椎间盘变性(Intervertebral disc degeneration (IDD)是一种进行性和多因素的病理过程,主要与腰痛和永久性残疾有关。焦亡是一种由炎症小体和半胱天冬酶激活引发的裂解程序性细胞死亡。与细胞凋亡不同,焦亡的特征在于质膜破裂和炎症介质的释放,加速细胞外基质(ECM)的破坏。研究表明,在 IDD 的进展中,含有), a progressive and multifactorial pathological process, is predominantly associated with low back pain and permanent disability. Pyroptosis is a type of lytic programmed cell death triggered by the activation of inflammasomes and caspases. Unlike apoptosis, pyroptosis is characterized by the rupture of the plasma membrane and the release of inflammatory mediators, accelerating the destruction of the extracellular matrix (ECM). Recent studies have shown that pyrin 结构域的 3 (NLRP3) 炎症小体介导的髓核 (NP) 细胞焦亡被激活。domain-containing 3 (NLRP3) inflammasome-mediated pyroptosis in nucleus pulposus (NP) cells is activated in the progression of IDD. Furthermore, targeting pyroptosis in IDD demonstrates the excellent capacity of ECM remodeling and its anti-inflammatory properties, suggesting that pyroptosis is involved in the IDD process. In this review, we briefly summarize the molecular mechanism of pyroptosis and the pathogenesis of IDD. We also focus on the role of pyroptosis in the pathological progress of IDD and its targeted therapeutic application.
- pyroptosis
- intervertebral disc degeneration
- NLRP3 inflammasome
- nucleus pulposus
1. Pyroptosis Triggers Cell Death in IDD
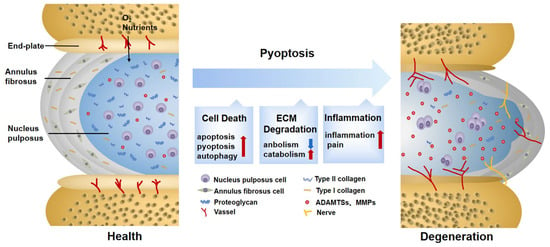
2. Pyroptosis Provokes ECM Disorder in IDD
The composition, synthesis, and reconstruction of the ECM are extremely important for the stability of the IVD internal environment. When the ECM metabolism is severely disturbed, the hydration capacity of the IVD decreases, becoming stiffer and more fragile, thus affecting biological function [70]. Previous observations have shown that pyroptosis can induce ECM degradation. For example, the downregulation of nicotinamide phosphoribosyl transferase blocks the priming phase of NLRP3 through the NF-κB and MAPK pathways, reducing the degradation of aggrecan and collagen II [71]. Cholesterol accumulates in IDD, showing a repression of aggrecan and collagen II, as well as an elevation of MMP13 and ADTAMTS5. This is related to the activation of endoplasmic reticulum (ER) stress, an activating phase of NLRP3 [72]. Although their signals differ, both of them are DAMPs, which not only activate pyroptosis in NP cells, but also reduce ECM synthesis and increase ECM degradation. In addition to directly disrupting the balance of ECM synthesis and decomposition by causing NP cell death, the inflammatory mediators and cellular contents released by pyroptosis are largely involved in ECM disorder as well. NP cells are instrumental in the ECM metabolism [73]. When healthy NP cells co-culture with pyroptotic ones, the expressions of MMP13, ADAMTS4, and ADAMTS5 are upregulated [22]. The fragment products they hydrolyze damage the stress-resistance capability of the ECM and provoke secondary inflammatory responses [74]. TNF-α and IL-1β are acknowledged factors driving the synthesis and release of MMPs and ADAMTSs [75], which are also in close contact with pyroptosis. The mediators mentioned above may result in ECM degradation caused by pyroptosis. Additionally, this phenomenon is also found in Modic EP changes and degenerated AF tissues and is thus not limited to NP cells [61,62].
In addition, ECM disorder also aggravates pyroptosis. P that anaerobic glycolysis in degenerated NP cells creates an acidic environment which activates ROS/NLRP3/caspase-1-mediated pyroptosis [23]. However, one study reached the opposite conclusion. With increased acidification, ECM degradation may be aggravated by exogenous factors rather than the decreased NLRP3 in NP cells [76]. On the other hand, the accumulation of metabolites, such as advanced glycation end products, hardens collagen fibers and facilitates the assembly of NLRP3 [77]. This shows promise in regard to restoring ECM disturbances and delaying IDD progression by inhibiting pyroptosis. An injection of VX-76, a caspase-1 inhibitor, can prevent the replacement of proteoglycans by fibrous tissue and slow the aging and fibrosis of the IVD effectively [69].
3.Pyroptosis Induces Secondary Inflammation in 焦亡诱发IDD继发性炎症
Increased secondary inflammatory cytokines are regarded as a significant feature of symptomatic IDD [78]. The inner region of the IVD lacks vascularized structures and is isolated from the host immune system. NP cells usually lack immune tolerance but are capable of phagocyting and secreting inflammatory factors. When exposed to an inflammatory environment, they will produce a solid autoimmune and inflammatory cascade [79,80]. The inflammation in IDD manifests in three ways, which are partly assigned to pyroptosis.
Changes in IVD structure and physiology lead to the production of inflammatory factors, which is the most distinctive feature of the first stage [81]. Characterized by a typical inflammatory cell death mode, pyroptosis unleashes proinflammatory factors in IVD cells either directly or indirectly [17]. As a direct product of pyroptosis, IL-1β is recognized as a key cytokine involved in discogenic back pain [82]. At the same time, when exposed to IL-1β, the expression of inflammatory factors such as IL-6 and IL-8 is significantly elevated [83]. It is not uncommon to see that some substances in IDD can trigger an inflammatory reaction by inducing pyroptosis. Extracellular ATP (eATP) is a representative co-activator of NLRP3 and P2X7R from the ATP-gated ion channel family. When strongly stimulated, P2X7R transfers to the cytoplasm to co-locate with NLRP3 and release transforming growth factor-β (TGF-β) and IL-1 . It is also related to IVD inflammation [84]. However, whether eATP causes inflammation directly or through pyroptosis remains to be further elaborated. Although IDD is mainly characterized by aseptic inflammation at this stage, infectious factors have gradually attracted attention in recent years. Among them, propionibacterium acnes account for 13% to 44% of the causes of IDD [85]. It was reported that propionibacterium acnes could induce the pyroptosis of NP cells through the ROS-NLRP3 signal pathway. Pyrolytic products aggravate the aggregation of inflammatory factors in IDD [22]. On the contrary, an injection of the NLRP3 inhibitor MCC950 successfully improves the inflammatory environment of IDD [86], suggesting that targeted pyroptosis may be a therapeutic strategy to prevent infection factors from aggravating the inflammatory progress of IDD. However, to date, in-depth insights into the specific antigens of the infectious factors inducing pyroptosis are still lacking. In addition, although pyroptosis is involved in a heightened inflammatory response in IDD, whether pyroptosis is the initial cause of IDD inflammation deserves further discussion.
The infiltration of leukocytes and the ingrowth of blood vessels and nerves are second-stage events. The results of this incremental process are closely related to the third stage, so we will expound them together. The third stage is identified by the pain symptoms caused by the sensitization of nerve endings and nociceptive neuron infiltration [81]. Sensory nerve fibers and blood vessels are usually distributed in the outer layer of AF for nutrient transport [87]. In the mouse lumbar-spine-instability model, vascular bundles and sensory nerves grew in the inner and outer regions of the AF, with calcitonin gene-related peptide (CGRP)-positive cells increasing [62]. This may be attributed to the fact that cytokines released by pyroptosis aggravate the loss of proteoglycans in the ECM and promotes angiogenesis [88]. CGRP is an important nociceptive neurotransmitter for controlling inflammation and pain [89]. Additionally, a large number of inflammatory factors released by cell rupture constantly stimulate sensory nerves, which is also a significant pain factor. A study utilizing NF-κB inhibitor Bay11-7082 on the lumbar-disc-herniation rat model found that the assembly of NLRP3 was blocked, and CGRP and pain-related behaviors such as allodynia and heat pain thresholds were ameliorated. This suggests that NF-κB, the standard signal of pain and pyroptosis, is involved in relieving neuropathic pain [90]. Moreover, although the relationship between ion channels and inflammation has not been fully explained, Ca
2+-依赖性Pizol1通道通过机械拉伸[91]激活,ASIC3通道由酸感应[23]标记,可以促进焦亡,这可能会影响IVD后期疼痛的致敏[92]。-dependent Pizol1 channels activated by mechanical stretching [91] and ASIC3 channels marked by acid sensing [23] can promote pyroptosis, which may influence the sensitization of pain in the IVD later [92].
