Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nina Pfisterer and Version 3 by Beatrix Zheng.
Pancreatic cancer is a highly lethal cancer and less than 10% of patients survive the 5-year mark. The molecular and biological underpinnings leading to this dismal prognosis are well-described, however, translation of these findings with subsequent improvement of the poor prognosis has been slow. The complex and dynamic accumulation of microbes, also called the microbiome, has recently attracted scientific interest in the pathogenesis of several diseases including pancreatic cancer. Since then, a limited number of significant findings were published pointing towards an important role of the microbiome in cancer, in particular pancreatic cancer.
- microbiome
- pancreatic ductal adenocarcinoma
- biomarker
1. The Role of the Microbiome in Pancreatic Carcinogenesis
In 2012, the International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans reported that about 13% of all global cancer cases are caused by so-called “oncomicrobes”. Eleven distinctly defined microbes evidently induce cancer, and there is experimental evidence for even more [1][36]. Contrary to these well-defined oncomicrobes in certain tumor entities, there is emerging evidence that the tumoral microbiome contributes to carcinogenesis in different ways. Figure 12 illustrates the established and putative associations between the microbiota and oncogenesis.
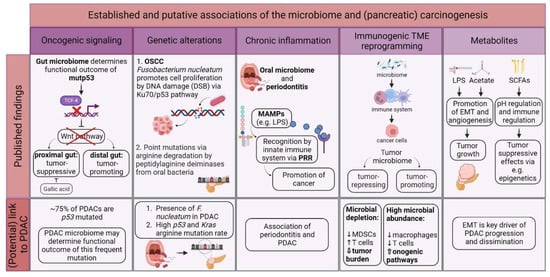
Figure 12. Potential involvement of the microbiome in (pancreatic) oncogenesis. There is growing evidence on how different microbiomes contribute to carcinogenesis, e.g., via promoting oncogenic signaling, direct and indirect genetic alterations, chronic inflammation, and interaction with the immune system and secretion of microbe-derived metabolites. However, most of these theories have yet to be validated in PDAC patients. Tumor microenvironment (TME); mutant p53 (mutp53); pancreatic ductal adenocarcinoma (PDAC); oral squamous cell carcinoma (OSCC); desoxyribonucleic acid (DNA); double-strand break (DSB); microbe-associated molecular pattern (MAMP); lipopolysaccharide (LPS); pattern recognition receptor (PRR); myeloid-derived suppressor cell (MDSC); short-chain fatty acid (SCFA); epithelial-to-mesenchymal transition (EMT); and pondus hydrogenii (pH).
2. Diagnostic Aspects of the Microbiome in PDAC
2.1. Difficulties in Establishing Screening Tools for PDAC
Considering the available descriptive and preliminary mechanistic findings on the PDAC tumor microbiome, the question of its potential diagnostic value and possible implication as a biomarker may arise. One of the main problems with PDAC is most often the late-stage diagnosis as the tumor is often locally advanced or metastasized. This is mostly due to a lack of early-stage symptoms. To date, a reliable screening method for pancreatic cancer is not available in the clinical routine [2][63]. Studies investigating different site-specific microbiomes, such as the oral and fecal microbiome, point towards a possible application of the microbiome as a diagnostic biomarker in PDAC [3][4][49,64].
2.2. The Orointestinal Microbiome as PDAC Biomarker
Indeed, there are numerous publications addressing the microbiome in the oral cavity and its diagnostic potential for PDAC, of which the latest are summarized in Table 12. One of the largest studies was published by Fan et al., which was a population-based nested case-control study on the predictive power of the oral microbiome to assess the risk for pancreatic cancer [5][65]. Over 730 oral wash samples from two prospective cohort studies were evaluated. The authors found oral pathogens such as Porphyromonas gingivalis to be associated with an increased pancreatic cancer risk. The pitfall of the microbial patterns of the oral cavity, however, is their rather pronounced heterogeneity and low specificity, as they may also be present in other cancer entities [6][66]. Microbiome studies present contradictory results concerning the microbial composition and differential abundances of these microbes (Table 12). This can be mainly ascribed to the different kinds of sampling methods, e.g., sputum, dorsal tongue, buccal, or gingival swabs. Furthermore, due to different sequencing approaches, i.e., depending on the selected variable (V) region of the 16S rRNA gene, the results significantly vary [7][67].
Table 12. Summary of studies regarding the oral, intestinal, and fecal microbiome of patients as a non-invasive biomarker for pancreatic cancer.
| Year | Authors | Study Design; Country of Conduction | Sample Type | Detection Method | Number of Patients | Change in Bacterial Composition | Ref. | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | Farrell et al. | Prospective study; USA | Saliva | Microarray, qRT-PCR | 38 PC 27 CP 38 HC |
Neisseria elongata, Streptococcus mitis | increased in PC cases | [3] | [49] | |||||||||||||||||||
| 2013 | Lin et al. | Cross-sectional study; USA | Oral wash samples | 16S rRNA amplicon sequencing (NGS) | 13 PC 3 CP 12 HC |
Bacteroides | increased in PC cases as compared with HC; | Corynebacterium, Aggregatibacter | decreased in PC cases as compared with HC | [8] | [68] | |||||||||||||||||
| 2013 | Michaud et al. | Prospective study; European countries | Blood | Immunoblot array | 405 PC 416 HC |
Plasma IgG against | Porphyromonas gingivalis | ATCC 53978 increased in PC cases | [9] | [48] | ||||||||||||||||||
| 2015 | Torres et al. | Cross-sectional study; USA | Saliva | 16S rRNA amplicon sequencing (V4 region) (NGS); qRT-PCR | 8 PC 78 other diseases (including pancreatic disease, non-pancreatic digestive disease/cancer, and non-digestive disease/cancer) 22 HC |
Leptotrichia:Porphyromonas | ratio increased in PC cases; | Neisseria, Aggregatibacter | decreased in PC cases | [10] | [69] | |||||||||||||||||
| 2016 | Fan et al. | Case-control study; USA | Oral wash samples | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 361 PDAC 371 HC |
Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans | increased in PDAC cases; | Leptotrichia | decreased in PDAC cases | [5] | [65] | |||||||||||||||||
| 2017 | Ren et al. | Prospective study; China | Feces | 16S rRNA amplicon sequencing (V3–V5 region) (NGS) | 85 PC 57 HC |
Veillonella, Klebsiella, Selenomonas | , LPS-producing bacteria ( | Prevotella, Hallella, Enterobacter, Cronobacter | ) increased in PC cases; | Bifidobacterium | , butyrate-producing bacteria ( | Coprococcus, Clostridium IV, Blautia, Flavonifractor, Anaerostipes bifidum, Butyricicoccus, Dorea, Gemmiger | ) decreased in PC cases | [11] | [70] | |||||||||||||
| 2017 | Olson et al. | Cross-sectional study; USA | Saliva | 16S rRNA amplicon sequencing (V4–V5 region) (NGS) | 40 PDAC 39 IPMN 58 HC |
Firmicutes | (e.g., | Streptococcus | ) increased in PDAC cases; | Proteobacteria | (e.g., | Haemophilus, Neisseria | ) decreased in PDAC cases as compared with HC | [12] | [71] | |||||||||||||
| 2018 | Pushalkar et al. | Case-control study; USA | Rectal swabs | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 32 PDAC 31 HC |
Proteobacteria, Actinobacteria, Fusobacteria, Verrucomicrobia, Synergistetes, Euryarchaeota | increased in PDAC cases | [13] | [16] | |||||||||||||||||||
| 2018 | Mei et al. | Case-control study; China | Duodenal mucosa | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 14 PC (pancreatic head cancer) 14 HC |
Acinetobacter, Aquabacterium, Oceanobacillus, Rahnella, Massilia, Delftia, Deinococcus, Sphingobium | increased in PC cases; | Porphyromonas, Paenibacillus, Enhydrobacter, Escherichia, Shigella, Pseudomonas | decreased in PC cases | [14] | [72] | |||||||||||||||||
| 2019 | Lu et al. | Case- control study; China | Tongue coat samples | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 30 PC (pancreatic head cancer) 25 HC |
Leptotrichia, Fusobacterium, Rothia, Actinomyces, Corynebacterium, Atopobium, Peptostreptococcus, Catonella, Oribacterium, Filifactor, Campylobacter, Moraxella, Tannerella | increased in PC cases; | Haemophilus, Porphyromonas, Paraprevotella | decreased in PC cases | [15] | [73] | |||||||||||||||||
| 2019 | del Castillo et al. | Cross-sectional study; USA | Tissue samples (pancreatic duct, duodenum, pancreas); swabs (bile duct, jejunum, stomach); feces |
16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 39 PC 12 periampullary cancer 18 non-cancer pancreatic conditions 8 non-cancer gastrointestinal conditions 34 HC |
Porphyromonas, Prevotella, Selenomonas, Gemella, Fusobacterium spp. | increased in cancer cases as compared with non-cancer cases; | Lactobacillus | decreased in cancer cases as compared with non-cancer cases | [16] | [17] | |||||||||||||||||
| 2019 | Half et al. | Case-control study; Israel | Feces | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 30 PDAC 6 pre-cancerous lesions 16 NAFLD 13 HC |
Veillonellaceae, Akkermansia, Odoribacter | increased in PDAC cases as compared with HC; | Clostridiacea, Erysipelotrichaeceae, Ruminococcaceae, Lachnospiraceae, Anaerostipes | decreased in PDAC cases as compared with HC | [17] | [74] | |||||||||||||||||
| 2020 | Vogtmann et al. | Case-control study; Iran | Saliva | 16S rRNA amplicon sequencing (V4 region) (NGS) | 273 PDAC 285 HC |
Enterobacteriaceae, Lachnospiraceae | G7, | Bacteroidaceae, Staphylococcaceae | increased in PDAC cases; | Haemophilus | decreased in PDAC cases | [18] | [75] | |||||||||||||||
| 2020 | Sun et al. | Case-control study; China | Saliva | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 10 PC 17 BPD 10 HC |
Fusobacteria | (e.g., | Fusobacterium periodonticum | ) | , Bacteroidetes, Firmicutes | increased in PC cases; | Proteobacteria | (e.g., | Neisseria mucosa | ) decreased in PC cases | [19] | [76] | |||||||||||
| 2020 | Kohi et al. | Case-control study; USA | Duodenal fluid | 16S and 18S rRNA amplicon sequencing (16S V3–V4 rRNA region, 18S ITS1 rRNA region) (NGS) | 74 PDAC 98 pancreatic cysts 134 HC |
Fusobacterium, Bifidobacterium | genera, | Enterococcus | increased in PDAC cases as compared with HC; | Escherichia/Shigella, Enterococcus, Clostridium sensu stricto 1, Bifidobacterium | increased in PDAC cases as compared with pancreatic cysts; | Fusobacterium, Rothia, Neisseria | increased in PDAC cases with short-term survival | [20] | [77] | |||||||||||||
| 2020 | Wei et al. | Case- control study; China | Saliva | 16S rRNA amplicon sequencing (V3–V4 region) (NGS) | 41 PDAC 69 HC |
Leptotrichia, Actinomyces, Lachnospiraceae, Micrococcaceae, Solobacterium, Coriobacteriaceae, Moraxellaceae, Streptococcus, Rothia, Peptostreptococcus, Oribacterium | increased in PDAC cases; | Porphyromonas gingivalis, Fusobacteriaceae, Campylobacter, Spirochaetaceae | , | Veillonella, Neisseria, Selenomona, Tannerella forsythia, Prevotella intermedia | decreased in PDAC cases | [21] | [78] | |||||||||||||||
| 2021 | Zhou et al. | Case-control study; China | Feces | Metagenomic shotgun sequencing (NGS) | 32 PDAC 32 AIP 32 HC |
Gammaproteobacteria | (e.g., | Escherichia coli | ) | , Veillonella | ( | V. atypica, V. parvula, V. dispar | ) | , Clostridium | (e.g., | Clostridium bolteae, Clostridium symbiosum | ) | , Fusobacterium nucleatum, Streptococcus parasanguinis, Prevotella stercorea | increased in PDAC cases as compared with HC; Butyrate-producing bacteria ( | Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia intestinalis, Coprococcus | ), | Ruminococcus, Dialister succinatiphilus | decreased in PDAC cases as compared with HC | [22] | [79] | |||
| 2021 | Matsukawa et al. | Case-control study; Japan | Feces | Whole-genome sequencing (including PCR) (NGS) | 24 PC (thereof 22 PDAC) 18 HC |
Klebsiella pneumoniae, Clostridium bolteae, Clostridium symbiosum, Streptococcus mutans, Alistipes shahii, Bacteroides, Parabacteroides, Lactobacillus | increased in PC cases | [23] | [80] | |||||||||||||||||||
| 2021 | Sugimoto et al. | Case-control study; Japan | Duodenal fluid | 16S rRNA terminal restriction fragment length polymorphism method (5’ FAM-labeled 516F and 1510R primers) | 22 benign pancreaticobiliary diseases (thereof 16 BPD) 12 pancreaticobiliary cancer (thereof 9 PC) |
Bifidobacterium, Clostridium | cluster XVIII increased in PC cases as compared with BPD | [24] | [81] | |||||||||||||||||||
| 2022 | Petrick et al. | Prospective study; USA | Oral wash samples | Metagenomic shotgun sequencing (NGS) | 148 PDAC (thereof 122 of African Americans, 26 of Caucasians) 441 HC (thereof 354 of African Americans, 87 of Caucasians) |
No significant changes in PDAC cases among African Americans; | Porphyromonas gingivalis | increased in PDAC cases among Caucasians; | Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia | increased in PDAC cases among never-smokers | [25] | [82] | ||||||||||||||||
| 2022 | Kartal et al. | Case- control study; Spain, Germany | Saliva | Metagenomic shotgun sequencing (NGS) (43 PDAC, 12 CP, 45 HC) 16S rRNA amplicon sequencing (V4 region) (NGS) (59 PDAC, 28 CP, 55 HC) |
59 PDAC 28 CP 55 HC (Spanish cohort only) |
No significant changes in PDAC cases | [4] | [64] | ||||||||||||||||||||
| Feces | Metagenomic shotgun sequencing (NGS) (101 PDAC, 29 CP, 82 HC) 16S rRNA amplicon sequencing (V4 region) (NGS) (51 PDAC, 23 CP, 46 HC) |
101 PDAC 29 CP 82 HC (thereof 57 PDAC, 29 CP and 50 HC from Spanish cohort; 44 PDAC and 32 HC from German cohort) |
Streptococcus, Akkermansia, Veillonella atypica, Fusobacterium nucleatum/hwasookii, Alloscardovia omnicolens | increased in PDAC cases as compared with HC; | Romboutsia timonensis, Faecalibacterium prausnitzii, Bacteroides coprocola, Bifidobacterium bifidum | decreased in PDAC cases as compared with HC | ||||||||||||||||||||||
| 2022 | Guo et al. | Case-control study; China | Feces | 16S rRNA amplicon sequencing (27F, 1492R primer) (NGS) | 36 resectable PDAC 36 unresectable PDAC |
Pseudonocardia, Cloacibacterium, Mucispirillum, Anaerotruncus | increased in unresectable PDAC cases; | Alistipes, Anaerostipes, Faecalibacterium, Parvimonas | decreased in unresectable PDAC cases | [26] | [83] | |||||||||||||||||
| 2022 | Nagata et al. | Case-control study; Japan, Spain, Germany | Saliva | Metagenomic shotgun sequencing (NGS) | 90 PDAC 280 HC (thereof 47 PDAC and 235 HC from Japanese cohort; others from Kartal et al., 2022) |
Firmicutes | (unknown | Firmicutes, Dialister | and | Solobacterium spp. | ) | , Prevotella spp. | ( | Prevotella pallens, Prevotella sp. | C561) increased in PDAC cases among Japanese cohort; | Streptococcus | spp. (e.g., | Streptococcus salivarius, Streptococcus thermophilus, Streptococcus australis | ) decreased in PDAC cases among Japanese cohort; No significant changes in PDAC cases among Spanish cohort; No correlation for oral species between the Japanese and Spanish datasets |
[27] | [84] | |||||||
| Feces | Metagenomic shotgun sequencing (NGS) | 144 PDAC 65 CP 150 IPMN 317 HC (thereof 43 PDAC, 65 CP, 150 IPMN and 235 HC from Japanese cohort; others from Kartal et al., 2022) |
Streptococcus oralis, Streptococcus vestibularis, Streptococcus anginosus, Veillonella atypica, Veillonella parvula, Actinomyces spp., Clostridium symbiosum, | unknown | Mogibacterium, Clostridium clostridioforme | increased in PDAC cases as compared with HC among Japanese cohort; Unknown | Lachnospiraceae, Eubacterium ventriosum, | unknown | Butyricicoccus, Faecalibacterium prausnitzii | decreased in PDAC cases as compared with HC among Japanese cohort; | Clostridium symbiosum, Streptococcus oralis | , unknown | Mogibacterium | increased in PDAC cases as compared with IPMN and CP among Japanese cohort; Significant correlation for gut species between the Japanese and Spanish datasets and between the Japanese and German datasets; | Streptococcus | spp. ( | S. anginosus | and | S. oralis | ), | Veillonella spp. | ( | V. parvula | and | V. atypica | ) increased in PDAC cases among all three cohorts; | Faecalibacterium prausnitzii | decreased in PDAC cases among all three cohorts |
United States of America (USA), quantitative real-time polymerase chain reaction (qRT-PCR), pancreatic cancer (PC), chronic pancreatitis (CP), healthy control (HC), Svedberg unit (S), ribosomal ribonucleic acid (rRNA), next-generation sequencing (NGS), immunoglobulin G (IgG), American Type Culture Collection (ATCC), variable (V), pancreatic ductal adenocarcinoma (PDAC), lipopolysaccharide (LPS), intraductal papillary mucinous neoplasm (IPMN), non-alcoholic fatty liver disease (NAFLD), benign pancreatic disease (BPD), internal transcribed spacer between 18S and 5.8S rRNA (ITS1), autoimmune pancreatitis (AIP), polymerase chain reaction (PCR), fluorescein amidite (FAM), forward (F), reverse (R).
Many studies have demonstrated a positive correlation between the pancreas and gut microbiome. For example, Ren et al. reported that the gut microbiome analyzed via stool samples was unique in PDAC and may serve as a non-invasive biomarker for the diagnosis of this disease [11][70]. Recently, Kartal et al. explored the fecal and salivary microbiota in PDAC patient samples from a Spanish and German case-control study as potential biomarkers; they found 27 fecal species that could be employed to identify PDAC throughout early and late stages with high accuracy. Thus, the authors suggested the fecal microbiome as a feasible early-stage PDAC biomarker, particularly in combination with carbohydrate antigen 19-9 [4][64]. However, these findings require validation in larger patient cohorts. Only a few months later, Nagata et al. reused the data from Kartal et al. and added their Japanese cohort dataset, which also included oral and gut bacteriophages [27][84]. Their aim was to further identity oral and gut metagenomic microbial signatures to predict PDAC. The authors found 30 gut and 18 oral species to be significantly associated with PDAC in their newly introduced Japanese cohort, and their metagenomic classifiers were also able to predict PDAC accurately. Consistently with Kartal et al., Nagata et al. found the gut microbiomes of European and Asian patients to present a globally robust and powerful biomarker for identifying PDAC.
Taken together, the orointestinal microbiome might be used as a non-invasive screening tool. However, the translational implication to the clinical setting remains unclear at present. Given the high cost of sequencing, a multiplex PCR or microarray for those identified bacteria might be more feasible. Furthermore, it must be discussed who will be screened, whether it be only high-risk patients or a broader screening population. Further studies with high sample numbers, such as one already completed in the U.S. (NCT03302637), will hopefully provide answers to these questions.
2.3. Blood-Derived Microbial Signatures as PDAC Biomarker
One of the most common sampling techniques in the clinical routine is blood drawing. Bacterial extracellular vesicles (bEV) are nano-sized, lipid membrane-delimited particles that contain different molecules, such as DNA, metabolites, proteins, and lipids. Recently, there is growing evidence that bEVs play an important role in bacteria–bacteria and bacteria–host communication [28][29][85,86]. These bEVs can be detected in the host’s blood, urine, bile, and stool. The exploitation of these vesicles for therapeutic and diagnostic purposes is still in its infancy [30][87]. One recent study revealed the diagnostic value of bEVs for differentiating between benign and malignant tumors [31][88]. Another Korean study performed a retrospective propensity score matching analysis showing a distinguishable composition of bEVs in blood by 16S rRNA sequencing [32][89]. Here again, environmental bacteria were detected in peripheral blood, emphasizing the need for thorough decontamination protocols for blood samples as well, in cases where bacterial DNA is found in very low concentrations [28][85]. Poore et al. demonstrated that microbial plasma profiles in over 10,000 patients, which were different from their respective healthy tissue signature, can predict different cancer types [33][90]. The authors used whole-genome and whole-transcriptome sequencing studies from TCGA. Moreover, pre-diagnosis blood samples from PDAC patients were subject to oral microbiota antibody measurements in a study by Michaud et al. Indeed, high levels of antibodies against Porphyromonas gingivalis, the pathogen responsible for periodontitis, was correlated with a two-fold increased PDAC risk [9][48].
