Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vasantha Kumar and Version 2 by Catherine Yang.
Advances in perinatal and neonatal care have led to improved survival of preterm infants into adulthood. However, the shift in focus to long-term health in adults born preterm requires a clear understanding of the impact of prematurity on developing organ systems and the development of adult-oriented disease. A less well-recognized area of risk for surviving preterm infants is their cardiometabolic health. Epidemiologic evidence has linked preterm birth to the development of systemic hypertension, type 2 diabetes, metabolic syndrome, heart failure, and ischemic heart disease. Of more significant concern is that the risk of cardiometabolic disorders is higher in adults born preterm compared to full-term infants.
- adults born preterm
- hypertension
- metabolic syndrome
- heart failure
- diabetes
1. Cardiac Structure and Function in Adults Born Preterm
Alterations in cardiac structure and function during development results in substantially higher lifetime risks for cardiovascular disease in adults born preterm. Studies have utilized both echocardiography [1][2][12,13] and cardiac magnetic resonance imaging (MRI) [3][4][5][14,15,16] as tools in the assessment of cardiac structure and function. Extremely preterm infants exhibit a unique cardiac phenotype characterized by smaller left ventricles with altered systolic and diastolic function by early childhood [2][13]. Additionally, these infants had an increase in blood pressure with a decrease in left ventricular (LV), and aortic size with preserved LV function in adolescence [1][12]. In an interesting cross-sectional cohort study, Goss et al., evaluated premature infants born at 28 weeks gestational age (GA) at adolescence (13 years of age) and as young adults (25 years of age) by cardiac MRI [3][14]. Premature infants in both cohorts had a smaller biventricular cardiac chamber than the term group on MRI [3][14]. In addition to lower biventricular mass, the left-ventricular diastolic volume (LVDV) index and the left-ventricular systolic volume (LVSV) index were significantly smaller than in term infants in both the adolescent and the adult cohort [3][14]. Cardiac strain analysis demonstrated a hypercontractile heart, primarily of the right ventricle (RV), in adults born preterm [3][14]. Although premature infants were born in the broader time-period (the 1980s through 2000s), all infants demonstrated a lower cardiac mass, suggesting that prematurity may contribute to alterations in the heart’s structure in adults born preterm.
In former premature infants born at 30-week gestation, Lewandowski et al. demonstrated a greater RV mass, smaller RV volume with a significantly lower RV systolic functional parameters than term infants [1][12]. Furthermore, these premature infants had a higher LV mass along with a smaller LV with significant reductions in systolic and diastolic function parameters as adults [4][15]. Higher biventricular mass and hypo contractile strain pattern by Lewandowski et al. [4][5][15,16], are in striking contrast to the findings of reduced LV mass and hypercontractile strain pattern on MRI observed by Goss et al. [3][14]. It is interesting to note that the gestational age was comparable at birth between the two studies (around 28–29 weeks GA). However, the infants were born at differing time-periods ranging from 1980s to early 2000s (Lewandowski et al.: 1982–1985 [4][5][15,16]; Goss et al. 2003–2004 [3][14] (adolescents); 1980s–1990s: adult cohort). Likewise, the infants had cardiac MRI at differing ages as adults (Lewandowski et al.: 20–40 years; Goss et al.: 25 years). Cardiac development is a consequence of multiple prenatal, postnatal, and environmental factors. Both the studies are important and consistent in concluding that cardiac development is significantly altered in adults born preterm. Future studies addressing the factors that predispose to predominant involvement of either RV or LV, would help us in developing therapeutic strategies to optimize cardiac development in premature infants.
Extremely premature infants (<24 weeks GA at birth) are at high risk for cardiorespiratory morbidities as children and adults; however, there is paucity of data on the evolution of cardiac structure and function in extremely premature infants as they grow as adults. Extremely low birth weight (ELBW) infants are exposed to oxygen-rich environments soon after birth. Hyperoxia and generation of reactive oxygen species (ROS) result in the cell-cycle arrest of cardiomyocytes [6][17]. In contrast, ROS scavenging or inhibition of DNA damage response in postnatal hypoxia prolongs the postnatal proliferative window of cardiomyocytes [6][17]. Additionally, the proliferation of neonatal and embryonic stem (ES)-cell-derived cardiac cells involves ROS-mediated signaling cascades associated with NADPH oxidase in the cardiovascular differentiation of embryonic stem cells [7][18]. The interplay between the degree of cardiomyocyte immaturity, oxygen administrated, and ROS generation may play an essential role in both the differentiation and proliferation of cardiac myocytes soon after birth. These early structural and proliferation changes in the myocardium may significantly impact cardiac mass and function as premature infants grow into adults, predisposing them to long-term cardiac vulnerability [8][19]. In a rat model of chronic lung disease of prematurity aged one-year, adult rats demonstrated significant RV hypertrophy and dysfunction with significant chronic pulmonary hypertension following postnatal hyperoxia [9][20]. Hyperoxia-exposed RV cardiomyocytes showed evidence of mitochondrial dysregulation and DNA damage, suggesting potential mitochondrial dysfunction as a cause of RV dysfunction [9][20]. Studies should further examine the interactions between hyperoxia, BPD, and prematurity and how they impact the differentiation and proliferation of cardiomyocytes, leading to RV dysfunction and long-term cardio-respiratory morbidity in preterm adults.
Prematurity is associated with global alterations in myocardial structure and function, which worsen over time and potentially produce cardiovascular disease as these infants grow into adults. VLBW survivors who demonstrate reduced physical activity and impaired lung function, have altered left ventricular structure and function, as noted by reduced mass, size, stroke volume, and cardiac output [10][21]. Beyond the effects of physical activity and body mass index, lung function and cardiac structure and function contributed equally to reduced exercise capacity [10][21]. Cardiac structural alterations have been noted as early as six years of age in ELBW infants [2][13]. The LV and the RV are equally affected by structural alterations, as demonstrated by cardiac resonance imaging [4][5][15,16]. Longitudinal studies suggest that alterations in cardiac structure precede alterations in cardiac function in adults born preterm. The pathophysiology of the cardiovascular morbidities in adults born preterm is illustrated in Figure 1.
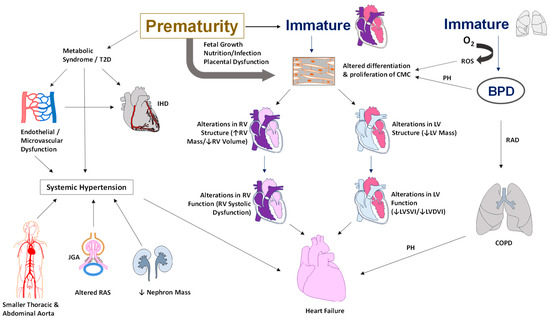
Figure 1. Illustration of the pathophysiology of cardiovascular morbidities in adults born preterm. Alterations in the cardiac structure include a smaller RV or LV systolic or diastolic volume and an increase in RV or LV mass. Functional changes include systolic and diastolic dysfunction, hypercontractile heart, and low cardiac output. Metabolic syndrome and a higher risk of type II diabetes contribute to endothelial dysfunction leading to systemic hypertension and ischemic heart disease. In addition, the smaller diameter of the thoracic and abdominal aorta, lower nephron mass, and alterations in sodium balance with activation of the renin-angiotensin system all contribute to the development of systemic hypertension in adults born preterm. Abbreviations: BPD—bronchopulmonary dysplasia, RAD—reactive airway disease, COPD—chronic obstructive airway disease, O2—oxygen, CMC—cardiomyocyte, RV—right ventricle, LV—left ventricle, T2D—type II diabetes, JGA—juxtaglomerular apparatus, RAS—renin-angiotensin system, PH—pulmonary hypertension, ROS—reactive oxygen species, IHD—ischemic heart disease, LVSVI—Left Ventricular Systolic Volume Index, LVDVI—Left Ventricular Diastolic Volume Index (copyright: Vasantha Kumar. H.S).
2. Risk for Cardiovascular Disease
Studies have shown that endothelial dysfunction, an established early marker of the development of hypertension and cardiovascular disease, may occur earlier in premature infants. Compared to term controls, reduced endothelial function has been noted in premature-born adults as measured by finger plethysmography [8][19] or flow-mediated dilatation [9][20]. However, the results must be interpreted with caution as factors such as dyslipidemia, impaired glucose responses, circulating sex hormone levels, and undetected atherosclerotic vascular disease alter endothelial function. Nevertheless, the evidence points to an increasing group of adults born preterm with risks of long-term complications and early adult death [11][22]. The adjusted hazard ratios (aHRs) for all-cause mortality were 1.44 (95% CI, 1.34–1.55) for moderate preterm birth (23 to 33 weeks gestation); 1.23 (95% CI, 1.18–1.29) for late preterm birth (34 to 36 weeks); and 1.12 (95% CI, 1.09–1.15) for early term deliveries (37 to 38 weeks). Preterm birth is associated with two-fold increased risks of death from cardiovascular disease (aHR, 1.89; 95% CI, 1.45–2.47) and diabetes (aHR, 1.98; 95% CI, 1.44–2.73) [11][22]. Accordingly, it is imperative to detect modifiable risk factors or early signs of cardiovascular disease in premature infants, to reduce the lifelong disease burden on individuals and society.
2.1. Diabetes and Cardiovascular Risk
In adult life, the risks for type 2 diabetes are higher in premature infants born less than 35 weeks of gestation and the risk is independent of fetal growth [12][23]. Additionally, VLBW infants had diminished sensitivity to insulin compared to term infants with comparable body size and composition as adults [13][24]. Nonetheless, higher insulin secretion compensates for depressed sensitivity to insulin in adults born preterm. The emphasis on healthy lifestyles and expeditious screening for type 2 diabetes is crucial as these infants may have predisposition to glucose intolerance as adults [13][24]. The metabolic profile of VLBW infants can be difficult to ascertain as higher insulin resistance is not necessarily accompanied by dysglycemia as adults [14][25]. In population-based studies, a 2-fold increase in the prevalence of type 2 diabetes has been noted by 40 to 60 years of age in infants born preterm [12][15][23,26]. In a recent meta-analysis, infants born preterm had a higher risk of diabetes and hypertension as adults [16][27]. Early diagnosis and management of prediabetes would contribute not only to decrease the disease progression towards type 2 diabetes [17][28] but also in reducing cardiovascular morbidity in these infants [18][29].
2.2. Metabolic Syndrome and Cardiovascular Risk
Body composition studies in former preterm infants have shown a lower lean body mass, an increase in body fat, and a higher risk of developing dysglycemia by the fourth decade of life [19][30]. Similar results relating to parameters of body composition have been reported in other studies in 20-year-old adults born preterm [20][31]. As body composition and metabolic disturbance may reveal with advancing age, long-term follow-up is essential for early diagnosis and appropriate intervention in these adults at increased risk for cardiovascular disease. Development of adipose tissue essentially occurs in the third trimester of pregnancy. Disturbances in adipocyte development during the third trimester could have consequences on adipocyte function throughout the life course in adults born preterm [21][32]. As obesity and body fat are major predictors of dysglycemia, it is essential to evaluate these infants and develop lifestyle interventions and wellness behaviors to modify body fat composition of the preterm population in young adulthood. However, there is paucity of data regarding when the lifestyle interventions should begin for maximum benefit in these infants. Nonetheless, it would be advantageous to start these interventions as early as possible to minimize the effects of adult-onset disease [19][30]. Prenatal and postnatal factors around the time of birth determine body composition relating to adiposity development and deposition [22][33]. The study highlights the emphasis on lifestyle interventions and wellness habits during the critical period of development to change modifiable factors during early childhood for potential benefits on cardiometabolic risk in later life.
Metabolic syndrome is a cluster of risk factors such as high blood pressure, excess body fat, high blood sugar and abnormal cholesterol levels that increase the risk for cardiovascular disease and stroke. Co-existence of more than one factor results in evolution of systemic inflammation [23][34] or ongoing oxidative stress [24][35], further increasing the risk for cardiovascular disease. A marked increase in risks factors such as hypertension, airflow obstruction and glucose intolerance has been noted in young adults born preterm [25][36]. However, the presence of these risk factors did not necessarily increase inflammatory or oxidative stress markers in these infants compared to full term infants [25][36], implying different pathogenetic mechanisms. The pathophysiology of the development of these risk factors may be rooted in interactions between developing organ systems over time. For instance, smaller kidneys noted in premature infants along with abnormal urine albumin to creatinine ratio and elevated angiopoietin-1 levels may suggest a renal etiology for elevated blood pressure [26][37]. An increase in the levels of antiangiogenic proteins such as soluble fms-like tyrosine kinase-1 may suggest microvascular hypoplasia and capillary rarefaction resulting in higher blood pressure [27][38]. Multiple developmental mechanisms impact cardiovascular development, further modified by postnatal factors and lifestyle changes as premature infants grow into adults. Epigenetic programming and neonatal conditions further complicate the development of cardiovascular risk factors leading to adult-onset diseases.
3. Adult-Onset Hypertension
Epidemiologic evidence has linked preterm birth with risks of hypertension [28][41] and type 2 diabetes [29][42], with smaller and less mature infants facing the most significant risks as young adults. In a systematic review, preterm infants with a mean GA of 30 weeks at birth had higher systolic blood pressures by 3.8 mmHg compared to term infants at 18 years of age [28][41]. Similarly, VLBW infants had higher systolic and diastolic blood pressures as adults, with female gender and preeclampsia being additional risk factors [30][9]. Young adults born <28 weeks gestation in the post-surfactant era had higher 24 h systolic (Mean: 4.5 mHg), diastolic (Mean: 3.4 mmHg) and mean (Mean: 3.6 mmHg) BP and an increase in ambulatory BP compared to term controls [31][43]. Additionally, the visit-to-visit variability to assess BP patterns may help identify young adults at increased risk of cardiovascular disease and all-cause mortality later in life [32][44]. Furthermore, the potential role of the so called “multiple office blood pressure measurement” still need to be studied [33][45]. Both healthy and sick preterm infants are at increased risk for cardiovascular morbidities such as high blood pressure in life [34][46]. In a systematic review of >17,000 preterm infants born < 37 gestational weeks adults born preterm had a significantly higher SBP (mean of 4.2 mm Hg), DBP (mean of 2.6 mm Hg), and low-density lipoproteins (LDL) as compared to adults born at term [35][47]. Women had relatively higher differences in blood pressure than men, suggesting that sex is an essential variable in preterm-born adults [35][47]. However, both men and women are at risk for adult-onset hypertension, and studies should enlighten whether sex is an essential variable for adult-onset hypertension in preterm adults. Interestingly, a recent study found that pulse-wave velocity, with advancing age, may behave differentially in women as compared to men [36][48].
Adult-onset hypertension can partly be explained by structural changes in the vessels in adults born preterm. Premature infants born around 30 weeks of gestation had 20% smaller thoracic and abdominal aortic lumens but similar carotid and brachial diameters as adults at 23 to 28 years of age by magnetic resonance [37][49]. Despite similar carotid size, the pulse wave velocity was increased, and carotid distensibility decreased in adults born preterm [37][49]. Variations in blood pressure and early aortic elastin and collagen developmental changes in the aorta in infants born preterm could explain some of the changes seen as adults. These changes could increase over the lifetime and may increase the risk of vascular aging in adults born preterm [37][49]. Higher serum creatinine and NGAL in the preterm group may indicate that preterm birth may affect kidney function and may partly explain more elevated blood pressure in preterm-born adults [38][50]. Close monitoring of blood pressure over time to facilitate early detection and timely management of hypertension is of utmost importance to improve the quality of life in this high-risk population.
4. Preterm Birth and Ischemic Heart Disease
Preterm birth (<37 weeks GA) has been linked with an increased risk of cardiometabolic disorders in adulthood, including hypertension [39][51], diabetes [40][52], and metabolic syndrome [13][14][24,25], which are the significant risk factors for ischemic heart disease. In a population-based cohort study from Sweden (age range of 18 to 43 years), gestational age was inversely correlated with the risks for ischemic heart disease (adjusted HR per additional week of gestation of 0.96; 95%CI, 0.93–0.98) [41][53]. The adjusted odds of ischemic heart disease for those born preterm (<37 gestational weeks) and those born early term (37–38 gestational weeks) were 1.44 (95% CI, 1.19–1.73) and 1.16 (95%CI, 1.02–1.31), respectively, compared to term infants. It is interesting to note that the risks for ischemic heart disease were correlated with gestational age in late adulthood (30 to 43 years) but not in early adulthood (18 to 29 years) [41][53]. The risks for ischemic heart disease in late adulthood were higher in preterm and early term infants compared to term infants (infants <37 weeks—adjusted HR: 1.53; 95% CI, 1.20–1.94; early term infants—adjusted HR: 1.19; 95% CI, 1.01–1.40, respectively). Of note, the increased risk for ischemic heart disease was mostly driven by infants born between 34- and 36-weeks’ gestation, due to limited number of babies < 34 weeks gestational age enrolled in the study [41][53]. It is interesting to note that on sex-stratified analysis, preterm birth was associated with significantly increased relative risk among women but not in men [41][53]. In one of the oldest longitudinal studies, with the birth cohort between 1924 and 1944, premature infants (<34 weeks versus 34–37 weeks at birth) were followed up to old age and found no difference in coronary heart disease in infants born preterm [42][54]. Differing results from the above two studies may be related to nutritional and environmental factors along with generational differences in the study population, as well as to the fact that in 1924–1944, preterm infants were likely to die of other diseases and complications before reaching an age where cardiovascular disease would have impacted life expectancy. Improved medical care and increased neonatal and pediatric survival might have relevantly shaped this result.
