods show promise for degrading pollutants and generating new kinds of energy (such as hydrogen and chemical) via the use of solar energy. All the relevant reactions are completed by the photo-generated holes and electrons; thus, they follow a similar principle. Since the first report of TiO2 in 1972 [1], different kinds of materials with a smaller band gap than that of TiO2 (3.0–3.2 eV) have been explored [2].
One of these materials is stannous tungstate (SnWO4), which is a potential catalyst due to its narrow band gap, suitable band position, and nontoxicity [3]. However, due to its low charge separation efficiency and poor stability [4], its photocatalytic and photoelectrochemical performance has not met theoretical expectations. To solve this problem, many researchers are committed to obtaining a better catalytic performance and higher solar energy efficiency by means of morphology control, doping, and multi-component compositing.
 Generally speaking, SnWO4 has good optical properties, such as a narrow band gap and a special band structure, which results in excellent application prospects for the fields of photocatalysis and photoelectrocatalysis (especially in the field of water splitting). However, it is necessary to pay attention to the instability and short carrier diffusion length of the material itself. Furthermore, because of the differences in crystal structure and band structure between the two crystal phases (α phase and β phase), their properties and synthesis methods are different. For instance, the photo-generated electrons (holes) of the β phase have more significant reduction (oxidation) activity than that of the α phase, so the β phase is suitable for photocatalytic degradation. The α phase has a wider light absorption range and higher carrier separation efficiency, so it has potential for use in photoelectrocatalysis.
Generally speaking, SnWO4 has good optical properties, such as a narrow band gap and a special band structure, which results in excellent application prospects for the fields of photocatalysis and photoelectrocatalysis (especially in the field of water splitting). However, it is necessary to pay attention to the instability and short carrier diffusion length of the material itself. Furthermore, because of the differences in crystal structure and band structure between the two crystal phases (α phase and β phase), their properties and synthesis methods are different. For instance, the photo-generated electrons (holes) of the β phase have more significant reduction (oxidation) activity than that of the α phase, so the β phase is suitable for photocatalytic degradation. The α phase has a wider light absorption range and higher carrier separation efficiency, so it has potential for use in photoelectrocatalysis.
2. Structure, Properties, and Synthesis of SnWO4
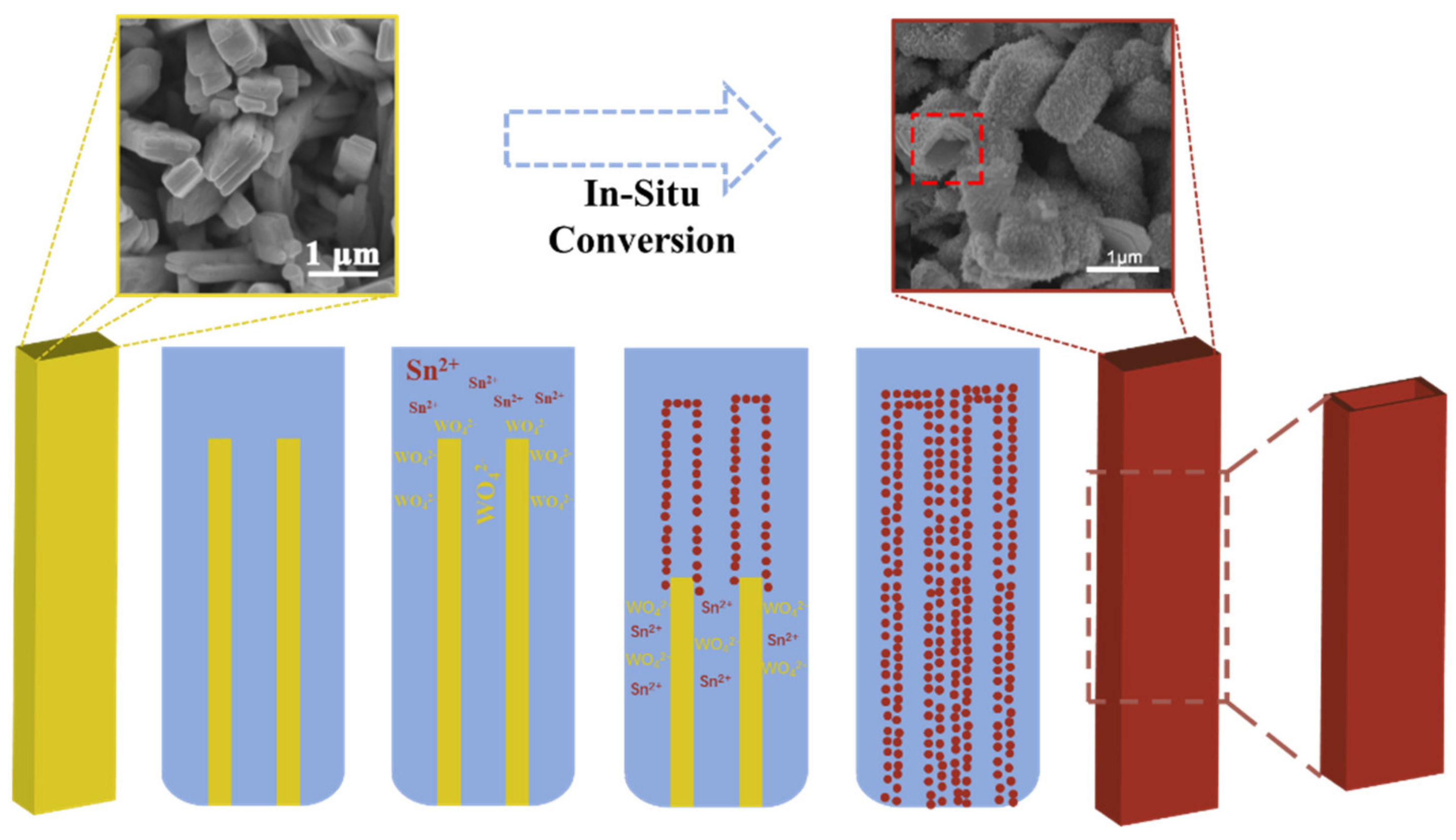
In the 1970s, Jeitschko and Sleight reported the crystalline structure and morphology of the low-temperature (α) and high-temperature (β) phases of stannous tungstate materials [5][6]. Orthorhombic α-SnWO4 exhibits a stable structure below 670 °C, and it tends to transform into cubic β-SnWO4 above 670 °C [7]. A reverse transition from the β-phase to the α-phase may occur under negative pressure, and the W is replaced by Mo [8]. In the crystal of α-SnWO4, W and O form WO6 octahedral structures that are interconnected in four corners, while O binds to Sn in a regular octahedral form [5]. For β-SnWO4, the W atoms of β- form WO42− tetrahedrons with O and disperse within the crystal, whereas near the Sn atoms, O is bound to it as a twisted octahedron [6]. This difference in structure leads to different properties for the α and β phases. The W-O bond in the WO42− tetrahedron (~1.75 Å) [5] is shorter than that in the WO6 octahedrons (~1.80–2.15 Å) [6]. It leads to a larger crystal field-splitting energy and a higher orbital energy of W in β-SnWO4. The conduction band edge of the α phase is mainly composed of a W 5d orbital, while the β phase contains an Sn 5p orbital, in addition to the W 5d orbital. The valence bands of both SnWO4 are formed by hybridizing the O 2p and Sn 5s orbitals, and the Sn-O bonds are almost identical in length (~2.20–2.80 Å) [5][6]. Thus, the positions of the valence bands are similar. These characteristics result in a narrower band gap for α-SnWO4 (~1.7 eV) than for β-SnWO4 (~2.7 eV) [3], and α-SnWO4 has a wider range of light absorption and a higher theoretical photocurrent density (~17 mA cm−2 [9]) than β-SnWO4. In addition, the β phase is a direct band gap and the α phase is an indirect band gap [3], which leads to a higher carrier separation efficiency for the α phase than the β phase [10]. Due to the wider band gap, the photo-generated electrons (holes) of β-SnWO4 show a more significant reduction (oxidation) activity. Thus, β-SnWO4 was used in photocatalysis, and only α-SnWO4 has been used in photoelectrochemical water splitting and sulfite oxidation. However, in addition to the excellent optical properties mentioned above, the use of stannous tungstate exhibits problems that urgently need to be solved. Since Sn is divalent and oxidizable in SnWO4, it is susceptible to photocorrosion, which is attributed to stannous, and the following reaction occurs: SnWO4 + H2O + 2h+ → SnO2 + WO3 + 2H+. Therefore, SnWO4 has poor stability, which limits its performance in practical applications [4]. At the same time, a recombination of carriers occurs in the diffusion process of SnWO4. Its diffusion length is short, which limits the number of photo-generated carriers that can arrive at the surface of the crystal, leading to unsatisfactory photocatalytic and photoelectrochemical performance. Various methods have been developed to synthesize SnWO4 materials, and their differences in thermodynamic stability lead to changes in the synthesis of α-SnWO4 and β-SnWO4. Because the α phase is a kind of crystalline phase at low temperatures, its synthesis conditions are milder. Many methods can be used to synthesize the α phase, e.g., hydrothermal methods, solid–solid reaction methods [3][11][12][13], and magnetron sputtering methods [10][14][15]. Each synthesis method has its own advantages. The hydrothermal method can be used to obtain crystals with a complete crystal phase, small particle size, and uniform distribution. A magnetron sputtering method can obtain dense and uniform high-quality thin films. The solid–solid reaction method possesses a simple process and a large output. However, the synthesis of the high-temperature crystalline β-phase is relatively harsh because it requires higher temperature conditions (which should be obtained by rapid cooling at more than 940 K). It has been reported that most β-SnWO4 powders are synthesized with a high-temperature solid phase method [11][12][16][17]. β-SnWO4 can also be obtained by using NaWO4 as a raw material through kinetic control under more mild conditions [18]. Jan Ungelenk and Claus Feldmann synthesized β-SnWO4 using a microemulsion method at low temperatures [19]. When W and O combine to form a discrete WO42− tetrahedron in NaWO4, the structure is similar to that of β-SnWO4, so Na2WO4 is likely to be directly converted to β-SnWO4. Additionally, assisted by hydrothermal [20][21] and microwave-assisted hydrothermal methods [22], α-SnWO4 can also be synthesized using NaWO4 and SnCl2 as the precursors. To synthesize the film for photoelectrochemical water spliting, an indirect or direct coating method can be used. In indirect coating, as-prepared SnWO4 powders are drop-cast onto conductive substrates. This is an easy-to-perform method, but the performance of the photoanode is weak due to the poor charge transfer caused by bad connections between the particles. For direct coating, Moritz Kölbach et al. employed a pulsed laser-deposited method that used an α-SnWO4 target formed by annealing WO3 (99.99%) and SnO (99.99%) powders [23]. Bozheyev et al. reported on the use of a magnetron co-sputtering method in which Sn (99.99%) and W (99.95%) were the targets, and the deposition was performed with the existence of O2 [24]. Recently, Gottesman used a new method—rapid thermal processing—to synthesize an α-SnWO4 film. The technique could be used to treat a sample at higher temperatures, without destroying the glass-based F:SnO2 (FTO), and a desired crystallinity with few defects was obtained [25]. Liu et al. used a chemical vapor deposition to convert WO3 into α-SnWO4, and the reaction is shown in Equation (1) [26]. An in situ hydrothermal conversion method was also reported, in which WO3 was used as a precursor to react with Sn2+ in the solution [27]. The possible reactions occurring in this method are illustrated as Equations (2)–(3) [28]. As a result, the α-SnWO4 film exhibits similar nanostructured array morphology to that of WO3 films due to the inherited behavior (Figure 1) [28]. The anion in hydrothermal conversion solutions may also affect the morphology of α-SnWO4 films [29].4WO3 + 3SnCl2 ⇌ 3SnWO4 + WCl6 (1)
WO3 + H2O ⇌ 2H+ + WO42− (2)
WO42− + Sn2+ ⇌ SnWO4 (3)

Figure 1. Schematic for in situ conversion from a rod-like WO3 array to a rod-like SnWO4 [28].
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38.
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608.
- Cho, I.-S.; Kwak, C.H.; Kim, D.W.; Lee, S.; Hong, K.S. Photophysical, Photoelectrochemical, and Photocatalytic Properties of Novel SnWO4 Oxide Semiconductors with Narrow Band Gaps. J. Phys. Chem. C 2009, 113, 10647–10653.
- Kölbach, M.; Pereira, I.J.; Harbauer, K.; Plate, P.; Höflich, K.; Berglund, S.P.; Friedrich, D.; van de Krol, R.; Abdi, F.F. Revealing the performance limiting factors in α-SnWO4 photoanodes for solar water splitting. Chem. Mater. 2018, 30, 8322–8331.
- Jeitschko, W.; Sleight, A. Stannous tungstate: Properties, crystal structure and relationship to ferroelectric SbTaO4 type compounds. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1974, 30, 2088–2094.
- Jeitschko, W.; Sleight, A.W. Synthesis, properties and crystal structure of β-SnWO4. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 3174–3178.
- Ke, J.; Younis, M.A.; Kong, Y.; Zhou, H.; Liu, J.; Lei, L.; Hou, Y. Nanostructured Ternary Metal Tungstate-Based Photocatalysts for Environmental Purification and Solar Water Splitting: A Review. Nano-Micro Lett. 2018, 10, 69.
- Gomes, E.O.; Gouveia, A.F.; Gracia, L.; Lobato, A.; Recio, J.M.; Andres, J. A Chemical-Pressure-Induced Phase Transition Controlled by Lone Electron Pair Activity. J. Phys. Chem. Lett. 2022, 13, 9883–9888.
- Schnell, P.; Kolbach, M.; Schleuning, M.; Obata, K.; Irani, R.; Ahmet, I.Y.; Harb, M.; Starr, D.E.; van de Krol, R.; Abdi, F.F. Interfacial Oxide Formation Limits the Photovoltage of α-SnWO4/NiOx Photoanodes Prepared by Pulsed Laser Deposition. Adv. Energy Mater. 2021, 11, 2003183.
- Harb, M.; Ziani, A.; Takanabe, K. Critical difference between optoelectronic properties of α- and β-SnWO4 semiconductors: A DFT/HSE06 and experimental investigation. Phys. Status Solidi B 2016, 253, 1115–1119.
- Kuzmin, A.; Anspoks, A.; Kalinko, A.; Timoshenko, J.; Kalendarev, R. Extended x-ray absorption fine structure spectroscopy and first-principles study of SnWO4. Phys. Scr. 2014, 89, 044005.
- Thangavel, S.; Venugopal, G.; Kim, S.-J. Enhanced photocatalytic efficacy of organic dyes using β-tin tungstate–reduced graphene oxide nanocomposites. Mater. Chem. Phys. 2014, 145, 108–115.
- Alexei, K.; Andris, A.; Aleksandr, K.; Janis, T.; Robert, K.; Lucie, N.; François, B.; Tetsuo, I.; Pascale, R. Pressure-induced insulator-to-metal transition in α-SnWO4. J. Phys. Conf. Ser. 2016, 712, 012122.
- Kuzmin, A.; Zubkins, M.; Kalendarev, R. Preparation and Characterization of Tin Tungstate Thin Films. Ferroelectrics 2015, 484, 49–54.
- Ziani, A.; Harb, M.; Noureldine, D.; Takanabe, K. UV-Vis optoelectronic properties of α-SnWO4: A comparative experimental and density functional theory based study. APL Mater. 2015, 3, 096101.
- Stoltzfus, M.W.; Woodward, P.M.; Seshadri, R.; Klepeis, J.-H.; Bursten, B. Structure and Bonding in SnWO4, PbWO4, and BiVO4: Lone Pairs vs. Inert Pairs. Inorg. Chem. 2007, 46, 3839–3850.
- Wojcik, J.; Calvayrac, F.; Goutenoire, F.; Mhadhbi, N.; Corbel, G.; Lacorre, P.; Bulou, A. Lattice Dynamics of β-SnWO4: Experimental and Ab Initio Calculations. J. Phys. Chem. C 2013, 117, 5301–5313.
- Pavithra, N.S.; Patil, S.B.; Kumar, S.R.K.; Alharthi, F.A.; Nagaraju, G. Facile synthesis of nanocrystalline β-SnWO4: As a photocatalyst, biosensor and anode for Li-ion battery. SN Appl. Sci. 2019, 1, 1123.
- Ungelenk, J.; Feldmann, C. Synthesis of faceted β-SnWO4 microcrystals with enhanced visible-light photocatalytic properties. Chem. Commun. 2012, 48, 7838–7840.
- Huang, J.; Liu, H.; Zhong, J.; Li, J. Enhanced simulated sunlight-driven photocatalytic performance of SnWO4 prepared in the presence of cetyltrimethylammonium bromide. Environ. Prog. Sustain. Energy 2020, 39, e13314.
- Alharthi, F.A.; Shashank, M.; Shashikanth, J.; Viswantha, R.; Alghamdi, A.A.; Algethami, J.; Alsaiari, M.A.; Jalalah, M.S.; Ganganagappa, N. Hydrothermal synthesis of α-SnWO4: Application to lithium-ion battery and photocatalytic activity. Ceram. Int. 2021, 47, 10242–10249.
- Barros, M.M.P.; Almeida, K.C.; Silva, S.A.; Botelho, G. Synthesis and characterization of α-SnWO4 powders obtained by microwave-assisted hydrothermal method. Cerâmica 2022, 68, 236–241.
- Kölbach, M.; Hempel, H.; Harbauer, K.; Schleuning, M.; Petsiuk, A.; Höflich, K.; Deinhart, V.; Friedrich, D.; Eichberger, R.; Abdi, F.F.; et al. Grain Boundaries Limit the Charge Carrier Transport in Pulsed Laser Deposited α-SnWO4 Thin Film Photoabsorbers. ACS Appl. Energy Mater. 2020, 3, 4320–4330.
- Bozheyev, F.; Akinoglu, E.M.; Wu, L.; Lu, H.; Nemkayeva, R.; Xue, Y.; Jin, M.; Giersig, M. Band gap optimization of tin tungstate thin films for solar water oxidation. Int. J. Hydrogen Energy 2020, 45, 8676–8685.
- Gottesman, R.; Peracchi, I.; Gerke, J.L.; Irani, R.; Abdi, F.F.; van de Krol, R. Shining a Hot Light on Emerging Photoabsorber Materials: The Power of Rapid Radiative Heating in Developing Oxide Thin-Film Photoelectrodes. ACS Energy Lett. 2022, 7, 514–522.
- Zhu, S.; Liu, D.; Li, J.; Kuang, Y. Chemical Vapor Deposition of Crystalized Nanoscale α-SnWO4 Thin Films and Their Photoelectrocatalytic Properties. ACS Appl. Energy Mater. 2022, 5, 14372–14380.
- Pyper, K.J.; Evans, T.C.; Bartlett, B.M. Synthesis of α-SnWO4 thin-film electrodes by hydrothermal conversion from crystalline WO3. Chin. Chem. Lett. 2015, 26, 474–478.
- Qiu, W.; Zhang, Y.; He, G.; Chen, L.; Wang, K.; Wang, Q.; Li, W.; Liu, Y.; Li, J. Two-Dimensional Long-Plate SnWO4 Photoanode Exposed Active Facets for Enhanced Solar Water Splitting. ACS Appl. Energy Mater. 2022, 5, 11732–11739.
- Liu, Y.; Qiu, W.; He, G.; Wang, K.; Wang, Y.; Chen, L.; Wu, Q.; Li, W.; Li, J. Nail-like α-SnWO4 Array Film with Increased Reactive Facets for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2022, 126, 15596–15605.
More
