Exosomes are a subpopulation of extravascular vesicles with a diameter of 30–150 nm. They are cellular-communication mediators, often reaching very distant organism tissues. Information is transferred by exosomal cargo, composed of a wide variety of macromolecules such as nucleic acids, proteins, and lipids. Exosomes possess natural specific cell targeting properties that are desirable in designing targeted macromolecules (DNA and RNA) and drug delivery systems (doxorubicin, paclitaxel, and taxol). In this context, exosomes can be defined as bio-derived drug transporting and protecting devices for the treatment of bacterial (toxoplasmosis and salmonellosis), viral (AIDS and hepatitis B), and cancer (lung, pancreatic, colon, brain, and breast) diseases. Extensive research proves that exosomes’ natural cargo can double-act, both increasing and decreasing the disease severity. In this case, the exosomes need to be prepared, namely, their origin and their cargo need to be screened and known. Thus, appropriate methods for intact and price-effective exosome isolation are needed with further exosome properties description. Among many utilized isolation methods, the most common are ultracentrifugation, polymer-based precipitation, and affinity precipitation-isolation systems, but novel microfluidic methods compromising high efficacy and purity are being developed.
1. Introduction
Exosomes can serve as a drug delivery platform for macromolecules (DNA, RNA, proteins, and lipids) and drugs in hastily developing pharmaceutical units. Currently, they are under extensive research to adjust their properties to overcome specific drug delivery obstacles
[1][2][1,2].
For over the last two decades, exosomes have been isolated from many cell types including normal and cancer cells, and their impact on other cells has been studied. It is a well-known fact that their main properties come from the cargo that can vary between distinct cells. These cargoes are cell-derived particles, namely nucleic acids, proteins, and lipids, which can influence other cells’ metabolism and functionality
[3][4][3,4]. Due to the similarity of exosomes to artificial lipid microvesicles, researchers started to incorporate drugs, macromolecules, and other substances, which, for clarity, are altogether stated as drugs in this
resear
chticle [5][6][5,6]. This attitude resulted in an improvement in therapy efficiency employing increased cell-drug response, a decrease in necessary drug concentration, and an unwanted systemic organism response. The possibility of additional targeted delivery is another advantage of exosomes
[7]. Some researchers proposed advanced combined delivery strategies that improve therapeutics efficiency by additional cell expression pattern manipulation with macromolecules such as RNA or DNA
[8][9][10][8,9,10].
2. Exosomes Natural Cargo and Structure
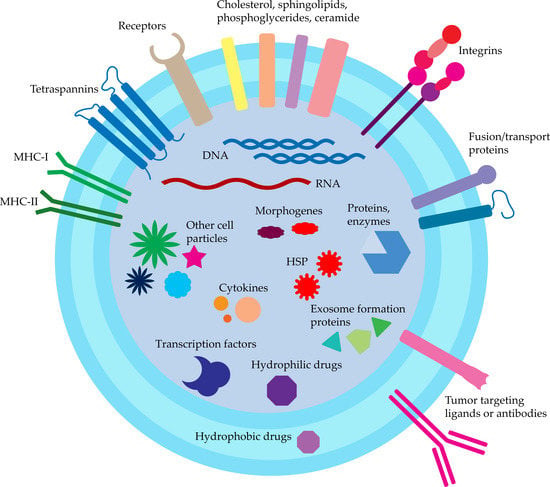
Exosomes are generally recognized as cell-to-cell signaling molecules containing numerous proteins, nucleic acids, cytokines, transcription factors, and other cell-derived particles (
Figure 12). Exosomes may deliver information not only to the local environment but also to greatly distanced cells and tissues. This may serve as a general signaling and communication pathway, but may also take part in oncogenes spread and further tumor progression and malignancy increase
[11][62]. To this date, exosomes presence was confirmed in body fluids such as amniotic liquid, blood serum, breast milk, epididymal fluid, saliva, urine, effusions of ascites and pleural, bronchoalveolar lavage fluid, synovial fluid, and cell culture supernatant in vitro
[12][13][14][15][63,64,65,66]. Due to these facts, exosomes are proposed as novel therapeutic and diagnostic (theranostics) devices
[6]. The excreted exosomes also serve as a way of unnecessary proteins and nucleic acids removal, e.g., during cell maturation (reticulocytes) or excretory system (guts and tubules of the kidney)
[16][17][18][67,68,69].
For proper usage of exosomes as a DDS,
pwe
ople first have to consider their natural cargo and the routes of their formation. Then, the characteristics of exosomes affinity and cell uptake should also be described. Exosomal cargo exhibit different purposes and functions, thanks to exosomes being basic transporters presenting outstanding properties themselves. This can be utilized in cell targeting and also as an additional enhancement of drugs. To date, ExoCarta estimates about 10,000 proteins, 3500 mRNA, and over 1100 distinct lipids found in exosomes. For the systematic review, many great databases arise such as Vesiclepedia and ExoCarta where exosome isolation procedures and sources are described along with identified cargo
[19][20][70,71]. The most important proteins for DDS design are those influencing exosomes transport and cell intake.
Proteins characteristic for exosomes are membrane-bound tetraspanins (CD9, CD63, CD81, and CD82), as well as EpCAM and Rab5, used routinely for isolation. Other commonly recognized proteins are receptors (CD46 and CD55), heat shock proteins (HSP; Hsc70, Hsp70, and Hsp90), proteins taking part in exosomes formation (Alix, TSG101), and membrane proteins responsible for fusion and transport (GTPases, annexins, and flotillin; ATP7A, ATP7B, MRP2, SLC1A4, SLC16A1, and CLIC1)
[6][21][6,44]. Detection of these markers with ELISA is used for confirmation of exosomes presence.
There are also reports about exosomes containing other particles such as cholesterol (B lymphocytes derived), sphingolipids, phosphoglycerides, and ceramide. The presence of the latter also distinguishes the exosomes from the lysosomes
[22][72].
Exosomes also transport morphogens (Hedgehog, Wingless, and Wingless-like) taking part in tissue patterning development described for
Drosophila melanogaster [13][64].
Nucleic acids are another major group of particles found in exosomes, namely DNA and RNA, which onward can take the functional role in cells. About ~1300 mRNA, 121 miRNA, and >100 small RNA were found in exosomes derived from mouse and human mast cells, while no DNA and rRNA were detected
[23][73]. Other miRNAs found in exosomes were lin-4 and let-7, as well as miR-181a and miR-155 in breast milk
[23][24][73,74].
Some of the exosomal miRNAs can be used as biomarkers in diagnostics (Table 12).
Figure 12.
Exosomes structure and cargo.
Table 12.
Examples of miRNA-level changes proposed for diagnosing diseases.
| miRNA Level Change |
Source of Exosomes |
Disease |
Source |
| ↑ miR-21 |
Serum |
Esophageal squamous cell cancer |
[25] | [75] |
| ↓ miR-21 |
Benign diseases |
|
| ↑ miR-21 |
Serum |
Hepatocellular carcinoma |
[26] | [76] |
| ↓ miR-21 |
Chronic hepatitis B or healthy volunteers as well |
|
↑ miR-1246
↑ miR-4644 |
Saliva |
Pancreatobiliary tract cancer |
[27] | [77] |
↑ bta-miR-142-5p
↑ bta-miR-223 |
Milk |
Detection of early mammary gland bacterial infection |
[28] | [78] |
↑miR-1290
↑ miR-375 |
Plasma |
Poor overall survival of castration-resistant prostate cancer patients |
[29] | [79] |
| ↑ miR-146a |
Urine |
Lupus nephritis patients. Also distinguishing between active and remission stages of the disease. |
[30] | [80] |
| ↑ miR-1910-3p |
Serum |
Breast cancer patients |
[31] | [81] |
| ↑ miR-423-5p |
Serum |
Lymph node metastasis of gastric cancer patients |
[32] | [82] |
| ↑ miR-106b |
Serum |
Lung cancer patients |
[33] | [83] |
To summarize, exosomal cargo can further improve its therapeutic properties when properly designed. This can be used for the fabrication of double-action exosomes, containing both therapeutic substances with the additional effect enhancement by incorporating proper nucleic acids, lipids, and proteins into exosomes. To this date, only a few concepts of this simultaneous strategy were proposed
[8][34][8,84].
3. Exosomes Innate Functions
Exosomes function will vary depending on their origin, cargo, and recipient cells. These innate properties can serve for further development and upgrading of exosome-based DDS. Exosomes’ most fundamental and widely recognized function is to mediate cell–cell signalization, thus regulating cell physiological functions and responses. This mechanism relies on the exchange of molecules described in the previous chapter.
Additionally, to date, many great articles arise with exosome function/effect description
[14][35][65,85]. Thus, in this
resear
ch, the researchersticle, we only mention properties influencing DDS. Exosomes derived from bone marrow, macrophages, and tumors were described as influencing the inflammatory response, either prolonging allograft rejection time or activating inflammatory cells to the target tumor
[36][37][86,87]. Exosomes also take part in the immune response and antigen presentation. Due to this fact, they can stimulate the immune response by MHC class I or II antigens presentation, which is not desirable in the case of DDS or allogenic therapeutics and can decrease their body circulation time. The current solution to this problem is exosomes isolated from mesenchymal or stromal stem cells
[38][88].
As mentioned, the natural exosomal cargo consists of various nucleic acids, proteins, and lipids, which can directly influence cell behavior and expression pattern. Similarly, artificial siRNA, mRNA, and miRNA delivered by exosomes will regulate cell expression as reported
[39][89]. In a similar manner, proteins can also act as procancerogenic agents, e.g., exosomes containing high levels of TGF-β and PGE2 will promote tumor growth
[40][90]. On the other hand, dendritic-cell-derived exosomes containing MHC class I molecules and tumor antigens are able to activate T-cell anticancer response
[37][87].
Exosomes are superior to artificial lipovesicles in terms of biocompatibility and internalization. They can also serve to reach hard-to-access and brain tissues due to the possibility of targeted delivery and blood–brain-barrier crossing, respectively
[41][28].
4. Targeted Delivery
Another factor influencing exosomes targeted delivery is the cell origin and membrane-incorporated targeting moieties
[42][43][91,92]. Isolated exosomes are proposed for targeted therapy and drug delivery to the same type of cells the exosomes were derived from. It is due to the fact that different membrane moieties are differently expressed by various cells. Surface moieties can be either expressed via genetic engineering of the endosomal sorting mechanism or fixed at the surface of released exosomes with click chemistry (
Table 23)
[35][43][85,92]. A similar approach is described for other DDS devices such as fibrin
[44][93].
Table 23.
Examples of moieties for exosome targeting.
| Moiety |
Targeted Cells/Tissue |
Source |
| Rabies viral glycoprotein |
Brain |
[45] | [94] |
| Mannose- and sialic acid- enriched glycoproteins |
Cancer cells |
[46] | [95] |
| Integrins α | 6 | β | 4 | and α | 6 | β | 1 |
Lung metastasis |
[47] | [96] |
| Integrins α | v | β | 5 |
Liver metastasis |
[48] | [97] |
| Cd63 |
Neuronal dendrites |
[49] | [98] |
| Phosphatidylethanolamine |
Glioblastoma cells |
[50] | [99] |
| Sphingomyelin |
Tumor microenvironment |
[51] | [100] |
| Metalloproteinase 15 |
Breast cancer cells (integrin α | v | β | 3 | ) |
[8] |
| Folate |
Breast cancer cells (FA receptors) |
[52] | [101] |
| CpG-STAT3 ASO |
Glioma microenvironment |
[53] | [102] |
There are also reports that certain particles, such as sphingolipids, can affect exosomes cell uptake
[56][105].
4.1. Cell Origin
Exosomes properties depend on source cells as they are closely related to overall cell processes necessary for metabolism. Crude exosomes can act as a double-agents for cancer development according to their source. Exosomes derived from normal cells will act as anticancer/antimetastatic in opposition to the reports that cancer exosomes will further increase proliferation, metastasis, and angiogenesis of cancer cells, promoting both in vitro and in vivo growth
[39][57][58][89,106,107]. Careful selection of the exosomes source is crucial for proper exosomal-based DDS development. Due to their bilateral mode of action, exosomes can both increase or restrain the proper DDS mode of action.
4.2. Cancer Cells
Targeted delivery of cancer exosomes is mediated by expressed surface proteins, e.g., tetraspanins, interacting with different cell types in a different manner
[59][108]. Tetraspanins also seem responsible for further malignancy spread; thus, therapeutic usage of this type of exosomes is not recommended
[60][109]. It is worth mentioning that cancerous exosomes were used for stimulating the immune response to prevent the remaining cancer cells to grow after resection. The immune response was mediated by tumor-specific antigens presented at the surface of exosomes administered to patients after surgery
[61][62][110,111]. Additionally, exosomes released from a dorsally implanted cell-graft composed of HT29 and HCT116 cell lines preferably locate at the stomach and intestine. This may be due to the origin of the cells composing the implant, which were derived from colorectal and colon tumors
[63][112]. This effect is also supported by higher autologous exosomes uptake by the colorectal cell line than allogenic cell-derived exosomes both in vitro and in vivo. Latter studies also revealed an increased accumulation of autologous exosomes in the tumor site
[64][113]. This effect can be overcome by expressing on the vesicles surface cancer-targeting particles such as integrins. Designed in this manner, exosomes will specifically target cells other than autologous cells, also increasing their uptake ratio
[65][114].
4.3. Normal Cells
Exosomes isolated from immune cells are recognized as safe, serving as delivery vehicles, and were proposed for use as vehicles for drugs, vaccines, and immunotherapy. The most common types of cells are dendritic cells, macrophages, monocytes, B cells, and T cells. These cells’ native function regulates the immunologic response and, thus, exosomes derived from them will also target cells recognized as hostile, e.g., cancer cells. Dendritic-cells-derived exosomes contain numerous membrane moieties that can induce cancer cells’ death themself. Macrophage culture purity needs to be maintained at a high profile due to the potential influence of pathogens on exosomes properties and mode of action
[66][115]. These exosomes also convert M2 macrophages to M1 type, increasing phagocytosis of tumor cells. Additionally, macrophage-derived exosomes stimulate the inflammatory response by the release of Th-1 type cytokines, which can be utilized in cancer vaccines, where exosomes may play the role of an adjuvant
[67][116]. Human dermal fibroblasts are also used as an exosomes source
[68][117].
4.4. Stem Cells
MSC-derived exosomes seem to be the most suitable immunologically for DDS design. These cells were isolated from various kinds of tissues such as adipose, liver, amniotic fluid, placenta, umbilical cord, and menstrual blood stem cells
[68][69][117,118]. They do not express MHC antigens at the surface, thus evading phagocytosis and fast clearance from organisms
[70][119]. Another advantage of MSCs is the production of exosomes in greater amounts than other cells. Currently, MSCs and dendritic cells are favored for use in clinical studies
[71][120].
4.5. Plants, Fruits, and Milk
Due to the high cost and problems with the isolation of large amounts of exosomes from the mentioned cells, plants and fruits are currently recognized as promising exosomes sources
[72][121]. In a similar manner, bovine milk is also an easily accessible, cost-effective source, containing significant amounts of microvesicles
[73][122]. They are generally stable in acidic environments; thus, they are great candidates for oral drugs development
[69][118]. There was no record of modifying plants or bovines in order to obtain modified exosomes.