Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Roberto Fernández de Luis.
Hexavalent chromium (Cr(VI)) is a highly mobile cancerogenic and teratogenic heavy metal ion. Among the varied technologies applied today to address chromium water pollution, photocatalysis offers a rapid reduction of Cr(VI) to the less toxic Cr(III). In contrast to classic photocatalysts, Metal-Organic frameworks (MOFs) are porous semiconductors that can couple the Cr(VI) to Cr(III) photoreduction to the chromium species immobilization.
- Metal-Organic frameworks
- photocatalysis
- hexavalent
- chromium
- adsorption
- water remediation
1. Introduction
Hexavalent chromium (Cr(VI)) is a highly toxic metal that is stabilized as chromate oxyanions in water (Figure 1). It induces well-known cancerogenic and teratogenic effects in living organisms due to its oxidative nature. In addition, the environmental, ecotoxicology and health impacts of Cr(VI) are intensified due to the industrial wastewater effluents derived from diverse manufacturing processes such as leather tanning, cooling tower blowdown, plating, electroplating, anodizing baths’ rinse waters, etc. [1,2,3][1][2][3].
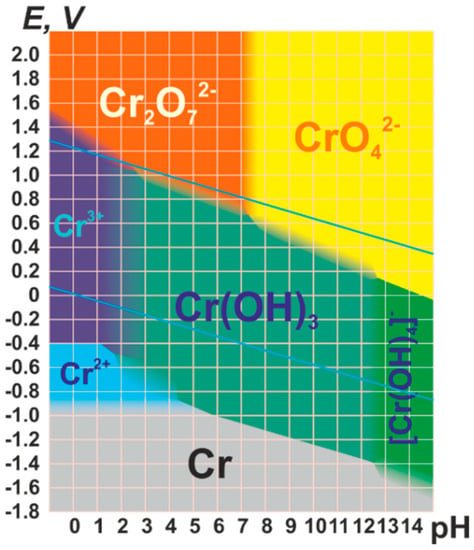
Figure 1. Pourbaix diagram of Cr. Reproduced from Denis Zihilin. Copyright CC BY-SA 3.0 License: https://creativecommons.org/licenses/by-sa/3.0/legalcode. “URL (accessed on 1 November 2022)”.
Depending on the pH and redox potential of the water, chromium ions can be stabilized in its hexavalent and trivalent forms, as visualized in the Pourbaix diagram (Figure 1). In parallel, the chromium oxidation state, along with the acidity/basicity of the media (i.e., pH), also governs the chromium speciation in water. Whilst trivalent chromium is usually stabilized as cationic oxo-aquo species with octahedral environments, its hexavalent form is usually found as neutral or negative oxyanions as tetrahedral H2CrO4, [HCrO4]−, [CrO4]2n− and [Cr2O7]2n−.
The redox properties of Cr(VI) ions enable applying different strategies for its removal through adsorption and photo-, chemo- or electroreduction [4,5,6][4][5][6]. Specifically, the most explored chromium removal technologies are precipitation–coagulation, ion exchange, membrane separation, adsorption, and reduction [7,8,9,10,11][7][8][9][10][11]. Among them, photocatalysis offers the possibility to rapidly reduce Cr(VI) to Cr(III) without the addition or production of any hazardous chemical by-product. Therefore, water remediation systems have to be able to capture and transform highly hazardous Cr(VI) into much less toxic Cr(III) species avoiding the release of chemical to the media [12].
2. Metal-Organic Frameworks for Hexavalent Chromium Photoreduction and Capture
2.1. Divalent Metal-Based Metal-Organic Framework Photocatalysts
Considering the photocatalytic ability of a ZnO semiconductor, the use of Zn-based MOFs in the photoreduction of chromium has been a natural step of exploration as a first approach to test the feasibility of MOF photocatalysts for hexavalent chromium detoxification (Table 1). The research on Cr(VI) photoreduction is limited to ZIF-8, BUC-21 (Zn(II)/anthracene), and NNU-36 materials and their composite structures when combined with inorganic semiconductors. In the specific case of the well-known ZIF-8 zeolitic imidazole framework (ZIF), although active for Cr(VI) photoreduction, its wide band gap (5.2 eV) severely limits its efficiency to harvest light and trigger the photocatalytic process. A band gap narrowing has been achieved by engineering Zn-MOF based on chromophore carboxyl-based organic linkers with aromatic ring systems. First, BUC-21, which is a Zn-MOF build up from Zn-paddlewheel and 1,3-dibenzyl-2-imidazolidone-4,5-dicarboxylic acid square planar “carboxylate-metal organic layers” pillared by a 4,4′-bipyridine (bpy) secondary linker, has been studied. The coordination environment of the paddlewheel units differs significantly from the one shown by the Zn(II) ions in ZIF-8, also inducing a shift in the optical band gap to 3.4 eV. The material exhibits a better Cr(VI) photoreduction response in comparison to the ZIF-8. Surprisingly, even if the long-term hydrolytic stability of Zn-MOFs is in question, BUC-21 exhibits excellent reusability [201][13].Table 1.
Divalent-metal-based MOF photocatalysts for Cr(VI) to Cr(III) reduction.
| Metal Center | MOFs | pH | Light Source | [Cr (VI)]0 (ppms) |
Photocatalyst Loading (g/L) |
Photo-Oxidation Efficiency |
Ref. | |
|---|---|---|---|---|---|---|---|---|
| Removal Percentage (%) |
Time (min) |
|||||||
| Zn | ZnO@ZIF-8 | 7 | UV | 20 | 1 | 88 | 240 | [202][14] |
| ZIF-8@Cd0.5Zn0.5S | 6 | Vis. | 20 | 1 | 100 | 10 | [203][15] | |
| MoO3@ZIF-8 | Vis. | 20 | 0.5 | 96 | 40 | [204][16] | ||
| ZIF-8@CuPd | 1 | Vis. | 20 | 0.20 | 89 | 60 | [205][17] | |
| BUC-21 | 2 | UV | 10 | 0.75 | 96 | 30 | [205][17] | |
| TNT@BUC-21 | 5 | UV | 10 | 0.16 | 100 | 20 | [206][18] | |
- (ii)
-
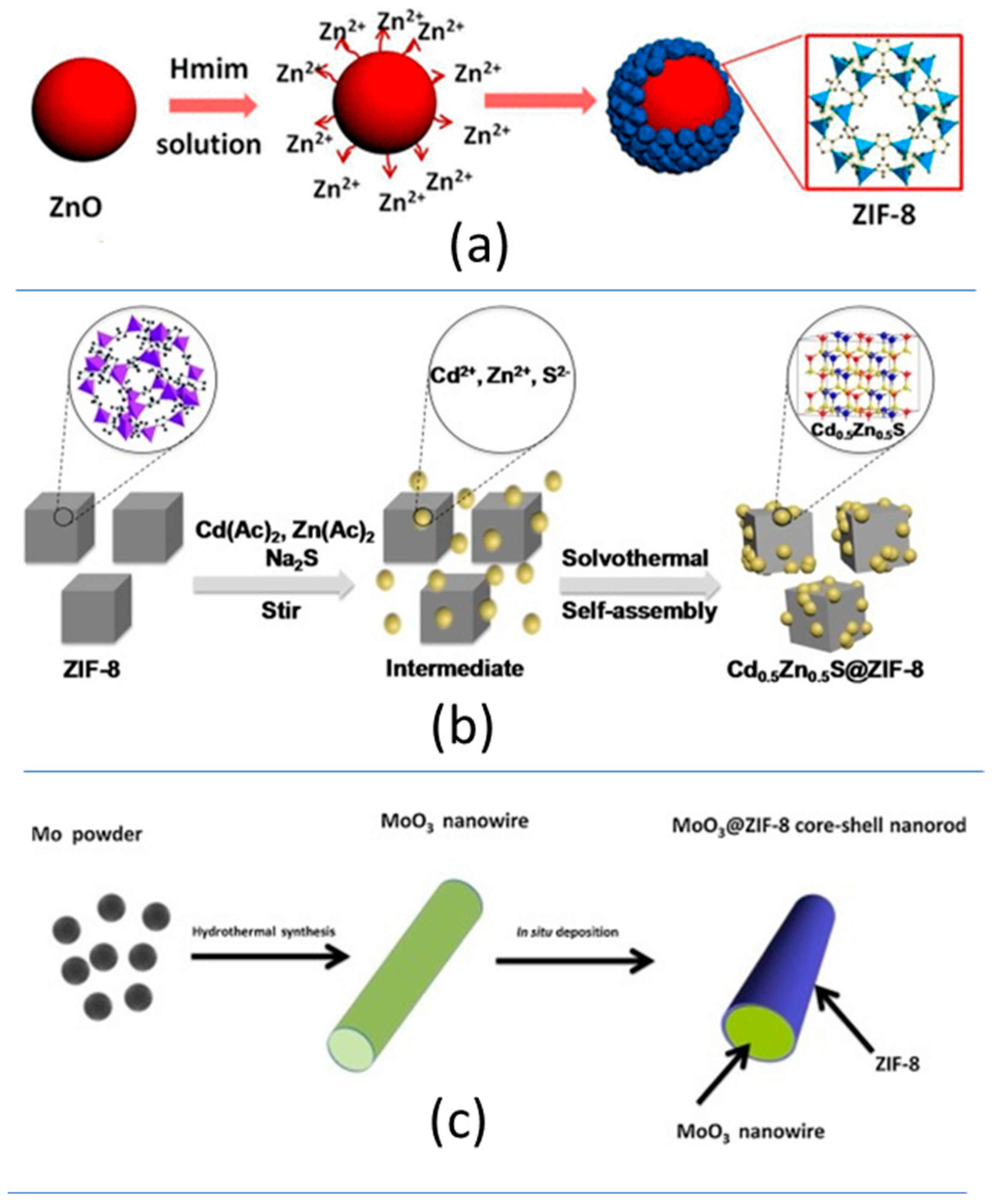
The direct growth of metal or sulphide nanoparticles at the surface of the ZIF-8 nanosized crystals (i.e., ZIF-8@CuPd [221][34] and ZIF-8@Cd0.5Zn0.5S [222][35]) (Figure 92b, Table 1).
- (iii)
-
The generation of semiconductor–MOF core–shell structures (i.e., ZnO@ZIF-8 nanoparticles, MoO3/ZIF-8 nanowires, and TiO2@BUC-21 nanotubes (Figure 92c, Table 1).In general terms, heterojunctions obtained from MOFs and inorganic/organic semiconductors lead to a shift in the optical band gap energy to the visible-light energy range that improves the light-harvesting capacity of these composites in comparison to their individual components. Merging the electronic structures at the interphase between the MOF and classic semiconductor materials also leads to improved photoconduction of the composites in comparison to their parent components. For some cases, the composite materials have been revealed as multifunctional photocatalysts able to couple the chromium photoreduction to the photo-oxidation of organic pollutants as methylene blue dye (Figure 92—Table 1). Although the chemical stability of the Zn-MOFs under the working conditions usually employed in photocatalysis have been confirmed in many works through X-ray diffraction after operation, and the recyclability of some of the Zn-MOFs indicates that a minor loss of activity is observed, it is important to be cautious when evaluating these conclusions, even when an important number of the studies have been performed at low-pH conditions. As explained before, the chemical and hydrolytic stability of Zn-carboxylate and Zn-imidazole bridges are limited, especially at acidic conditions, and the Zn-MOFs usually lose their porosity and long-range ordering when exposed to moisture conditions or immersed in water in the short- to mid-term. So, even if the materials could resist the chemico-physical conditions of the Cr(VI) to Cr(III) reactions in usual experiments carried out at lab-scale, this does not preclude the MOF being partially dissolved or disintegrated during the process. The environmental impact of a partial leaching of the MOF during the operation will depend on the toxicity of their components, which is especially concerning when sophisticated organic linkers based on aromatic rings or pyridyl moieties are used to build up the MOF structure.
2.2. Trivalent-Metal-Based Metal-Organic Framework Photocatalysts
Among the trivalent transition metals employed for the synthesis of MOFs, iron, chromium, and aluminum are the most investigated ones (Table 2). Depending on the synthesis conditions and the connectivity and functional groups of the organic linkers, Fe(III), Al(III), and Cr(III)-MOFs can crystallize in a wide variety of structures with high-to-intermediate hydrolytic and chemical stabilities (Figure 103).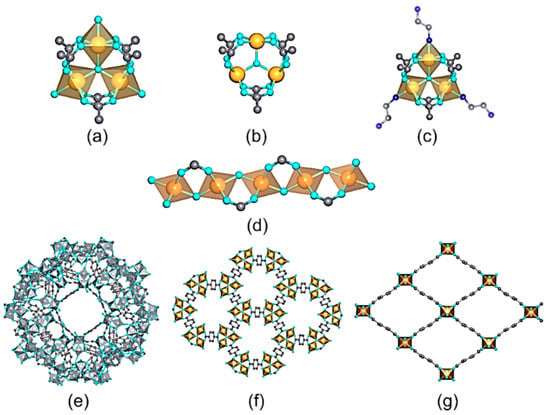 Figure 103. Inorganic structural units found in trivalent MOFs used for metal ion recovery in aqueous media. (a) M3(µ3-O)(R─CO2)6AlS2 (A = Cl, OH, F) trimers; S = Solvent (b), M3(µ3-O)(R–CO2)6A1 trimer after solvent removal (c), M3(µ3-O)(R–CO2)6Al(en)2; trimers after their decoration with en (ethylenediamine) molecules, (d) [M(µ2-A)(R–CO2)2]n chains. Crystal structures of (e) MIL-100, (f) MIL-88, and (g) MIL-53 materials.Table 2.Trivalent-metal-based MOF photocatalysts for Cr(VI) to Cr(III) reduction.
Figure 103. Inorganic structural units found in trivalent MOFs used for metal ion recovery in aqueous media. (a) M3(µ3-O)(R─CO2)6AlS2 (A = Cl, OH, F) trimers; S = Solvent (b), M3(µ3-O)(R–CO2)6A1 trimer after solvent removal (c), M3(µ3-O)(R–CO2)6Al(en)2; trimers after their decoration with en (ethylenediamine) molecules, (d) [M(µ2-A)(R–CO2)2]n chains. Crystal structures of (e) MIL-100, (f) MIL-88, and (g) MIL-53 materials.Table 2.Trivalent-metal-based MOF photocatalysts for Cr(VI) to Cr(III) reduction.Metal MOFs pH Light
Source *[Cr (VI)]0 Loading
(g/L)Photo-Oxidation
EfficiencyRef. Efficiency (%) Time
(min)Fe MIL-53 4 Vis. 20 1 100 40 [223][39] MIL-88B-NH2 2 Vis. 8 0.5 100 45 [224][40] MIL-53-NH2 15 60 MIL-101-NH2 100 60 MIL-100/HPMo 5% 4 Vis. 20 1 100 8 [225][41] MIL-53/rGO 4 Vis. 20 1 100 80 [226][42] BUC-21 and g-C3N4 2 SL 10 MIL-100/Au 1% 0.25 100 60 [ 4 207 ][ Vis. 19] 20 1 BUC-21 and Bi24O31Br10 2 Vis. 10 0.25 99 120 [208][20] 20 [ 227 ] [ 43 NNU-36 2 Vis. 10 0.38 95.3 60 [209][21] MOF-Zn-BPEA 3 Vis. 10 0.38 92 50 [210][22] Zn-MOF [a] 2 SL 20 1 93 90 [206][18] ] MIL-100/Pd 1% 100 16 MIL-100/Pt 1% 8 MIL-68/AgBr 30%/Ag 1.5% 4 Vis. 20 0.25 99.9 6 [228][44] MIL-88B-NH2/Ag/AgCl 2 - 20 0.5 85.7 45 [229][45] MIL-101/Pd-Cu NR Vis. NR NR 100 30 MIL 53/g-C3N4 3% [ 211 ] [ 2–3 23 ] Vis. 10 0.4 100 180 [ 230 ][46] Zn-PA-MOF 2–6 UV 20 0.4 98 MIL-101-NH2 90 10%/g-C3N4 2–3 Vis. 10 0.5 76.0 60 [231][47][212][24] Cd BUC-66 2 UV 10 0.075 98 30 [213,214][25][26] Co BUC-67 99 30 Cd Cd(4-Hptz)2.(H2O)2]n 3 UV 10 0.175 100 50 [210][22] [a] 1 mL EtOH as scavenger. Vis. = visible light, SL = sun light, WL = white light.Representing a step forward, the incorporation of visible-light-responsive bipyridine-like linkers, such as 9,10-bis(4-pyridylethynyl)-anthracene (BPEA) acting as a pillars of 2D Zn-carboxylate layers, allows expanding the optical band gap of pillared MOFs to the visible-light region, and thus improving the photocatalytic efficiency of the material under sunlight illumination [215][27]. The photocatalytic activity of Zn-MOFs is not only limited to robust 3D and 3D porous structures, but to one-dimensional ladder-like Zn(II)/BPEA coordination polymers [216][28]. The incorporation of a chromophore ligand is a well-known and widely applied strategy to tune the visible-light-harvesting capacity of MOF and, thus, improve their Cr(VI) photoreduction efficiency. The luminescence properties of anthracene-like organic linkers have been applied as well to detect hexavalent chromium as the presence of chromate anions induces a quenching of the luminescence signal. Although scarcely explored, other divalent Cd(II) [213][25], Co(II) [214][26], and Cu(II) [217][29] MOFs have been also successfully tested for hexavalent chromium photoreduction purposes. In terms of band gap energy, there is a clear advantage when applying Co(II)- or Cu(II)-based MOFs for photocatalysis, since their light-harvesting capacity is shifted to the visible range. In addition, copper is a well-known active metal center for the oxidative catalytic degradation of organic pollutants such as phenols. For instance, as the oxidative degradation by copper sites depends on the generation of oxygen radicals such as hydrogen peroxide, exciton generation through light illumination can induce an enhancement in ROS generation, Cu center activation, and finally, organic pollutant oxidation [218,219,220][30][31][32]. The engineering of MOF-based heterojunctions has allowed tuning the optical band gap energy to visible-light capture and improving the overall properties and photocatalytic performance of Zn-MOFs. The soft synthesis conditions of divalent MOFs make the in situ growing of the MOF at the surface of different materials relatively straightforward [150][33]. Three are the main strategies that have been explored to construct heterostructured MOF materials for chromium photoreduction:- (i)
-
The physical mixture through ball milling of MOF and other semiconductor inorganic or carbon-based materials (i.e., BiSurprisingly, the application of tetravalent MOFs for chromium photoreduction purposes has been limited to the benchmark UiO-66 and MIL-125 materials, and of their modifications. There is room for exploration in this specific research subject, since the structural and chemical versatility of tetravalent-based MOFs could open the perspective to understanding the effect of many key features of these materials (i.e., surface area, pore space, presence of defects, connectivity of the inorganic units, post-synthetic functionalization of the pore space, multivariate chemistry…) into their photocatalytic performance. For instance, for readers that could be interested in gaining a deeper understanding of the structural and chemical versatility of tetravalent-based MOFs, they may consult references [184,185,186,187,188,189][67][68][69][70][71][72]. Regarding the UiO-66 and MIL-125 materials employed for Cr(VI) photoreduction, they share a common topology arising from the similar connectivity of their inorganic and organic building units (Figure 125). Both compounds share the terephthalic-like linkers as their organic building blocks, and slightly differ in the characteristics of their inorganic building blocks. UiO-66 is built of hexanuclear [Zr6(μ3-O)4(μ3-OH)4]12+ clusters. The zirconium oxide nodes of UiO-66 can connect up to 12 carboxylate groups belonging to BDC linkers. Half of the eight oxygen atoms in the hydroxylated version of this SBU are bound to three zirconium atoms as individual atoms, and the remaining oxygen atoms are bound to three zirconium atoms in hydroxide form (Figure 125a). UiO-66 crystallizes as a face-centered-cubic structure of F m−3m symmetry with a lattice parameter of 20.7 Å. The structure contains two types of cages: tetrahedron and octahedron pores of 7.5 Å and 12 Å, respectively. Ti-MIL-125 shares the same topology and connectivity of their inorganic and organic building units with UiO-66. Therefore, similar tetrahedral and octahedral cages and surface areas have been reported for this compound as well. The main difference lies on the inorganic nodes of the crystal structure. Titanium oxo-clusters in MIL-125 consist of a ring structure of eight-edge-shared Ti-octahedra capped by twelve carboxylate groups belonging to the BDC organic linkers. Overall, the connectivity of the Ti-oxo clusters (12-c) and the organic linkers (2-c) gives rise to an fcu topology with a slightly distorted tetragonal symmetry in comparison to the one of UiO-66. Readers may consult the recent review of Y. Bai et al. [249][73], L. Feng et al. [250][74], and Y. Chen et al. [251][75] for the crystal structure, defective chemistry, chemical and thermal stability, and potential applications of the UiO-66 family. Similarly, the investigation on MIL-125 has been intense since its discovery by Ferey’s lab that wrote specific reviews of titanium-based MOFs [252,253,254][76][77][78]. Taking into account the chemical stability of tetravalent metal-based MOFs, they have been started to be employed as photocatalysts for chromium photoreduction, as summarized in Table 3.2-CO2)X hexanuclear clusters. (d) Zr one-dimensional units found in MIL-140 structure. Crystal structures of the zirconium MOFs used for metal ion adsorption, (e) UiO-66, (f) MOF-808, (g) DUT-67, (h) MOF-545.Table 3.Tetravalent-metal-based MOF photocatalysts for Cr(VI) to Cr(III) reduction.
Metal MOFs pH Illumination Source [Cr (VI)]0
(ppms)Photocatalyst
Loading
(g/L)Photo-Oxidation
EfficiencyRef. Removal
Percentage (%)Time
(min)Zr UIO-66-NH2 2 Vis. 10 0.5 97 80 [173][79] UiO-66 2 UV/Vis. 10 0.5 35 170 [255][80] UiO-66-NH2 100 100 UiO-66-NO2 12 170 UiO-66-Br 22 170 ] UiO-66-NH2/rGO 2 Visible 10 0.5 100 100 [256][81] UiO-66/g-C3N4 2 Visible 10 0.5 99 40 [257][82] MIL-100/Bi 12 O 17 C l2 200 mg 2 WL 10 0.5 99.3 120 [ 237 ][53] UiO-66(OH)2/H2BDC-(OH)2 20% 2 UV-LED 10 0.4 100 40 [258][83] UiO-66-NH2-100/PTCDA-10 2 LED-Visible 10 0.375 100 100 [259][84] UiO-66/BiOBr/Cotton fibers 2.5 Visible 5 2 99 80 [260][85] UiO-66-NH2−def 2 Visible 5 0.35 100 100 [46][86] UiO-66-NH2/Zr/Hf/
-Al2O3 membrane2 Visible 5 - 98 120 [261][87] Ti MIL-125/NH2 2.1 80 60 [222][35] MIL-101-NH2/g-C3N4 7 SL 20 MIL-125/MoS2 1 66 90 [ 232 ] [48] 6 Visible 48 0.4 20 70 [ 242][58] 2 91 MIL-125/Ag2S 38 MIL-68 3 Vis. 20 0.25 100 5 [233][ MIL-125/CdS 49 ] 40 MIL-53/WO3 2.5 SL 45 1 94 240 [234][50] MIL-100/WO3 80 wt.%/120 2 LED-Vis 5 0.25 100 60 [235][51] NH2-MIL 88B/TiO2 7 Vis. 10 0.5 MIL-125/CuS 60 MIL-125-NH2/NTU-9 3 Visible 10 1 100 90 [213][25] 98.6 35 5 70 [ 201 ] [ 13 ] MIL-53/Bi12O17Cl2 100 mg 2 WL 10 0.5 99.2 120 [236 8 ] [ 80 52 TiO2/MIL-125/core shell 2 Visible 5 0.3 100 60 [243][59] MIL-100/PANI 9% 2 NH2 WL -MIL-125/BiOI 210 0.25 100 90 Visible[238][54] 40 1 100 120 [ 211 ] [23] Fe-MOF/MoS2 1.5% 2 Vis. 20 1 98.8 60 [215][27] MIL-53 4 Vis. 20 0.5 51 30 [216][28] MIL-53/CQDs/2% Au 100 20 MIL-53/CQDs/2% Ag - - MIL-53/CQDs/2% Pd 80 30 MIL-101-NH2/Sand-Cl (50%) 2 Vis.
(1000 W)20 1.0 98.8 60 [239][55] MIL-101-NH2/Al2O3 2 SL 5 0.3 100 8 [240][56] STA-12-Mn-Fe 2 SL 20 0.25 100 30 [241][57] MIL-125-NH2/BiO 2 Vis. 40 1 100 120 [242][58] Cr MIL-101/Pt NR Vis. NR NR 100 40 [243][59] MIL-101/Pd 240 MIL-101/Pd-Cu NR Vis. NR NR 100 30 [211][23] In MIL-68 2 Vis. 20 1 97 180 [244][60] MIL-68-NH2/In0.4Fe0.6 2 Vis. 20 0.4 99 120 [245][61] * Vis. = visible light, SL = sun light, WL = white light.Although Fe-MOFs exhibit lower chemical and hydrolytic stability than chromium and aluminum homologues, this family of MOFs has been the most profoundly explored in terms of their photocatalytic activity to face hexavalent chromium pollution. The cost-effectiveness, facile fabrication, environmental friendliness, and excellent photosensitivity of the Fe-MOFs make these materials highly appealing for environmental remediation purposes. Even if the chemical strength of Fe-MOFs is lower than chromium, aluminum, or zirconium homologues, the environmental risks derived from the leakage of their components to water media is null or very low if the adequate organic linkers are selected. Furthermore, in addition to the usual photocatalytic mechanisms triggered by the illumination of the iron MOFs, Fenton-like functions of iron-based materials can generate additional radical species via metal-redox-related pathways. For instance, the ROS generated via photocatalysis can activate the Fenton catalysis as well. Among the Fe(III)-based MOFs studied for the photoreduction of Cr(VI), MIL-88B, MIL-53, MIL-101, and MIL-68 (build up from the1,4-benzenedicarboxylic acid (BDC)) and MIL-100 (assembled from trimethyl 1,3,5-benzenetricarboxylate (BTC)) are the most applied ones. Although they share common or very similar building blocks, their crystal structures differ significantly in terms of surface area, connectivity of the iron-oxo units, and connectivity of the pore space. For instance, MIL-53 and MIL-68 structures exhibit inorganic chains of corner-shared iron-oxo units with an octahedral environment that are connected through the BDC organic linkers to form a three-dimensional structure with one-dimensional pores (Figure 103). In comparison, the crystal structures of MIL-100 and MIL-88 compounds are built up from the archetypal trimeric Fe-units, which are connected in a three-dimensional framework via the BTC and BDC organic linkers (Figure 92). For more detailed information of the topology, porosity, and properties of these materials, readers may consult reference [156][62]. In comparison, the crystal structure of Fe-MIL-53 is constructed from one-dimensional Fe-oxo inorganic chains. These 1D interconnected paths within MIL-53 make it a better photoconductor in comparison to MIL-100 and MIL-88. In addition, MIL-53 shows excellent performance at slightly acidic (pH 4) to highly acidic conditions (pH 1), it is functional under visible-light illumination, and its reusability gives rise to a negligible loss of its efficiency (Table 2). Among the underlying mechanisms that can explain this performance, the direct excitation of iron-oxo cluster is one of the most interesting proposals, as reported by Laurier et al. [246][63]. Thus, the iron-oxo chains in MIL-53 adsorb incident photons under visible-light irradiation, and the photogenerated charge carriers migrate to the surface of the MOF particles participating in the redox reaction. Afterwards, the reduction of Cr(VI) to Cr(III) absorbed on the surface is driven by the photogenerated electrons (ECB = −0.40V vs. NHE at pH 6.8 and ECr(VI)/Cr(III) = + 0.51V vs. NHE, pH 6.8). Fenton mechanisms can also play an important role by directly generating (i) electrons able to potentiate the Cr(VI) photoreduction, and (ii) reactive oxygen species able to activate the iron sites to induce a Fenton parallel reaction [247][64]. In particular, the good functionality of Fe-MIL-68 under neutral to slightly acidic conditions (pH = 7–5), or its outstanding performance under acidic environments <5, further evidences the versatility of Fe-MOFs to function under varied environmental conditions (Table 2). There is plenty of room for innovation in terms of organic linkers’ functionalization in Fe-MOFs applied for chromium photoreduction. To date, most of the chemical encodings are limited to the incorporation of amino groups to the organic linkers, as are the cases of MIL-53-NH2, MIL-88B-NH2, and MIL101-NH2 materials (Table 2). Fe-MOFs, in comparison to their non-functionalized variants, is attributed to the dual excitation pathways achieved through (i) the photoinduced exciting of amine functionality followed by the electron transfer to the Fe3-μ3-oxo clusters and (ii) the direct excitation of Fe3-μ3-oxo clusters (Table 2). Just as an illustrating example, MIL-88B fails to complete the Cr(VI) reduction under visible-light irradiation (i.e., 20%) while MIL-88B-NH2 reaches the complete Cr(VI) to Cr(III) photoreduction under the same conditions. In fact, MIL-88B-NH2 outperforms the efficiency for the photocatalytic reduction of Cr(VI) under visible-light illumination of other amino-functionalized MOFs (e.g., (Zr)UiO-66-NH2 and (Ti)MIL-125-NH2), commercial inorganic and organic photocatalysts (N-doped TiO2, g-C3N4), and even of the benchmark P25-TiO2 photocatalyst when irradiated under UV–visible light (Figure 114).Fe-MOF hybrid photocatalysts have also been combined with carbon (rGO), g-C3N4, metal oxides (H3PMo12O40 (HPMo)), [9] WO3, TiO2, Bi12O17Cl2, and metal-halide and noble-metal nanoparticles (carbon quantum dots (CQDs)/Au, Ag, Pd, Pd, Pt, Au, and Ag—Table 2) to engineer advanced heterojunctions. The complexity of the heterojunctions has been steadily increased by (i) incorporating carbon/metal or metal halite/metal nanoantenna at the surface of the MOF particles, or by (ii) a direct physical mixture of MOFs and TiO2, metal/halides, and WO3 semiconductors or PANI polymeric conductors (Table 2). In parallel, (iii) the encapsulation of polyoxometalate species within the pore space of the MOF has been explored as well [248][66]. The construction of advanced heterojunctions based on carbon-based 2D materials has been explored with MIL-53 and MIL-101 materials. The doping degree of RGO or g-C3N4 is a key parameter to control in order to enhance the light-harvesting and photocatalytic efficiency of MIL-53, since surpassing a given threshold, the activity of these heterostructure materials starts to decline. MIL-101 has been modified with carboxylated g-C3N4 to improve the interfacial bonding of heterojunctions, reaching moderate chromium photoreduction efficiency in a third of the time compared to non-carboxylated systems. In general, the heterostructure MOF-metal oxide photocatalysts exhibit better performance than carbon-based homologue materials. This is the case of MIL-53/WO3, MIL-100/HPMo, and MIL-100/WO3 materials, whose heterojunctions exhibit a better interplay to couple the photocatalytic degradation and adsorption functions (Table 2). It has been observed that the best catalytic performance of metal-oxide/MOF heterostructures is obtained at pH < pHpzc. The stability, the potential reusability, and the negligible effect of organic interferents for Fe-MOF/WO3 have been duly confirmed as well. For example, MIL-100/WO3 (80 wt.–120 wt.%) displayed outstanding stability and reusability during five successive cycling experiments on Cr(VI) photoreduction in synthetic water at pH 2 and enhanced Cr(VI) removal efficiency in the presence of low-weight organic molecules. It is important to mention that the same photocatalyst is partially inhibited with the presence of inorganic competing ions such as NO3−, Cl−, and SO42−. An alternative way to achieve Cr(VI) photoreduction in neutral-pH conditions has been reported by the modification of MIL-88B with TiO2 (i.e., 98.6% at pH 7 after 35 min of irradiation with visible light) (Table 2). In contrast, the modification of MIL-53 and MIL-100 with Bi12O17Cl2 improved the catalytic response under acidic-pH conditions for composites with a weight ratio (1:1) (Fe-MOF: Bi12O17Cl2). The weight ratio of MOF/metal oxides plays an important role in the modulation of the photocatalytic performance and tuning of the heterojunctions’ efficiencies, even if those are obtained from a physical mixture of the two components of the system (Table 2). The encapsulation of metal nanoparticles, or the direct crystallization of the metal nanoparticles at the surface of the MOF nanoparticles, offers an alternative approach to engineer advanced heterojunctions. This strategy also endows hetero-photocatalysts of hot-spots arising from the plasmonic functions of noble-metal nanosystems. That is, surface plasmons open the perspective to generating localized-heat points and active catalytic sites able to potentiate the photocatalysis in a synergic manner. For instance, the complete reduction of Cr(VI) under visible irradiation was achieved in short reaction times when combining MIL-100 with gold, palladium, or platinum metal nanoparticles (Table 2). Similarly, the Cr-MIL-101 photocatalytic efficiency for reducing Cr(VI) under visible-light illumination conditions has been improved by anchoring Pt, Pd, and Cu nanoparticles on its surface. However, as is well known in metal-based catalysts applied for petrochemical applications, the agglomeration of the metal nanoparticles, or their size, induces a decay in the Cr(VI) photoreduction performance of these composite catalysts. Organic conductors as PANI polymers have also been mechanically integrated with MOF nanoparticles (i.e., MIL-100). The benefits arising from the combination of metal or organic-based electronic conductors into the framework of ordered porous materials have been duly demonstrated during the last decade. Nevertheless, it is important to note that wresearchers are far from fully understanding the underpinning mechanisms at the interphase/heterojunction between these two types of materials. Overall, considering the state-of-the-art of Fe-MOFs for chromium photoreduction, the key parameters that potentiate their performance are: (i) acidic working conditions (i.e., pH < 4), (ii) the presence of hole-trapping agents (i.e., oxalic acid), (iii) amine functionalization of the frameworks, and (iv) the concentration and size of the metal oxide, metal, or carbon nanomaterials integrated within the MOFs to engineer their photo-response. Recent studies point out the benefits of integrating MOF materials in sand [239][55] and alumina (Table 2). Multivariate chemistry is and will be an active research area to boost the photoreduction efficiency of the MOFs over Cr(VI), as explained in the next section of this work (Table 2). An illustrative example is the bimetallic Fe/In MOF (MIL-68-NH2 (InαFe1-α)). The efficiency of these photocatalysts is highly dependent on the Fe(III)/In(III) content. For an α of 0.8, the reduction efficiency was lower even than the initial In-MOF, while an optimum balance between the cations, i.e., MIL-68-NH2 (In0.4Fe0.6), gave rise to 3.6 times faster photo-reduction than for MIL-68-NH2 (In) (Table 2).
2.3. Tetravalent-Metal-Based Metal-Organic Framework Photocatalysts
Tetravalent-based MOFs stand out in terms of chemical and hydrolytic stability in comparison with most of the trivalent- and divalent-metal-based homologues. Generally speaking, these background characteristics arise from the high connectivity of their archetypal inorganic building blocks: the poly-nuclear zirconium and titanium oxo-hydroxy clusters and chain-like subunit. Since the discovery of the archetypal UiO-66 and MIL-125 zirconium and titanium, their tailor function pre- and post-synthetic encoding has exponentially increased (Figure 125).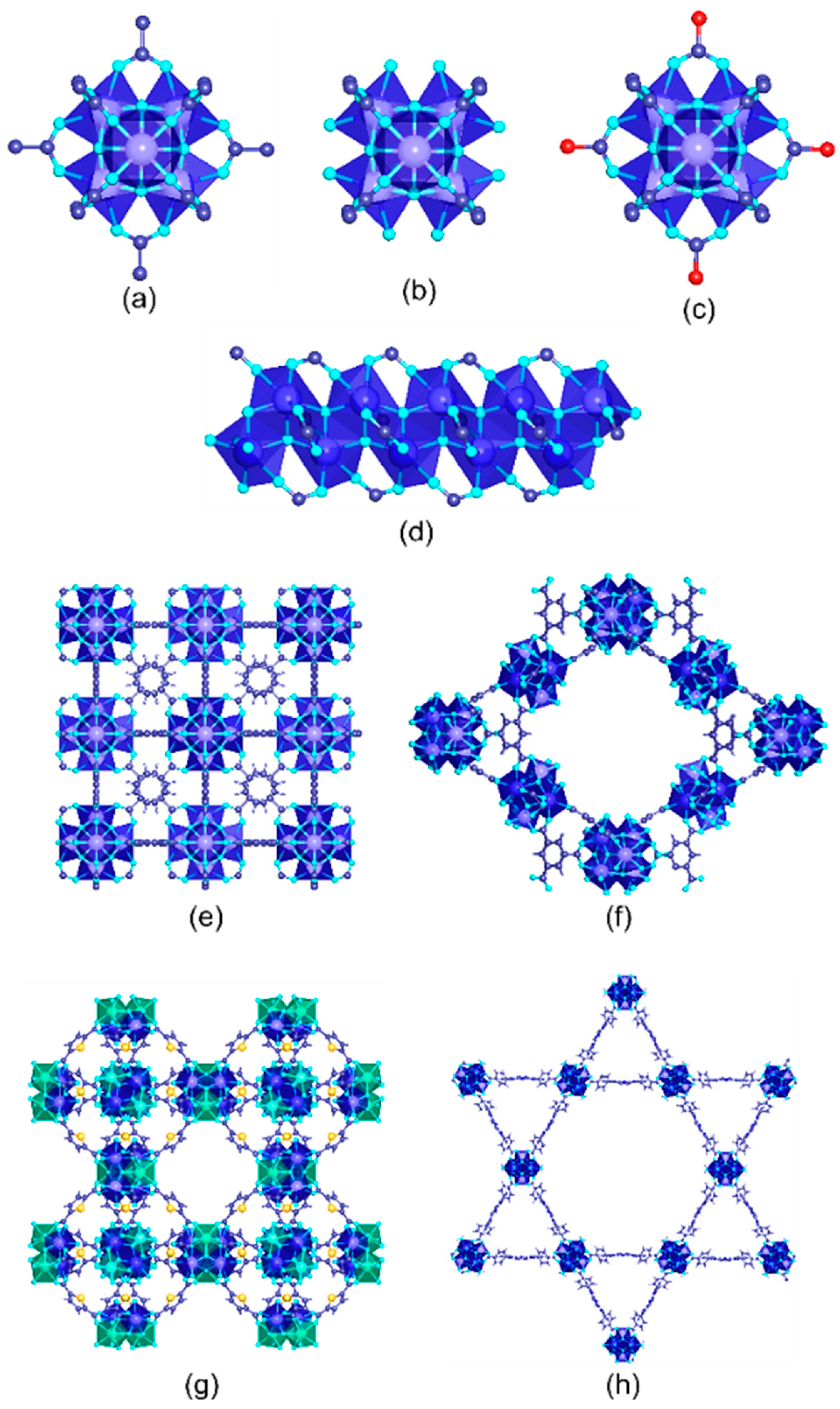 Figure 125. Inorganic structural units found in tetravalent zirconium MOFs used for metal ion recovery in aqueous media. (a) Zr6O4(OH)4(CO2)12, (b) defective Zr6O4(OH)4(CO2)12, and (c) functionalized Zr6O4(OH)4(R1-CO2)12-X(RRegarding the application of UiO-66 for photocatalysis, most of the investigations have been focused on its chemical modification to prevent the recombination of electron–hole pairs and shift the photo-absorption edge from UV (3–5% of total sunlight) to the visible-light region. By applying a similar strategy reported for divalent and trivalent based-MOFs, Shen et al. (2013) [173][79] improved the Cr(VI) to Cr(III) photoreduction efficiency of UiO-66 by encoding amino groups into its framework (i.e., UiO-66-NH2). Due to the typical yellow color of the amino-terephthalic acid, and of the UiO-66-NH2 sample, the band gap energy was shifted to the visible-light region, unlocking the capacity of the material to drive the hexavalent chromium photoreduction under sunlight illumination. Extending this initial study, the same authors (Shen et al.) explored the light-harvesting and photocatalytic activity of UiO-66-NO2 and UiO-66-Br variants (Table 3). The photoactivity of the UiO-66 frameworks was clearly related to their band gap energy, the amine variant being the one with the lower band gap energy and the most efficient one to photo-reduce chromium hexavalent species under visible-light illumination. Nevertheless, the correlation between the structure and the photoactivity of the UiO-66 frameworks was unclear. The metal substitution at the zirconium hexanuclear units of the UiO-66-NH2 framework has also been explored, both by post-synthetically doping the linker-defective positions of the structure with titanium ions [262][88]. It is interesting to note that for Ti/Zr-UiO-66, the improvement in the hexavalent chromium photoreduction does not arise from an energy band gap modification, since both Zr and Zr/Ti compounds exhibit similar light-harvesting characteristics, but from the improved photoconduction (i.e., reduced electron–hole recombination) of the Zr/Ti variant. Recently, UiO-66-(OH)2 has been revealed as the most efficient variant within the UiO-66 family to photo-reduce Cr(VI) to Cr(III). Its low band gap energy, together with its fast and efficient photoconductions, has given rise to a 100% conversion in less than 40 min under UV-Vis light illumination. It is important to note at this point that the hydroxyl variant of the UiO-66 framework shows an intermediate hydrolytic stability, so although the reusability tests indicate a negligible loss of activity, it would be key to assess to what extent the materials are releasing some of their inorganic or organic components to the media. In comparison to UiO-66, the conduction band (CB) potential of the titanium-oxo cluster in MIL-125 is more positive than the ones reported for the zirconium hexanuclear clusters. This feature induces an efficient electron transfer from the photoexcited organic linker to the titanium-oxo cluster (Table 3). The main drawback of MIL-125 is its wide band gap, limiting its light absorption only to the UV region. Amine functionalization of the MIL-125 photocatalysts has been the first approach to shift its band gap to the visible-light region, arising from the electron donation of the N2p to the aromatic ring of the amino-terephthalate linker. In addition, the amino groups within MIL-125-NH2 act as a photosensitizer, improving the photocatalytic behavior in the reduction from Cr(VI) to Cr(III). It is well known that the electron transfer from the linkers to the Ti-oxo clusters generated Ti3+-Ti4+ pairs that play an additional role in transferring the electrons to the hexavalent chromium, or in generating radical oxidative or reductive species at the surface of the MIL-125 particles (Table 3). Although the photocatalytic efficiency of MOFs has been widely proved, and the strategies to enhance their performance duly identified, until recently, their potential to work as dual sorbents/photocatalysts for the photoreduction and capture of Cr(VI) and Cr(III) has been widely overlocked. The first investigation in this regard was reported by P.G.-Saiz and coworkers for Ti and Zr benchmark UiO-66 and MIL-125 materials [46][86]. In order to elucidate the fate of Cr(III) ions during the photo-transformation of Cr(VI), the authors monitored both the Cr(VI) and Cr(III) concentration in the water solution during the Cr(VI) adsorption in dark conditions, and after triggering the photocatalysis through UV-Vis illumination. Their findings demonstrate that even though MIL-125 was the best photocatalyst in terms of Cr(VI) reduction rate, the material was not able to fully retain the Cr(III) photo-transformed species. In contrast, the UiO-66-NH2 variant showed a full retention of the Cr(III) ions during photocatalysis, although the kinetics were slightly slower than those of MIL-125 (Figure 136).It is interesting to highlight at this point a previous study of the same authors where they assessed the adsorption capacity of UiO-66 amine and hydroxyl variants for Cr(VI) and Cr(III) adsorption [135][90]. In this work, they reported an experimental approach to determine the Cr(VI), Cr(V) and Cr(III) speciation within the MOFs after adsorption. This approach can be easily adapted in the future to study the chromium speciation evolution during photocatalysis as well (Figure 147).
Figure 125. Inorganic structural units found in tetravalent zirconium MOFs used for metal ion recovery in aqueous media. (a) Zr6O4(OH)4(CO2)12, (b) defective Zr6O4(OH)4(CO2)12, and (c) functionalized Zr6O4(OH)4(R1-CO2)12-X(RRegarding the application of UiO-66 for photocatalysis, most of the investigations have been focused on its chemical modification to prevent the recombination of electron–hole pairs and shift the photo-absorption edge from UV (3–5% of total sunlight) to the visible-light region. By applying a similar strategy reported for divalent and trivalent based-MOFs, Shen et al. (2013) [173][79] improved the Cr(VI) to Cr(III) photoreduction efficiency of UiO-66 by encoding amino groups into its framework (i.e., UiO-66-NH2). Due to the typical yellow color of the amino-terephthalic acid, and of the UiO-66-NH2 sample, the band gap energy was shifted to the visible-light region, unlocking the capacity of the material to drive the hexavalent chromium photoreduction under sunlight illumination. Extending this initial study, the same authors (Shen et al.) explored the light-harvesting and photocatalytic activity of UiO-66-NO2 and UiO-66-Br variants (Table 3). The photoactivity of the UiO-66 frameworks was clearly related to their band gap energy, the amine variant being the one with the lower band gap energy and the most efficient one to photo-reduce chromium hexavalent species under visible-light illumination. Nevertheless, the correlation between the structure and the photoactivity of the UiO-66 frameworks was unclear. The metal substitution at the zirconium hexanuclear units of the UiO-66-NH2 framework has also been explored, both by post-synthetically doping the linker-defective positions of the structure with titanium ions [262][88]. It is interesting to note that for Ti/Zr-UiO-66, the improvement in the hexavalent chromium photoreduction does not arise from an energy band gap modification, since both Zr and Zr/Ti compounds exhibit similar light-harvesting characteristics, but from the improved photoconduction (i.e., reduced electron–hole recombination) of the Zr/Ti variant. Recently, UiO-66-(OH)2 has been revealed as the most efficient variant within the UiO-66 family to photo-reduce Cr(VI) to Cr(III). Its low band gap energy, together with its fast and efficient photoconductions, has given rise to a 100% conversion in less than 40 min under UV-Vis light illumination. It is important to note at this point that the hydroxyl variant of the UiO-66 framework shows an intermediate hydrolytic stability, so although the reusability tests indicate a negligible loss of activity, it would be key to assess to what extent the materials are releasing some of their inorganic or organic components to the media. In comparison to UiO-66, the conduction band (CB) potential of the titanium-oxo cluster in MIL-125 is more positive than the ones reported for the zirconium hexanuclear clusters. This feature induces an efficient electron transfer from the photoexcited organic linker to the titanium-oxo cluster (Table 3). The main drawback of MIL-125 is its wide band gap, limiting its light absorption only to the UV region. Amine functionalization of the MIL-125 photocatalysts has been the first approach to shift its band gap to the visible-light region, arising from the electron donation of the N2p to the aromatic ring of the amino-terephthalate linker. In addition, the amino groups within MIL-125-NH2 act as a photosensitizer, improving the photocatalytic behavior in the reduction from Cr(VI) to Cr(III). It is well known that the electron transfer from the linkers to the Ti-oxo clusters generated Ti3+-Ti4+ pairs that play an additional role in transferring the electrons to the hexavalent chromium, or in generating radical oxidative or reductive species at the surface of the MIL-125 particles (Table 3). Although the photocatalytic efficiency of MOFs has been widely proved, and the strategies to enhance their performance duly identified, until recently, their potential to work as dual sorbents/photocatalysts for the photoreduction and capture of Cr(VI) and Cr(III) has been widely overlocked. The first investigation in this regard was reported by P.G.-Saiz and coworkers for Ti and Zr benchmark UiO-66 and MIL-125 materials [46][86]. In order to elucidate the fate of Cr(III) ions during the photo-transformation of Cr(VI), the authors monitored both the Cr(VI) and Cr(III) concentration in the water solution during the Cr(VI) adsorption in dark conditions, and after triggering the photocatalysis through UV-Vis illumination. Their findings demonstrate that even though MIL-125 was the best photocatalyst in terms of Cr(VI) reduction rate, the material was not able to fully retain the Cr(III) photo-transformed species. In contrast, the UiO-66-NH2 variant showed a full retention of the Cr(III) ions during photocatalysis, although the kinetics were slightly slower than those of MIL-125 (Figure 136).It is interesting to highlight at this point a previous study of the same authors where they assessed the adsorption capacity of UiO-66 amine and hydroxyl variants for Cr(VI) and Cr(III) adsorption [135][90]. In this work, they reported an experimental approach to determine the Cr(VI), Cr(V) and Cr(III) speciation within the MOFs after adsorption. This approach can be easily adapted in the future to study the chromium speciation evolution during photocatalysis as well (Figure 147).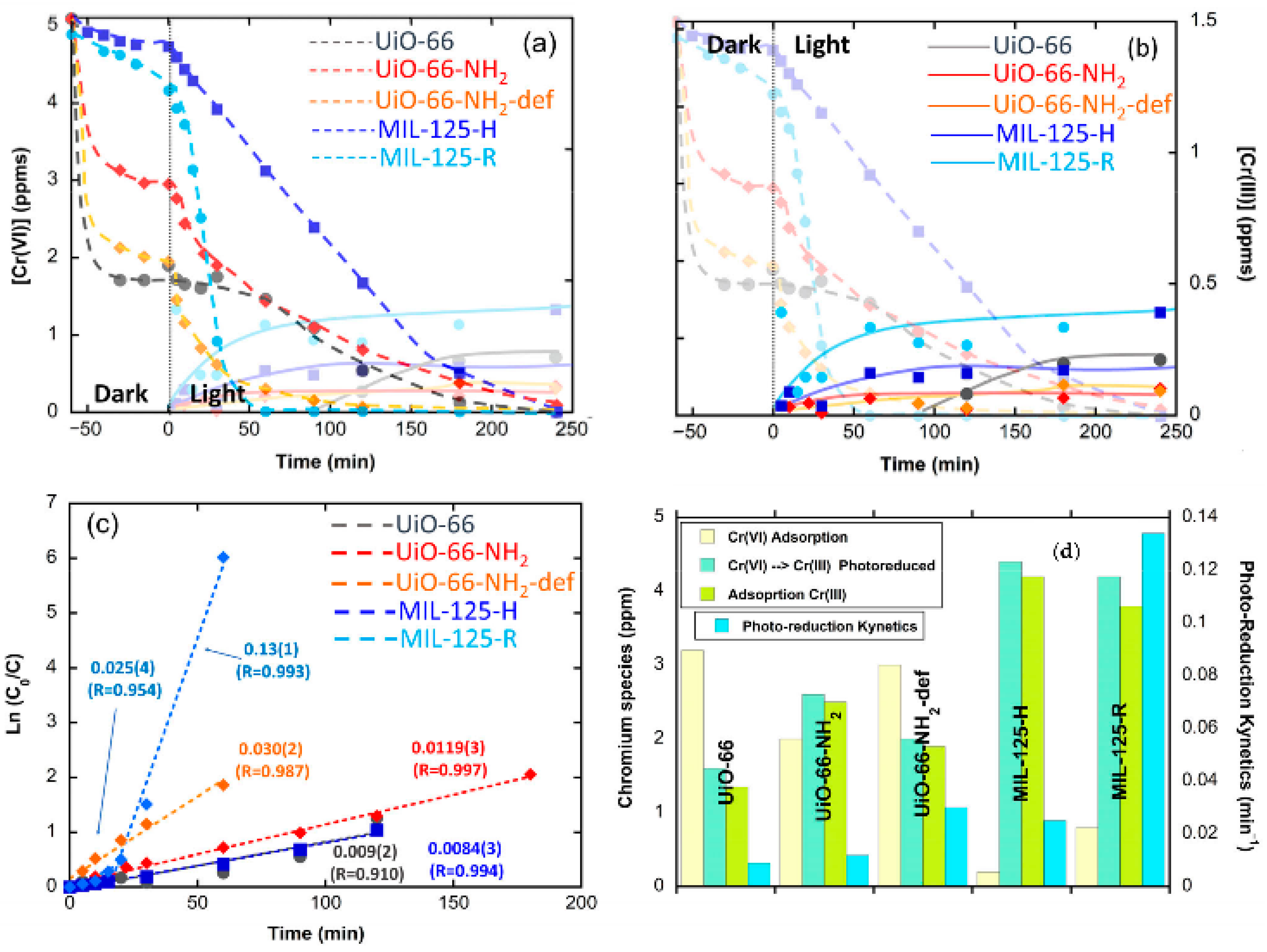 Figure 136. (a,b) Adsorption and photocatalytic reduction of Cr(VI) in the different MOF samples under UVA light: (a) Detail of the Cr(VI) and (b) Cr(III) concentration evolutions. (c) Fitting of the photoreduction kinetics. (d) Summary of Cr(VI) adsorbed at the MOF at dark conditions, the total amount of Cr(VI) photoreduced to Cr(III), the amount of photoreduced Cr(III) adsorbed at the MOF, and the photoreduction rate of the studied materials. Reproduced with permission from reference [64][89].The engineering of advanced heterojunctions has been also applied for zirconium and titanium MOFs. Shen et al. [28][92] induced an electrostatic self-assembly of UiO-66-NH2 and GO, and posteriorly, reduced GO to rGO via hydrothermal treatment. The heterostructured UiO-66-NH2/rGO improves the visible-light absorption and the efficiency to separate the photo-generated electron–hole pairs, due to the electron conductivity of graphene functionalities. Overall, UiO-66-NH2/rGO exhibits significantly improved photocatalytic activity under visible-light illumination in comparison to UiO-66-NH2. Similarly, UiO-66/C3N4 composites showed a limited recombination of photo-induced charge carriers due to the enhanced mobility of photogenerated electrons induced by g-C3N4 sheets (Table 3). In comparison to its zirconium counterpart, the heterostructured photocatalysts constructed from MIL-125 materials have been mainly based on metal oxide or metal sulphide nanoparticles. The formation of a heterojunction system consisting of narrow-gap semiconductors, such as MoS2, Ag2S, CdS, and CuS on MIL-125, shifts the absorption of the heterostructured materials to the visible region (Table 3). In this case, metal sulphide nanoparticles also have a similar sensitizer effect to that NH2 encoding into the framework [263,264][93][94]. Additionally, E. Dhivya et al. (Table 3) reported the synthesis of a heterostructure system involving two Ti-based MOFs (NH2-MIL-125 and NTU-9 (Ti)) for increasing the charge separation. In this case, the 1,4-dioxido-2,5-benzenedicarboxylate organic linkers of NTU-9 expand even more the light-harvesting capacity of the system to the visible-light region (i.e., 2.54 eV and 1.29 eV band gaps). The combined system exhibits the highest photocatalytic performance in Cr(VI) reduction due to the efficient transfer of the photogenerated electrons on the charge band of NTU-9 to the empty valence band of MIL-125-NH2.
Figure 136. (a,b) Adsorption and photocatalytic reduction of Cr(VI) in the different MOF samples under UVA light: (a) Detail of the Cr(VI) and (b) Cr(III) concentration evolutions. (c) Fitting of the photoreduction kinetics. (d) Summary of Cr(VI) adsorbed at the MOF at dark conditions, the total amount of Cr(VI) photoreduced to Cr(III), the amount of photoreduced Cr(III) adsorbed at the MOF, and the photoreduction rate of the studied materials. Reproduced with permission from reference [64][89].The engineering of advanced heterojunctions has been also applied for zirconium and titanium MOFs. Shen et al. [28][92] induced an electrostatic self-assembly of UiO-66-NH2 and GO, and posteriorly, reduced GO to rGO via hydrothermal treatment. The heterostructured UiO-66-NH2/rGO improves the visible-light absorption and the efficiency to separate the photo-generated electron–hole pairs, due to the electron conductivity of graphene functionalities. Overall, UiO-66-NH2/rGO exhibits significantly improved photocatalytic activity under visible-light illumination in comparison to UiO-66-NH2. Similarly, UiO-66/C3N4 composites showed a limited recombination of photo-induced charge carriers due to the enhanced mobility of photogenerated electrons induced by g-C3N4 sheets (Table 3). In comparison to its zirconium counterpart, the heterostructured photocatalysts constructed from MIL-125 materials have been mainly based on metal oxide or metal sulphide nanoparticles. The formation of a heterojunction system consisting of narrow-gap semiconductors, such as MoS2, Ag2S, CdS, and CuS on MIL-125, shifts the absorption of the heterostructured materials to the visible region (Table 3). In this case, metal sulphide nanoparticles also have a similar sensitizer effect to that NH2 encoding into the framework [263,264][93][94]. Additionally, E. Dhivya et al. (Table 3) reported the synthesis of a heterostructure system involving two Ti-based MOFs (NH2-MIL-125 and NTU-9 (Ti)) for increasing the charge separation. In this case, the 1,4-dioxido-2,5-benzenedicarboxylate organic linkers of NTU-9 expand even more the light-harvesting capacity of the system to the visible-light region (i.e., 2.54 eV and 1.29 eV band gaps). The combined system exhibits the highest photocatalytic performance in Cr(VI) reduction due to the efficient transfer of the photogenerated electrons on the charge band of NTU-9 to the empty valence band of MIL-125-NH2.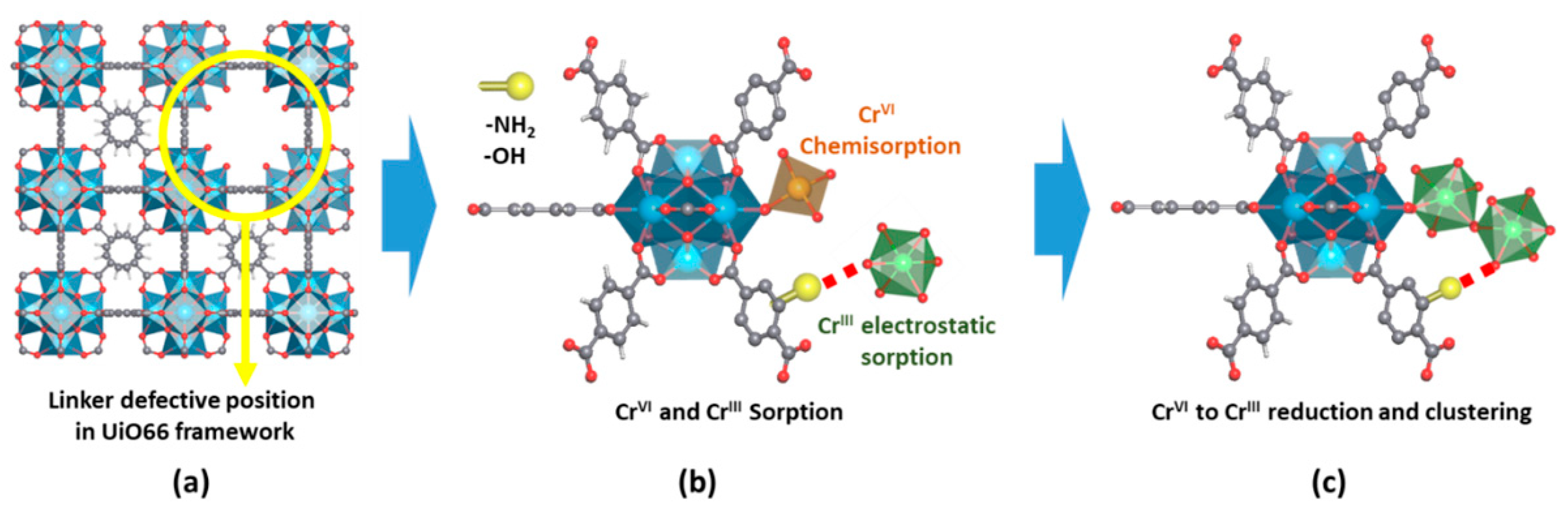 Figure 147. (a) Local structure of a linker defect position in UiO-66. (b) Detail of the possible Cr(VI) and Cr(III) adsorption positions at an under-coordinated defect position and organic linkers of zirconium hexanuclear clusters. (c) Cr(VI) to Cr(III) reduction induced by the presence of electron-donor groups at the organic linkers or photoactivity of the material. Reproduced with permission from reference [65][91].
Figure 147. (a) Local structure of a linker defect position in UiO-66. (b) Detail of the possible Cr(VI) and Cr(III) adsorption positions at an under-coordinated defect position and organic linkers of zirconium hexanuclear clusters. (c) Cr(VI) to Cr(III) reduction induced by the presence of electron-donor groups at the organic linkers or photoactivity of the material. Reproduced with permission from reference [65][91].3. Multivariate Metal-Organic Frameworks for Chromium Photoreduction
Multivariate reticular chemistry offers an opportunity to tailor and balance the light harvesting, photoconduction capacity, and oxygen radicals generation to achieve a fast and efficient chromium photoreduction. The variance of the different functional groups encoded within the ordered pore space of MOFs opens the avenue to obtain synergistic effects. For instance, multivariate MOFs (MTV-MOFs) possess more than two functionalities randomly distributed within the framework that work together in a cooperative or coupled fashion, outperforming—as an ensemble—their homogenous and periodic counterparts [264,265,266][94][95][96]. MTV-MOFs must not be mixed up with multicomponent MOFs, where the multiple linkers are topologically different from one another in terms of length and connectivity, and thus, can be distinguished individually in a crystalline lattice. Indeed, the fundamental criteria of MTV-MOFs are specific functionalities occupying a similar location in the framework and a changeable percentage of each functionality. This way, the introduction of varied functional groups can be achieved without altering the underlying backbone of their structure, obtaining a “heterogeneity within the order”. MTV-MOFs are classified as mixed-ligand (ML) and mixed-metal (MM) MOFs. Just as an illustrative example of the versatility of MTV materials, in 2010, O. M. Yaghi et al. reported the first ML-MTV-MOF, incorporating a terephthalate linker and its eight derivatives within one pure phase of a MOF-5 compound [267][97]. Since then, the application of MTV-MOFs has been expanded to many research areas, including Cr(VI) photoreduction. It is important to mention at this point that X. S. Wang et al. [268][98]. Have recently reviewed the application of MTV-MOF materials for chromium photoreduction purposes. Below, wresearchers have tried to highlight these works focused on MTV-MOF reticular materials that have been published after the seminar compilation developed by the abovementioned authors. Recently, Valverde et al. [136][99] designed a multivariate UiO-66 to develop dual sorbent photocatalysis for the removal of Cr(VI) in wastewater. Many studies have explored how replacing the original terephthalate (TPA) linker of the UiO-66 framework for some of its derivatives can endow the material with chemical (i.e., dihydroxyterephtalate (TPA-(OH)2)) and photocatalytic (i.e., aminoterephtalate (TPA-NH2)) capacity to reduce Cr(VI) to Cr(III), as with the chemical affinity to adsorb both Cr(VI) (i.e., TPA-NH2) and Cr(III) (i.e., TPA-(OH)2). However, both UiO-66-NH2 and UiO-66-(OH)2 lack the chemical robustness to work under highly acidic or caustic conditions that only the nitro-functionalized UiO-66-NO2 can tolerate (Figure 158). Multivariate reticular chemistry offers an opportunity to tailor and balance all the targeted characteristics to achieve a fast and efficient Cr(VI) to Cr(III) photoreduction via the synergistic combination of different functional groups. In this study, Valverde et al. employed multivariate functionalization strategy to tune multiple chemical characteristics of the UiO-66 structure, such as the light harvesting, the adsorption capacity over Cr(VI) and Cr(III) species, photoconduction efficiency, and Cr(VI) to Cr(III) chemical reduction and photoreduction properties (Figure 158).In parallel, they explored how the compositional variance in MTV-MOFs affects their hydrolytic stability in comparison to the one of their parent single-functionalized frameworks. From the overall performance to photo-reduce and capture chromium ions, the balanced multivariate functionalization of the UiO-66-NH2/-(OH)2/-NO2 framework resulted in a dual sorbent/photocatalyst with: (i) efficient chemical/photo-reduction of Cr(VI) to Cr(III) and (ii) retention through adsorption of the resulting Cr(III) ions. Regarding the mechanisms for the chemical and photocatalytic transformation and immobilization of chromium in the UiO-66 MTV-MOFs, it is important to note that the modification of the chromium oxidation state is linked to variation in its coordination environment. Overall, Cr(VI), stabilized as CrO42− chromate anions, gains three electrons and incorporates two hydroxyl or water molecules within its coordination environment during its reduction to Cr(III). During this process, the highly reactive and transient intermediate Cr(V) species are formed as well, as A. Valverde et al. [136][99] proved through EPR. The authors stated that the first step of the immobilization and transformation of Cr(VI) to Cr(III) into the UiO-66 frameworks is the adsorption of chromate anions (Figure 169a,b). Two possible mechanisms explain the chromate adsorption capacity of the UiO-66 frameworks, their covalent immobilization to the linker-defective positions located at the zirconium hexanuclear clusters, or their electrostatic interaction with hydroxyl, but especially, with amine-protonated groups. Reached at this point, two possible paths for the Cr(VI) to Cr(III) reduction are possible: (i) photocatalysis and (ii) chemical reduction. The chemical encoding of the UiO-66 frameworks determines the efficiency and combination of the separated paths.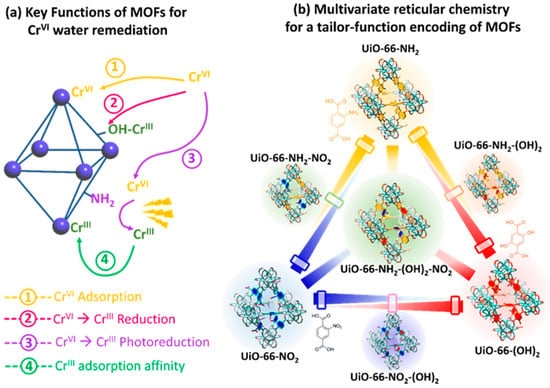 Figure 158.Illustration of the potentials of MTV-MOFs designed for improved chromium photoreduction and adsorption.During photocatalysis, the light-harvesting capacity of the UiO-66 frameworks (i.e., band gap) promotes the electron and hole separation. Nevertheless, the conduction and transfer of electron/hole pairs are governed by the photoconduction efficiency of the materials (Figure 169(c.1)). At this point, the chemical variance of MTV materials makes the difference. In fact, they realized that the incorporation of an electron donor and withdrawing groups within the same framework improves the photoconduction and fastens the Cr(VI) to Cr(III) photo-transformation. The chromate anions stabilized within the porous scaffold are steadily reduced to CrV and Cr(III) while they are immobilized into their adsorption position (Figure 169(c.2)). As the photocatalytic transformation of Cr(VI) to Cr(III) evolves, the photoreduction process is repeated, leading to the clustering of Cr(III) ions within the framework (Figure 169(c.3)). It is important to note that if the single or MTV-UiO-66 lacks hydroxyl groups, transient Cr(V) species will be stabilized within the material after operation. In contrast, when hydroxyl functionalities are encoded in UiO-66, the Cr(VI) adsorption process (Figure 169(b,d.1)) is coupled to its chemical reduction to Cr(III) via electron-rich quinone groups coming from hydroxyl functionalities (Figure 169(d.2)). Finally, they stated that as the chemical reduction, or its combination with photocatalysis, evolves, chromium ions are stabilized as clustered Cr(III) ions with the frameworks (Figure 169(d.3)). Regardless of whether photocatalysis, chemical reduction or their combination is the process that triggers the Cr(VI) transformation to Cr(III), the presence of hydroxyl ions is key to destabilizing Cr(V) transient species and transforming them into Cr(III) ions. This does not preclude the presence of Cr(VI) and Cr(III)ions within hydroxyl-functionalized frameworks, but the absence of highly reactive and toxic Cr(V). MM-MTV-MOFs are complex to synthetize. For these materials, different metals are mixed in the inorganic secondary building units (SBU). However, this often results in the synthesis of mixed MOF phases, rather than a single MM-MTV-MOF. This issue can be overcome by choosing metals with similar valences and making sure that they can form the same SBU. Another strategy to overcome this issue is the transmetalation of the material. With this post-synthetic modification, heterometallic MM-MOFs can be obtained, which cannot be achieved through normal synthesis because of the different reactivity of the metal ions [269,270][101][102]. MM-MTV-MOFs are highly promising as photocatalysts, since they offer two metal reaction centers in apparent proximity to the distinct photocatalytic performance. Even if they have not been studied yet for Cr(VI) photoreduction, they have been shown to enhance the photocatalytic activity to reduce CO2. Indeed, the presence of a second metal as an electron mediator in these materials promotes EHP transference from the excited state of the linker to metal ion clusters [271][103]. Sun et al. [272][104] introduced Ti via post-synthetic metal exchange in (Zr) UiO-66-NH2, and their material showed enhanced photocatalytic performance for both CO2 reduction and hydrogen evolution under visible light. MM-MOFs and ML-MOFs can also be merged in a single material. For instance, Navarro Amador et al. [273][105] obtained a material based on (Zr) UiO-67 with mixed ligands and mixed metals. The synthesis of the material was made through the combination of two synthetic pathways: first, the solvothermal synthesis with two different linkers (the original linker biphenyl-4,4′-dicarboxylic acid, and a similar one modified with Ru to be used as light antenna). Afterwards, Ti was included via post-synthetic metal exchange on the coordination node of UiO-67, and a material able to remove organic pollutants from an aqueous solution and to catalyze the degradation of the pollutant under visible-light irradiation was obtained. Concretely, the material showed to be active towards the degradation of methylene blue with a good improvement due to the modifications on the structure even when the exchange was not complete, proving the interaction between the light antenna and the catalytic center, since the materials with just one of the mentioned modifications did not show a big improvement in the catalytic activity. These studies show the promising materials that can be obtained via the multivariate strategy, and that could be extended to Cr(VI) photoreduction soon.
Figure 158.Illustration of the potentials of MTV-MOFs designed for improved chromium photoreduction and adsorption.During photocatalysis, the light-harvesting capacity of the UiO-66 frameworks (i.e., band gap) promotes the electron and hole separation. Nevertheless, the conduction and transfer of electron/hole pairs are governed by the photoconduction efficiency of the materials (Figure 169(c.1)). At this point, the chemical variance of MTV materials makes the difference. In fact, they realized that the incorporation of an electron donor and withdrawing groups within the same framework improves the photoconduction and fastens the Cr(VI) to Cr(III) photo-transformation. The chromate anions stabilized within the porous scaffold are steadily reduced to CrV and Cr(III) while they are immobilized into their adsorption position (Figure 169(c.2)). As the photocatalytic transformation of Cr(VI) to Cr(III) evolves, the photoreduction process is repeated, leading to the clustering of Cr(III) ions within the framework (Figure 169(c.3)). It is important to note that if the single or MTV-UiO-66 lacks hydroxyl groups, transient Cr(V) species will be stabilized within the material after operation. In contrast, when hydroxyl functionalities are encoded in UiO-66, the Cr(VI) adsorption process (Figure 169(b,d.1)) is coupled to its chemical reduction to Cr(III) via electron-rich quinone groups coming from hydroxyl functionalities (Figure 169(d.2)). Finally, they stated that as the chemical reduction, or its combination with photocatalysis, evolves, chromium ions are stabilized as clustered Cr(III) ions with the frameworks (Figure 169(d.3)). Regardless of whether photocatalysis, chemical reduction or their combination is the process that triggers the Cr(VI) transformation to Cr(III), the presence of hydroxyl ions is key to destabilizing Cr(V) transient species and transforming them into Cr(III) ions. This does not preclude the presence of Cr(VI) and Cr(III)ions within hydroxyl-functionalized frameworks, but the absence of highly reactive and toxic Cr(V). MM-MTV-MOFs are complex to synthetize. For these materials, different metals are mixed in the inorganic secondary building units (SBU). However, this often results in the synthesis of mixed MOF phases, rather than a single MM-MTV-MOF. This issue can be overcome by choosing metals with similar valences and making sure that they can form the same SBU. Another strategy to overcome this issue is the transmetalation of the material. With this post-synthetic modification, heterometallic MM-MOFs can be obtained, which cannot be achieved through normal synthesis because of the different reactivity of the metal ions [269,270][101][102]. MM-MTV-MOFs are highly promising as photocatalysts, since they offer two metal reaction centers in apparent proximity to the distinct photocatalytic performance. Even if they have not been studied yet for Cr(VI) photoreduction, they have been shown to enhance the photocatalytic activity to reduce CO2. Indeed, the presence of a second metal as an electron mediator in these materials promotes EHP transference from the excited state of the linker to metal ion clusters [271][103]. Sun et al. [272][104] introduced Ti via post-synthetic metal exchange in (Zr) UiO-66-NH2, and their material showed enhanced photocatalytic performance for both CO2 reduction and hydrogen evolution under visible light. MM-MOFs and ML-MOFs can also be merged in a single material. For instance, Navarro Amador et al. [273][105] obtained a material based on (Zr) UiO-67 with mixed ligands and mixed metals. The synthesis of the material was made through the combination of two synthetic pathways: first, the solvothermal synthesis with two different linkers (the original linker biphenyl-4,4′-dicarboxylic acid, and a similar one modified with Ru to be used as light antenna). Afterwards, Ti was included via post-synthetic metal exchange on the coordination node of UiO-67, and a material able to remove organic pollutants from an aqueous solution and to catalyze the degradation of the pollutant under visible-light irradiation was obtained. Concretely, the material showed to be active towards the degradation of methylene blue with a good improvement due to the modifications on the structure even when the exchange was not complete, proving the interaction between the light antenna and the catalytic center, since the materials with just one of the mentioned modifications did not show a big improvement in the catalytic activity. These studies show the promising materials that can be obtained via the multivariate strategy, and that could be extended to Cr(VI) photoreduction soon.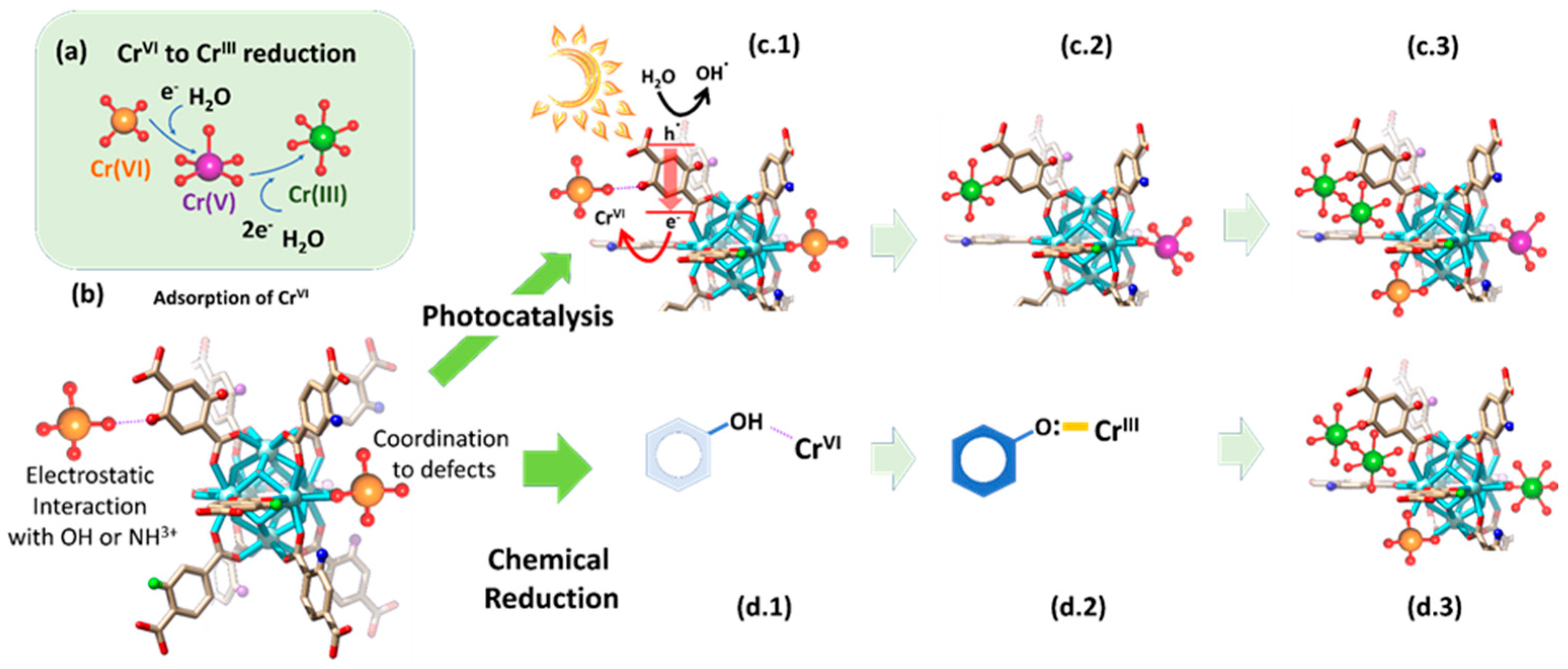 Figure 169. Cr(VI) to Cr(III) chemical and photoreduction mechanisms in MTV-UiO-66 dual sorbent/photocatalysts. (a) Overall Cr(VI) to Cr(III) reduction including Cr(V) intermediate species. (b) Adsorption mechanisms for Cr(VI) oxyanions within the MTV-UiO-66 frameworks. (c.1) Light-triggered generation of electron and holes and the concurrent electron transfer from the UiO-66-R framework to Cr(VI) oxyanions. (c.2,c.3) Evolution of the chromium species into the porous frameworks during photocatalysis. (d.1) Electrostatic interaction between hydroxyl groups and Cr(VI) ions. (d.2) Chemical reduction of Cr(VI) to Cr(III) and their stabilization into electron-rich quinone groups. (d.3) Chromium speciation within the UiO-66 framework after the chemical reduction of Cr(VI) to Cr(III), and their coordination to electron-rich quinone groups derived from hydroxyl functions. Possible chromium speciation without Cr(V) transient species within UiO-66 framework containing hydroxyl groups. Reproduced with permission from reference [73][100].
Figure 169. Cr(VI) to Cr(III) chemical and photoreduction mechanisms in MTV-UiO-66 dual sorbent/photocatalysts. (a) Overall Cr(VI) to Cr(III) reduction including Cr(V) intermediate species. (b) Adsorption mechanisms for Cr(VI) oxyanions within the MTV-UiO-66 frameworks. (c.1) Light-triggered generation of electron and holes and the concurrent electron transfer from the UiO-66-R framework to Cr(VI) oxyanions. (c.2,c.3) Evolution of the chromium species into the porous frameworks during photocatalysis. (d.1) Electrostatic interaction between hydroxyl groups and Cr(VI) ions. (d.2) Chemical reduction of Cr(VI) to Cr(III) and their stabilization into electron-rich quinone groups. (d.3) Chromium speciation within the UiO-66 framework after the chemical reduction of Cr(VI) to Cr(III), and their coordination to electron-rich quinone groups derived from hydroxyl functions. Possible chromium speciation without Cr(V) transient species within UiO-66 framework containing hydroxyl groups. Reproduced with permission from reference [73][100].
References
- Sun, W.; Xu, X.; Lv, Z.; Mao, H.; Wu, J. Environmental Impact Assessment of Wastewater Discharge with Multi-Pollutants from Iron and Steel Industry. J. Environ. Manag. 2019, 245, 210–215.
- Marinho, B.A.; Cristóvão, R.O.; Boaventura, R.A.R.; Vilar, V.J.P. As(III) and Cr(VI) Oxyanion Removal from Water by Advanced Oxidation/Reduction Processes—A Review. Environ. Sci. Pollut. Res. 2019, 26, 2203–2227.
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health Hazards of Hexavalent Chromium (Cr (VI)) and Its Microbial Reduction. Bioengineered 2022, 13, 4923–4938.
- Jin, Q.; Dai, M.; Zhan, X.; Wang, S.; He, Z. Carbon Nanotubes and Graphene Composites Used in Cr(VI) Detection Techniques: A Review. J. Alloys Compd. 2022, 922, 166268.
- De Ruiter, G.; Gupta, T.; van der Boom, M.E. Selective Optical Recognition and Quantification of Parts Per Million Levels of Cr6+ in Aqueous and Organic Media by Immobilized Polypyridyl Complexes on Glass. J. Am. Chem. Soc. 2008, 130, 2744–2745.
- Zhang, J.; Li, S. Sensors for Detection of Cr(VI) in Water: A Review. Int. J. Environ. Anal. Chem. 2021, 101, 1051–1073.
- Xing, Y.; Chen, X.; Wang, D. Electrically Regenerated Ion Exchange for Removal and Recovery of Cr(VI) from Wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443.
- Sandoval-Olvera, I.G.; González-Muñoz, P.; Palacio, L.; Hernández, A.; Ávila-Rodríguez, M.; Prádanos, P. Ultrafiltration Membranes Modified by PSS Deposition and Plasma Treatment for Cr(VI) Removal. Sep. Purif. Technol. 2019, 210, 371–381.
- Yang, L.; Xiao, Y.; Liu, S.; Li, Y.; Cai, Q.; Luo, S.; Zeng, G. Photocatalytic Reduction of Cr(VI) on WO3 Doped Long TiO2 Nanotube Arrays in the Presence of Citric Acid. Appl. Catal. B Environ. 2010, 94, 142–149.
- Gan, M.; Huang, D.; Chen, F.; Zhang, K.; Zhu, J. Enhanced Cr(VI) Reduction and Cr(III) Coprecipitation through the Synergistic Effect between Sulfide Minerals and Chemoautotrophic Decomposer. J. Environ. Chem. Eng. 2021, 9, 105942.
- Arslan, H.; Eskikaya, O.; Bilici, Z.; Dizge, N.; Balakrishnan, D. Comparison of Cr(VI) Adsorption and Photocatalytic Reduction Efficiency Using Leonardite Powder. Chemosphere 2022, 300, 134492.
- Galani, A.; Mamais, D.; Noutsopoulos, C.; Anastopoulou, P.; Varouxaki, A. Biotic and Abiotic Biostimulation for the Reduction of Hexavalent Chromium in Contaminated Aquifers. Water 2022, 14, 89.
- Yuan, R.; Yue, C.; Qiu, J.; Liu, F.; Li, A. Highly Efficient Sunlight-Driven Reduction of Cr(VI) by (Fe) Heterostructures under Neutral Conditions. Appl. Catal. B Environ. 2019, 251, 229–239.
- Wang, X.; Liu, J.; Leong, S.; Lin, X.; Wei, J.; Kong, B.; Xu, Y.; Low, Z.-X.; Yao, J.; Wang, H. Rapid Construction of Heterostructures with Size-Selective Photocatalysis Properties. ACS Appl. Mater. Interfaces 2016, 8, 9080–9087.
- Qiu, J.; Zhang, X.-F.; Zhang, X.; Feng, Y.; Li, Y.; Yang, L.; Lu, H.; Yao, J. Constructing Nanocomposites through Self-Assembly Strategy to Enhance Cr(VI) Photocatalytic Reduction. J. Hazard. Mater. 2018, 349, 234–241.
- Zhang, Y.; Park, S.-J. Facile Construction of MoO3@ZIF-8 Core-Shell Nanorods for Efficient Photoreduction of Aqueous Cr(VI). Appl. Catal. B Environ. 2019, 240, 92–101.
- Wang, F.-X.; Yi, X.-H.; Wang, C.-C.; Deng, J.-G. Photocatalytic Cr(VI) Reduction and Organic-Pollutant Degradation in a STable 2D Coordination Polymer. Chin. J. Catal. 2017, 38, 2141–2149.
- Wang, X.; Liu, W.; Fu, H.; Yi, X.-H.; Wang, P.; Zhao, C.; Wang, C.-C.; Zheng, W. Simultaneous Cr(VI) Reduction and Cr(III) Removal of Bifunctional MOF/Titanate Nanotube Composites. Environ. Pollut. 2019, 249, 502–511.
- Yi, X.-H.; Wang, F.-X.; Du, X.-D.; Wang, P.; Wang, C.-C. Facile Fabrication of BUC-21/g-C3N4 Composites and Their Enhanced Photocatalytic Cr(VI) Reduction Performances under Simulated Sunlight. Appl. Organomet. Chem. 2019, 33, e4621.
- Zhao, C.; Wang, Z.; Li, X.; Yi, X.; Chu, H.; Chen, X.; Wang, C.-C. Facile Fabrication of BUC-21/Bi24O31Br10 Composites for Enhanced Photocatalytic Cr(VI) Reduction under White Light. Chem. Eng. J. 2020, 389, 123431.
- Zhao, H.; Xia, Q.; Xing, H.; Chen, D.; Wang, H. Construction of Pillared-Layer MOF as Efficient Visible-Light Photocatalysts for Aqueous Cr(VI) Reduction and Dye Degradation. ACS Sustain. Chem. Eng. 2017, 5, 4449–4456.
- Yi, X.-H.; Wang, F.-X.; Du, X.-D.; Fu, H.; Wang, C.-C. Highly Efficient Photocatalytic Cr(VI) Reduction and Organic Pollutants Degradation of Two New Bifunctional 2D Cd/Co-Based MOFs. Polyhedron 2018, 152, 216–224.
- Dai, D.; Qiu, J.; Zhang, L.; Ma, H.; Yao, J. Amino-Functionalized Ti-Metal-Organic Framework Decorated BiOI Sphere for Simultaneous Elimination of Cr(VI) and Tetracycline. J. Colloid Interface Sci. 2022, 607, 933–941.
- Kaur, H.; Sinha, S.; Krishnan, V.; Koner, R.R. Photocatalytic Reduction and Recognition of Cr(VI): New Zn(II)-Based Metal-Organic Framework as Catalytic Surface. Ind. Eng. Chem. Res. 2020, 59, 8538–8550.
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-Light-Promoted Photocatalytic Hydrogen Production by Using an Amino-Functionalized Ti(IV) Metal-Organic Framework. J. Phys. Chem. C 2012, 116, 20848–20853.
- Yadav, M.; Xu, Q. Catalytic Chromium Reduction Using Formic Acid and Metal Nanoparticles Immobilized in a Metal-Organic Framework. Chem. Commun. 2013, 49, 3327.
- Li, W.-Q.; Wang, Y.-X.; Li, Y.-M.; He, C.-S.; Lai, B.; Chen, F.; Wang, H.-J.; Zhou, X.-G.; Mu, Y. Metal Organic Framework Decorated with Molybdenum Disulfide for Visible-Light-Driven Reduction of Hexavalent Chromium: Performance and Mechanism. J. Clean. Prod. 2021, 318, 128513.
- He, Z.; Liang, R.; Zhou, C.; Yan, G.; Wu, L. Carbon Quantum Dots (CQDs)/Noble Metal Co-Decorated MIL-53(Fe) as Difunctional Photocatalysts for the Simultaneous Removal of Cr(VI) and Dyes. Sep. Purif. Technol. 2021, 255, 117725.
- Wang, S.; Meng, F.; Sun, X.; Bao, M.; Ren, J.; Yu, S.; Zhang, Z.; Ke, J.; Zeng, L. Bimetallic Fe/In Metal-Organic Frameworks Boosting Charge Transfer for Enhancing Pollutant Degradation in Wastewater. Appl. Surf. Sci. 2020, 528, 147053.
- Huang, L.; Sun, D.-W.; Pu, H. Photosensitized Peroxidase Mimicry at the Hierarchical 0D/2D Heterojunction-Like Quasi Metal-Organic Framework Interface for Boosting Biocatalytic Disinfection. Small 2022, 18, 2200178.
- Sun, Y.; Jiang, X.; Liu, Y.; Liu, D.; Chen, C.; Lu, C.; Zhuang, S.; Kumar, A.; Liu, J. Recent Advances in Cu(II)/Cu(I)-MOFs Based Nano-Platforms for Developing New Nano-Medicines. J. Inorg. Biochem. 2021, 225, 111599.
- Birhanlı, E.; Noma, S.A.A.; Boran, F.; Ulu, A.; Yeşilada, Ö.; Ateş, B. Design of Laccase—Metal-Organic Framework Hybrid Constructs for Biocatalytic Removal of Textile Dyes. Chemosphere 2022, 292, 133382.
- Haase, F.; Hirschle, P.; Freund, R.; Furukawa, S.; Ji, Z.; Wuttke, S. Beyond Frameworks: Structuring Reticular Materials across Nano-, Meso-, and Bulk Regimes. Angew. Chem. Int. Ed. 2020, 59, 22350–22370.
- Zhang, Y.; Park, S.-J. Stabilization of Dispersed CuPd Bimetallic Alloy Nanoparticles on ZIF-8 for Photoreduction of Cr(VI) in Aqueous Solution. Chem. Eng. J. 2019, 369, 353–362.
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Jiang, L.; Li, H. Facile Synthesis of Amino-Functionalized Titanium Metal-Organic Frameworks and Their Superior Visible-Light Photocatalytic Activity for Cr(VI) Reduction. J. Hazard. Mater. 2015, 286, 187–194.
- Yang, D.; Babucci, M.; Casey, W.H.; Gates, B.C. The Surface Chemistry of Metal Oxide Clusters: From Metal-Organic Frameworks to Minerals. ACS Cent. Sci. 2020, 6, 1523–1533.
- Ji, Z.; Li, T.; Yaghi, O.M. Sequencing of Metals in Multivariate Metal-Organic Frameworks. Science 2020, 369, 674–680.
- Schrimpf, W.; Jiang, J.; Ji, Z.; Hirschle, P.; Lamb, D.C.; Yaghi, O.M.; Wuttke, S. Chemical Diversity in a Metal-Organic Framework Revealed by Fluorescence Lifetime Imaging. Nat. Commun. 2018, 9, 1647.
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a Highly Efficient Bifunctional Photocatalyst for the Simultaneous Reduction of Cr(VI) and Oxidation of Dyes. J. Hazard. Mater. 2015, 287, 364–372.
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Meng, X.; Liu, H.; Ye, J. An Amine-Functionalized Iron(III) Metal-Organic Framework as Efficient Visible-Light Photocatalyst for Cr(VI) Reduction. Adv. Sci. 2015, 2, 1500006.
- Liang, R.; Chen, R.; Jing, F.; Qin, N.; Wu, L. Multifunctional Polyoxometalates Encapsulated in MIL-100(Fe): Highly Efficient Photocatalysts for Selective Transformation under Visible Light. Dalton Trans. 2015, 44, 18227–18236.
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-Reduced Graphene Oxide Nanocomposites by a Simple Self-Assembly Strategy for Increasing Interfacial Contact: Efficient Visible-Light Photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 9507–9515.
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. (Fe) (M = Au, Pd, Pt) Nanocomposites Fabricated by a Facile Photodeposition Process: Efficient Visible-Light Photocatalysts for Redox Reactions in Water. Nano Res. 2015, 8, 3237–3249.
- Liang, R.; He, Z.; Lu, Y.; Yan, G.; Wu, L. High-Efficiency Sandwich-like Hierarchical (Fe) Photocatalysts: Step-Scheme Photocatalytic Mechanism for Enhanced Photoactivity. Sep. Purif. Technol. 2021, 277, 119442.
- Bao, M.; Liu, Y.; Sun, X.; Ren, J.; Zhang, Z.; Ke, J.; Zeng, L. Plasmonic Ag/AgCl/NH2-MIL-88B (Fe) Inorganic-Organic Hybridized Heterojunction as Visible-Light-Driven Photocatalyst for Hexavalent Chromium Reduction. J. Alloys Compd. 2021, 862, 158195.
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal Organic Framework G-C 3 N 4/MIL-53(Fe) Heterojunctions with Enhanced Photocatalytic Activity for Cr(VI) Reduction under Visible Light. Appl. Surf. Sci. 2017, 425, 107–116.
- Chen, F.; Wang, H.; Hu, H.; Gan, J.; Su, M.; Xu, H.; Wei, C. Construction of NH2-MIL-101(Fe)/g-C3N4 Hybrids Based on Interfacial Lewis Acid-Base Interaction and Its Enhanced Photocatalytic Redox Capability. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127710.
- Pattappan, D.; Kavya, K.V.; Vargheese, S.; Kumar, R.T.R.; Haldorai, Y. Graphitic Carbon Nitride/NH2-MIL-101(Fe) Composite for Environmental Remediation: Visible-Light-Assisted Photocatalytic Degradation of Acetaminophen and Reduction of Hexavalent Chromium. Chemosphere 2022, 286, 131875.
- Jing, F.; Liang, R.; Xiong, J.; Chen, R.; Zhang, S.; Li, Y.; Wu, L. MIL-68(Fe) as an Efficient Visible-Light-Driven Photocatalyst for the Treatment of a Simulated Waste-Water Contain Cr(VI) and Malachite Green. Appl. Catal. B Environ. 2017, 206, 9–15.
- Oladipo, A.A. MIL-53 (Fe)-Based Photo-Sensitive Composite for Degradation of Organochlorinated Herbicide and Enhanced Reduction of Cr(VI). Process Saf. Environ. Prot. 2018, 116, 413–423.
- Wang, J.-W.; Qiu, F.-G.; Wang, P.; Ge, C.; Wang, C.-C. Boosted Bisphenol A and Cr(VI) Cleanup over Z-Scheme WO3/MIL-100(Fe) Composites under Visible Light. J. Clean. Prod. 2021, 279, 123408.
- Li, H.; Zhao, C.; Li, X.; Fu, H.; Wang, Z.; Wang, C.-C. Boosted Photocatalytic Cr(VI) Reduction over Z-Scheme MIL-53(Fe)/Bi12O17Cl2 Composites under White Light. J. Alloy. Compd. 2020, 844, 156147.
- Zhao, C.; Wang, J.; Chen, X.; Wang, Z.; Ji, H.; Chen, L.; Liu, W.; Wang, C.-C. Bifunctional Bi12O17Cl2/MIL-100(Fe) Composites toward Photocatalytic Cr(VI) Sequestration and Activation of Persulfate for Bisphenol A Degradation. Sci. Total Environ. 2021, 752, 141901.
- Chen, D.-D.; Yi, X.-H.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.-C. Polyaniline Modified MIL-100(Fe) for Enhanced Photocatalytic Cr(VI) Reduction and Tetracycline Degradation under White Light. Chemosphere 2020, 245, 125659.
- Sadeghian, S.; Pourfakhar, H.; Baghdadi, M.; Aminzadeh, B. Application of Sand Particles Modified with NH2-MIL-101(Fe) as an Efficient Visible-Light Photocatalyst for Cr(VI) Reduction. Chemosphere 2021, 268, 129365.
- Zhao, Q.; Yi, X.-H.; Wang, C.-C.; Wang, P.; Zheng, W. Photocatalytic Cr(VI) Reduction over MIL-101(Fe)–NH2 Immobilized on Alumina Substrate: From Batch Test to Continuous Operation. Chem. Eng. J. 2022, 429, 132497.
- Garazhian, Z.; Farrokhi, A.; Rezaeifard, A.; Jafarpour, M.; Khani, R. The Enhanced Visible-Light-Induced Photocatalytic Activities of Bimetallic Mn–Fe MOFs for the Highly Efficient Reductive Removal of Cr(VI). RSC Adv. 2021, 11, 21127–21136.
- Dhivya, E.; Magadevan, D.; Palguna, Y.; Mishra, T.; Aman, N. Synthesis of Titanium Based Hetero MOF Photocatalyst for Reduction of Cr(VI) from Wastewater. J. Environ. Chem. Eng. 2019, 7, 103240.
- Li, Y.-X.; Wang, C.-C.; Fu, H.; Wang, P. Marigold-Flower-like TiO2/MIL-125 Core−shell Composite for Enhanced Photocatalytic Cr(VI) Reduction. J. Environ. Chem. Eng. 2021, 9, 105451.
- Liang, R.; Shen, L.; Jing, F.; Wu, W.; Qin, N.; Lin, R.; Wu, L. NH2-Mediated Indium Metal-Organic Framework as a Novel Visible-Light-Driven Photocatalyst for Reduction of the Aqueous Cr(VI). Appl. Catal. B Environ. 2015, 162, 245–251.
- Guerrini, L.; Graham, D. Molecularly-Mediated Assemblies of Plasmonic Nanoparticles for Surface-Enhanced Raman Spectroscopy Applications. Chem. Soc. Rev. 2012, 41, 7085.
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) Reduction in Metal-Organic Frameworks: A Mini-Review. Appl. Catal. B Environ. 2016, 193, 198–216.
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-Based Metal-Organic Frameworks As Visible Light Photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491.
- Li, W.-Q.; Wang, Y.-X.; Chen, J.-Q.; Hou, N.-N.; Li, Y.-M.; Liu, X.-C.; Ding, R.-R.; Zhou, G.-N.; Li, Q.; Zhou, X.-G.; et al. Boosting Photo-Fenton Process Enabled by Ligand-to-Cluster Charge Transfer Excitations in Iron-Based Metal Organic Framework. Appl. Catal. B Environ. 2022, 302, 120882.
- Emam, H.E.; Darwesh, O.M.; Abdelhameed, R.M. In-Growth Metal Organic Framework/Synthetic Hybrids as Antimicrobial Fabrics and Its Toxicity. Colloids Surf. B Biointerfaces 2018, 165, 219–228.
- Ye, G.; Hu, L.; Gu, Y.; Lancelot, C.; Rives, A.; Lamonier, C.; Nuns, N.; Marinova, M.; Xu, W.; Sun, Y. Synthesis of Polyoxometalate Encapsulated in UiO-66(Zr) with Hierarchical Porosity and Double Active Sites for Oxidation Desulfurization of Fuel Oil at Room Temperature. J. Mater. Chem. A 2020, 8, 19396–19404.
- Custelcean, R.; Moyer, B.A. Anion Separation with Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2007, 2007, 1321–1340.
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-Organic Framework-Based Materials: Superior Adsorbents for the Capture of Toxic and Radioactive Metal Ions. Chem. Soc. Rev. 2018, 47, 2322–2356.
- Howarth, A.J.; Liu, Y.; Hupp, J.T.; Farha, O.K. Metal-Organic Frameworks for Applications in Remediation of Oxyanion/Cation-Contaminated Water. CrystEngComm 2015, 17, 7245–7253.
- Kumar, P.; Pournara, A.; Kim, K.-H.; Bansal, V.; Rapti, S.; Manos, M.J. Metal-Organic Frameworks: Challenges and Opportunities for Ion-Exchange/Sorption Applications. Prog. Mater. Sci. 2017, 86, 25–74.
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal-Organic Framework Technologies for Water Remediation: Towards a Sustainable Ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947.
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910.
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-Based Metal-Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem. Soc. Rev. 2016, 45, 2327–2367.
- Feng, L.; Day, G.S.; Wang, K.-Y.; Yuan, S.; Zhou, H.-C. Strategies for Pore Engineering in Zirconium Metal-Organic Frameworks. Chem 2020, 6, 2902–2923.
- Chen, Y.; Zhang, X.; Mian, M.R.; Son, F.A.; Zhang, K.; Cao, R.; Chen, Z.; Lee, S.-J.; Idrees, K.B.; Goetjen, T.A.; et al. Structural Diversity of Zirconium Metal-Organic Frameworks and Effect on Adsorption of Toxic Chemicals. J. Am. Chem. Soc. 2020, 142, 21428–21438.
- Wang, S.; Cabrero-Antonino, M.; Navalón, S.; Cao, C.; Tissot, A.; Dovgaliuk, I.; Marrot, J.; Martineau-Corcos, C.; Yu, L.; Wang, H.; et al. A Robust Titanium Isophthalate Metal-Organic Framework for Visible-Light Photocatalytic CO2 Methanation. Chem 2020, 6, 3409–3427.
- Wang, S.; Reinsch, H.; Heymans, N.; Wahiduzzaman, M.; Martineau-Corcos, C.; De Weireld, G.; Maurin, G.; Serre, C. Toward a Rational Design of Titanium Metal-Organic Frameworks. Matter 2020, 2, 440–450.
- Assi, H.; Mouchaham, G.; Steunou, N.; Devic, T.; Serre, C. Titanium Coordination Compounds: From Discrete Metal Complexes to Metal-Organic Frameworks. Chem. Soc. Rev. 2017, 46, 3431–3452.
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. Multifunctional NH2-Mediated Zirconium Metal-Organic Framework as an Efficient Visible-Light-Driven Photocatalyst for Selective Oxidation of Alcohols and Reduction of Aqueous Cr(VI). Dalton Trans. 2013, 42, 13649.
- Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic Effects of Ligand Substitution on Metal-Organic Framework Photocatalysts: The Case Study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121.
- Shen, L.; Huang, L.; Liang, S.; Liang, R.; Qin, N.; Wu, L. Electrostatically Derived Self-Assembly of NH2-Mediated Zirconium MOFs with Graphene for Photocatalytic Reduction of Cr(VI). RSC Adv. 2014, 4, 2546–2549.
- Yi, X.-H.; Ma, S.-Q.; Du, X.-D.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.-C. The Facile Fabrication of 2D/3D Z-Scheme g-C3N4/UiO-66 Heterojunction with Enhanced Photocatalytic Cr(VI) Reduction Performance under White Light. Chem. Eng. J. 2019, 375, 121944.
- Li, Y.-H.; Yi, X.-H.; Li, Y.-X.; Wang, C.-C.; Wang, P.; Zhao, C.; Zheng, W. Robust Cr(VI) Reduction over Hydroxyl Modified UiO-66 Photocatalyst Constructed from Mixed Ligands: Performances and Mechanism Insight with or without Tartaric Acid. Environ. Res. 2021, 201, 111596.
- Wei, X.; Wang, C.-C.; Li, Y.; Wang, P.; Wei, Q. The Z-Scheme NH2-UiO-66/PTCDA Composite for Enhanced Photocatalytic Cr(VI) Reduction under Low-Power LED Visible Light. Chemosphere 2021, 280, 130734.
- Chen, Z.; Zhao, J.; Chen, J.; Zhang, Y.; Chen, D.; Wang, Q.; Xia, D. UiO-66/BiOBr Heterojunction Functionalized Cotton Fabrics as Flexible Photocatalyst for Visible-Light Driven Degradation of Dyes and Cr(VI). Sep. Purif. Technol. 2021, 258, 118007.
- Saiz, P.G.; Valverde, A.; Gonzalez-Navarrete, B.; Rosales, M.; Quintero, Y.M.; Fidalgo-Marijuan, A.; Orive, J.; Reizabal, A.; Larrea, E.S.; Arriortua, M.I.; et al. Modulation of the Bifunctional CrVI to CrIII Photoreduction and Adsorption Capacity in ZrIV and TiIV Benchmark Metal-Organic Frameworks. Catalysts 2021, 11, 51.
- Du, X.-D.; Yi, X.-H.; Wang, P.; Zheng, W.; Deng, J.; Wang, C.-C. Robust Photocatalytic Reduction of Cr(VI) on UiO-66-NH2(Zr/Hf) Metal-Organic Framework Membrane under Sunlight Irradiation. Chem. Eng. J. 2019, 356, 393–399.
- Feng, Y.; Chen, Q.; Cao, M.; Ling, N.; Yao, J. Defect-Tailoring and Titanium Substitution in Metal-Organic Framework UiO-66-NH 2 for the Photocatalytic Degradation of Cr(VI) to Cr(III). ACS Appl. Nano Mater. 2019, 2, 5973–5980.
- Borthakur, P.; Boruah, P.K.; Das, M.R.; Artemkina, S.B.; Poltarak, P.A.; Fedorov, V.E. Metal Free MoS2 2D Sheets as a Peroxidase Enzyme and Visible-Light-Induced Photocatalyst towards Detection and Reduction of Cr(VI) Ions. New J. Chem. 2018, 42, 16919–16929.
- Saiz, P.G.; Iglesias, N.; González Navarrete, B.; Rosales, M.; Quintero, Y.M.; Reizabal, A.; Orive, J.; Fidalgo Marijuan, A.; Larrea, E.S.; Lopes, A.C.; et al. Chromium Speciation in Zirconium-Based Metal-Organic Frameworks for Environmental Remediation. Chem. Eur. J. 2020, 26, 13861–13872.
- Liu, Y.; Mi, X.; Wang, J.; Li, M.; Fan, D.; Lu, H.; Chen, X. Two-Dimensional SnS 2 Nanosheets Exfoliated from an Inorganic–Organic Hybrid with Enhanced Photocatalytic Activity towards Cr(VI) Reduction. Inorg. Chem. Front. 2019, 6, 948–954.
- Zhao, X.; Li, J.; Li, X.; Huo, P.; Shi, W. Design of Metal-Organic Frameworks (MOFs)-Based Photocatalyst for Solar Fuel Production and Photo-Degradation of Pollutants. Chin. J. Catal. 2021, 42, 872–903.
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Zeng, G. Photodeposition of Metal Sulfides on Titanium Metal-Organic Frameworks for Excellent Visible-Light-Driven Photocatalytic Cr(VI) Reduction. RSC Adv. 2015, 5, 32531–32535.
- Luo, T.-Y.; Liu, C.; Gan, X.Y.; Muldoon, P.F.; Diemler, N.A.; Millstone, J.E.; Rosi, N.L. Multivariate Stratified Metal-Organic Frameworks: Diversification Using Domain Building Blocks. J. Am. Chem. Soc. 2019, 141, 2161–2168.
- Dong, Z.; Sun, Y.; Chu, J.; Zhang, X.; Deng, H. Multivariate Metal-Organic Frameworks for Dialing-in the Binding and Programming the Release of Drug Molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216.
- Osborn Popp, T.M.; Yaghi, O.M. Sequence-Dependent Materials. Acc. Chem. Res. 2017, 50, 532–534.
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple Functional Groups of Varying Ratios in Metal-Organic Frameworks. Science 2010, 327, 846–850.
- Wang, X.-S.; Chen, C.-H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of Adsorption and Photosensitivity Capabilities into a Cationic Multivariate Metal-Organic Framework for Enhanced Visible-Light Photoreduction Reaction. Appl. Catal. B Environ. 2019, 253, 323–330.
- Valverde, A.; Payno, D.; Lezama, L.; Wuttke, S.; Fernández de Luis, R. Multivariate Functionalization of UiO-66 for Photocatalytic Water Remediation. Adv. Sustain. Syst. 2022, 6, 2200024.
- Besharat, F.; Ahmadpoor, F.; Nasrollahzadeh, M. Graphene-Based (Nano)Catalysts for the Reduction of Cr(VI): A Review. J. Mol. Liq. 2021, 334, 116123.
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-Metal MOFs: Unique Opportunities in Metal-Organic Framework (MOF) Functionality and Design. Angew. Chem. 2019, 131, 15330–15347.
- Lalonde, M.; Bury, W.; Karagiaridi, O.; Brown, Z.; Hupp, J.T.; Farha, O.K. Transmetalation: Routes to Metal Exchange within Metal-Organic Frameworks. J. Mater. Chem. A 2013, 1, 5453.
- Granadeiro, C.M.; Ribeiro, S.O.; Karmaoui, M.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Production of Ultra-Deep Sulfur-Free Diesels Using a Sustainable Catalytic System Based on UiO-66(Zr). Chem. Commun. 2015, 51, 13818–13821.
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a Mediator for Enhancing Photocatalytic Performance via Post-Synthetic Metal Exchange in Metal-Organic Frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059.
- Navarro Amador, R.; Carboni, M.; Meyer, D. Sorption and Photodegradation under Visible Light Irradiation of an Organic Pollutant by a Heterogeneous UiO-67–Ru–Ti MOF Obtained by Post-Synthetic Exchange. RSC Adv. 2017, 7, 195–200.
More
