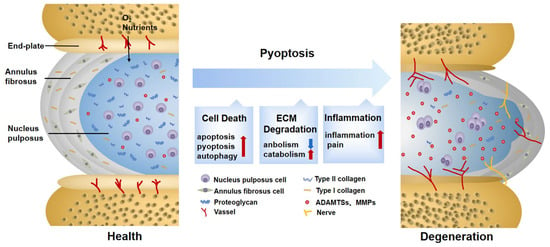
椎间盘变性(Intervertebral disc degeneration (IDD), a progressive and multifactorial pathological process, is predominantly associated with low back pain and permanent disability. Pyroptosis is a type of lytic programmed cell death triggered by the activation of inflammasomes and caspases. Unlike apoptosis, pyroptosis is characterized by the rupture of the plasma membrane and the release of inflammatory mediators, accelerating the destruction of the extracellular matrix (ECM). Recent studies have shown that DD)是一种进行性和多因素的病理过程,主要与腰痛和永久性残疾有关。焦亡是一种由炎症小体和半胱天冬酶激活引发的裂解程序性细胞死亡。与细胞凋亡不同,焦亡的特征在于质膜破裂和炎症介质的释放,加速细胞外基质(ECM)的破坏。研究表明,在 IDD 的进展中,含有 pyrin domain-containing 3 (NLRP3) inflammasome-mediated pyroptosis in nucleus pulposus (NP) cells is activated in the progression of IDD. Furthermore, targeting pyroptosis in IDD demonstrates the excellent capacity of ECM remodeling and its anti-inflammatory properties, suggesting that pyroptosis is involved in the IDD process. Here, the molecular mechanism of pyroptosis and the pathogenesis of IDD are briefly summarized. Researchers also focus on the role of pyroptosis in the pathological progress of IDD and its targeted therapeutic application.结构域的 3 (NLRP3) 炎症小体介导的髓核 (NP) 细胞焦亡被激活。
1. Pyroptosis Triggers Cell Death in IDD
Cell death, especially
nucleus pulposus (NP
) cell death, is one of the most critical factors triggering intervertebral disc (IVD) degeneration (IDD). It leads to a loss of cell function and a decline in nutrient synthesis and repair capability and, moreover, increases inflammation and oxidative stress
[1][7]. All of this aggravates the degenerative cascade in IVDs. A positive correlation between NLRP3
(NOD-like
receptor (NLR) family, pyrin containing domain 3) expression and the degenerative score was found in 45 clinical samples
[2][61]. This result demonstrated that pyroptosis is widely involved in IDD progression, which is mainly induced by NLRP3 inflammasome (
Figure 1).
Figure 1. Changes in normal and degenerative intervertebral discs. The intervertebral disc is composed of nucleus pulposus (NP), annulus fibrosus (AF), and endplate (EP), together constituting a closed buffer system against stress. While in IDD, cell death, ECM degradation, and secondary verification aggravate the vicious cycle, which is closely associated with pyroptosis. ECM: extracellular matrix; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; MMPs: matrix metalloproteinases.
Besides directly causing cell death, pyroptosis and other programmed-cell-death pathways are jointly cross-regulated in the complex and harsh degenerative environment. It was found that, after lumbar spine instability surgery, the height of the IVD was reduced in mice, and fissures and folds were formed between the AF layers, with osteophyte formation in the EP. The TUNEL assay and pyroptosis-related protein detection showed that both apoptosis and pyroptosis in the IDD-model group were potentiated
[3][62]. Studies on anti-inflammatory protein A20 further revealed that when pyroptosis was inhibited, the expression of inflammatory cytokines coupled with apoptosis was also markedly reduced
[4][63]. The above results suggest that there is an interplay between apoptosis and pyroptosis in IDD. The molecular mechanism behind the interplay may be explained by PANoptosis
[5][64], which is characterized by pyroptosis, apoptosis, and necroptosis. Li et al.
[6][65] first discovered PANoptosis, consisting of pyroptosis and apoptosis, in IDD lesions. They used different concentrations of TNF-α to treat NP cells and found that both pyroptosis and apoptosis were upregulated. However, pyroptosis was predominant at low concentrations, whereas apoptosis was predominant at high concentrations, implying that the major type of cell death may vary during different periods of IDD progression
[6][65]. This interconverting relationship may be partially explained by the altered concentrations of inflammatory mediators in the IDD microenvironment and the activation of the CASP3-GSDME pathway in the crosstalk of pyroptosis and apoptosis
[7][66]. Moreover, the relationship between pyroptosis and autophagy is also intriguing. In bromodomain-containing protein 4-inhibited rat NP cells, enhanced autophagy was able to reduce pyroptosis to relieve degenerative development. Whether autophagy enhancement is linked with attenuated pyroptosis requires further investigation; however, it has been demonstrated that the NF-κB signaling pathway is involved
[8][67]. Similarly, one study found a tendency for autophagic vacuoles to increase with higher microtubule-associated protein 1 light chain 3 (LC3)-II/LC3-I ratios in NLRP3 knockout NP cells, demonstrating that pyroptosis and autophagy are antagonistic to each other
[9][68]. Moreover, some researchers have tried to explore the mechanism underlying this phenomenon. One study pointed out that autophagy protects against LPS-induced NP cell pyroptosis. The explicit regulatory relationship between them depends on SQSTM1/p62, one of the autophagy-related proteins, co-localizing with GSDMD-N. It degraded GSDMD-N through the autophagy–lysosome pathway, thereby attenuating pyroptosis
[10][69]. In conclusion, the interplay between pyroptosis and other programmed cell deaths of NP cells in IDD currently remains questionable. It is suggested that subsequent experiments focus on this aspect, which may have a significant role in revealing the progression of IDD.
2. Pyroptosis Provokes ECM Disorder in IDD
The composition, synthesis, and reconstruction of the ECM
are extremely important for the stability of the 的组成、合成和重建对于IVD
internal environment. When the ECM metabolism is severely disturbed, the hydration capacity of the IVD decreases, becoming stiffer and more fragile, thus affecting biological function [11]. Previous observations have shown that pyroptosis can induce 内部环境的稳定性极为重要。当ECM代谢受到严重干扰时,IVD的水合能力下降,变得更僵硬和脆弱,从而影响生物学功能[70]。先前的观察表明,焦亡可以诱导ECM
degradation. For example, the downregulation of nicotinamide phosphoribosyl transferase blocks the priming phase of 降解。例如,烟酰胺磷酸核糖转移酶的下调通过N
LRP3 through the NF-κB and MAPK pathways, reducing the degradation of aggrecan and collagen II [12]. Cholesterol accumulates in F-κB和MAPK途径阻断NLRP3的启动阶段,从而减少聚集聚糖和胶原蛋白II的降解[71]。胆固醇在IDD
, showing a repression of aggrecan and collagen 中积聚,显示聚集聚糖和胶原蛋白II
, as well as an elevation of MMP13 and 的抑制,以及MMP13和ADTAMTS5
. This is related to the activation of endoplasmic reticulum (ER) stress, an activating phase of NLRP3 [13]. Although their signals differ, both of them are 的升高。这与NLRP3的激活期ER应激的激活有关[72]。尽管它们的信号不同,但它们都是DAMP
s, which not only activate pyroptosis in ,不仅可以激活NP
cells, but also reduce ECM synthesis and increase ECM degradation. In addition to directly disrupting the balance of ECM synthesis and decomposition by causing NP cell death, the inflammatory mediators and cellular contents released by pyroptosis are largely involved in ECM disorder as well. NP cells are instrumental in the ECM metabolism [14]. When healthy 细胞中的焦亡,还可以减少ECM合成并增加ECM降解。除了通过引起NP细胞死亡直接破坏ECM合成和分解的平衡外,焦亡释放的炎症介质和细胞内容物也在很大程度上参与ECM紊乱。NP细胞有助于ECM代谢[73]。当健康的NP
cells co-culture with pyroptotic ones, the expressions of 细胞与焦解细胞共培养时,MMP13
, 、ADAMTS4
, and 和ADAMTS5
are upregulated [15]. The fragment products they hydrolyze damage the stress-resistance capability of the 的表达上调[22]。它们水解的片段产物会破坏ECM
and provoke secondary inflammatory responses [16]. 的抗应激能力,并引发继发性炎症反应[74]。TNF-α
and 和IL-1β
are acknowledged factors driving the synthesis and release of MMPs and 是公认的驱动MMP和ADAMTSs
[17], which are also in close contact with pyroptosis. The mediators mentioned above may result in 合成和释放的因素[75],它们也与焦亡密切相关。上述介质可能导致焦亡引起的ECM
degradation caused by pyroptosis. Additionally, this phenomenon is also found in 降解。此外,这种现象也见于Modic EP
changes and degenerated AF tissues and is thus not limited to NP cells [2][3].
In addition, 改变和退行性AF组织,因此不仅限于NP细胞[61,62]。
此外,ECM
disorder also aggravates pyroptosis. Previous research found that anaerobic glycolysis in degenerated 紊乱也会加重焦亡。先前的研究发现,退化NP
cells creates an acidic environment which activates 细胞中的厌氧糖酵解会产生酸性环境,激活ROS/NLRP3/
caspase-1-mediated pyroptosis [18]. However, one study reached the opposite conclusion. With increased acidification, 半胱天冬酶-1介导的焦亡[23]。然而,一项研究得出了相反的结论。随着酸化加剧,外源性因素可能加剧ECM
degradation may be aggravated by exogenous factors rather than the decreased 降解,而不是NP细胞NLRP3
in 降低[76]。另一方面,代谢物(如晚期糖基化终产物)的积累使胶原纤维变硬并促进N
P cells [19]. On the other hand, the accumulation of metabolites, such as advanced glycation end products, hardens collagen fibers and facilitates the assembly of NLRP3
[20]. This shows promise in regard to restoring 的组装[77]。这在通过抑制焦亡来恢复ECM
disturbances and delaying 紊乱和延缓IDD
progression by inhibiting pyroptosis. An injection of VX-76, a caspase-1 inhibitor, can prevent the replacement of proteoglycans by fibrous tissue and slow the aging and fibrosis of the IVD effectively [10].
进展方面显示出希望。注射半胱天冬酶-1抑制剂VX-76可防止纤维组织替代蛋白聚糖,有效延缓IVD的衰老和纤维化[69]。
3. Pyroptosis 焦亡诱发Induces Secondary Inflammation in IDD继发性炎症
继发性炎症细胞因子增加被认为是症状性I
ncreased secondary inflammatory cytokines are regarded as a significant feature of symptomatic IDD
[21]. The inner region of the 的重要特征[78]。IVD
lacks vascularized structures and is isolated from the host immune system. 的内部区域缺乏血管化结构,并与宿主免疫系统隔离。NP
cells usually lack immune tolerance but are capable of phagocyting and secreting inflammatory factors. When exposed to an inflammatory environment, they will produce a solid autoimmune and inflammatory cascade [22][23]. The inflammation in 细胞通常缺乏免疫耐受性,但能够吞噬细胞和分泌炎症因子。当暴露于炎症环境时,它们会产生固体自身免疫性和炎症级联反应[79,80]。IDD
manifests in three ways, which are partly assigned to pyroptosis.
Changes in 的炎症表现为三种方式,部分归因于焦亡。
IVD
structure and physiology lead to the production of inflammatory factors, which is the most distinctive feature of the first stage [24]. Characterized by a typical inflammatory cell death mode, pyroptosis unleashes proinflammatory factors in 结构和生理学的改变导致炎症因子的产生,这是第一阶段最显著的特征[81]。焦亡的特征是典型的炎症细胞死亡模式,直接或间接地在IVD
cells either directly or indirectly [25]. As a direct product of pyroptosis, 细胞中释放促炎因子[17]。作为焦亡的直接产物,IL-1β
is recognized as a key cytokine involved in discogenic back pain [26]. At the same time, when exposed to 被认为是参与椎间盘性背痛的关键细胞因子[82]。同时,当暴露于IL-1β
, the expression of inflammatory factors such as 时,IL-6
and IL-8 is significantly elevated [27]. 和IL-8等炎症因子的表达明显升高[83]。I
t is not uncommon to see that some substances in IDD
can trigger an inflammatory reaction by inducing pyroptosis. Extracellular ATP (eATP) is a representative co-activator of NLRP3 and P2X7R from the ATP-gated ion channel family. When strongly stimulated, P2X7R transfers to the cytoplasm to co-locate with NLRP3 and release transforming growth factor-β (TGF-β) and IL-1. It is also related to IVD inflammation [28]. However, wh中的某些物质可以通过诱导焦亡来引发炎症反应的情况并不少见。细胞外ATP(eATP)是来自ATP门控离子通道家族的NLRP3和P2X7R的代表共激活剂。当受到强烈刺激时,P2X7R转移到细胞质中与NLRP3共定位并释放转化生长因子β(TGF-β)和IL-1。它也与IVD炎症有关[84]。然而,e
ther eATP
causes inflammation directly or through pyroptosis remains to be further elaborated. Although IDD is mainly characterized by aseptic inflammation at this stage, infectious factors have gradually attracted attention in recent years. Among them, propionibacterium acnes account for 13% to 44% of the causes of IDD [29]. It was reported that propionibacterium acnes could induce the pyroptosis of NP cells through the 是否直接或通过焦亡引起炎症仍有待进一步阐述。虽然IDD现阶段主要以无菌性炎症为特征,但近年来感染因素逐渐引起人们的关注。其中,痤疮丙杆菌占IDD病因的13%-44%[85]。据报道,痤疮丙酸杆菌可通过ROS-NLRP3
signal pathway. 信号通路诱导NP
yrolytic products aggravate the aggregation of inflammatory factors in IDD [15]. On the contrary, an injection of the 细胞的焦亡。热解产物会加重IDD中炎症因子的聚集[22]。相反,注射NLRP3
inhibitor 抑制剂MCC950
successfully improves the inflammatory environment of IDD [30], suggesting that targeted pyroptosis may be a therapeutic strategy to prevent infection factors from aggravating the inflammatory progress of 成功改善了IDD的炎症环境[86],表明靶向焦亡可能是防止感染因素加重IDD
. However, to date, in-depth insights into the specific antigens of the infectious factors inducing pyroptosis are still lacking. 炎症进展的治疗策略。然而,迄今为止,仍然缺乏对诱导焦亡的感染因子的特定抗原的深入见解。此外,尽管焦亡与I
n addition, although pyroptosis is involved in a heightened inflammatory response in IDD, whether pyroptosis is the initial cause of IDD inflammation deserves further discussion.
The infiltration of leukocytes and the ingrowth of blood vessels and nerves are second-stage events. The results of this incremental process are closely related to the third stage, so researchers will expound them together. The third stage is identified by the pain symptoms caused by the sensitization of nerve endings and nociceptive neuron infiltration [24]. Sensory nerve fibers and blood vessels are usually distributed in the outer layer of DD的炎症反应增强有关,但焦亡是否是IDD炎症的最初原因值得进一步讨论。白细胞的浸润以及血管和神经的向内生长是第二阶段的事件。这个渐进过程的结果与第三阶段密切相关,因此将一起阐述。第三阶段由神经末梢致敏和伤害性神经元浸润引起的疼痛症状确定[81]。感觉神经纤维和血管通常分布在AF
tissue for nutrient transport [31]. In the mouse lumbar组织的外层,用于营养输送[87]。在小鼠腰椎-
spine-instability model, vascular bundles and sensory nerves grew in the inner and outer regions of the 脊柱不稳定模型中,血管束和感觉神经在AF
, with calcitonin gene-related peptide (CGRP)-positive cells increasing [3]. This may be attributed to the fact that cytokines released by pyroptosis aggravate the loss of proteoglycans in the 的内部和外部区域生长,降钙素基因相关肽(CGRP)阳性细胞增加[62]。这可能是由于焦亡释放的细胞因子会加剧ECM
and promotes angiogenesis [32]. 中蛋白聚糖的丢失并促进血管生成[88]。CGRP
is an important nociceptive neurotransmitter for controlling inflammation and pain [33]. Additionally, a large number of inflammatory factors released by cell rupture constantly stimulate sensory nerves, which is also a significant pain factor. A study utilizing 是控制炎症和疼痛的重要伤害性神经递质[89]。此外,细胞破裂释放的大量炎症因子不断刺激感觉神经,这也是一个显著的痛因。一项利用NF-κB
inhibitor 抑制剂Bay11-7082
on the lumbar-disc-herniation rat model found that the assembly of NLRP3 was blocked, and CGRP and pain-related behaviors such as allodynia and heat pain thresholds were ameliorated. This suggests that NF-κB, the standard signal of pain and pyroptosis, is involved in relieving neuropathic pain [34]. Moreover, although the relationship between ion channels and inflammation has not been fully explained, 对腰椎间盘突出症大鼠模型的研究发现,NLRP3的组装被阻断,CGRP和疼痛相关行为(如异常性疼痛和热痛阈值)得到改善。这表明NF-κB(疼痛和焦亡的标准信号)参与缓解神经性疼痛[90]。此外,虽然离子通道与炎症之间的关系尚未得到充分解释,但Ca
2+-
dependent 依赖性Pizol1
channels activated by mechanical stretching [35] and 通道通过机械拉伸[91]激活,ASIC3
channels marked by acid sensing [18] can promote pyroptosis, which may influence the sensitization of pain in the 通道由酸感应[23]标记,可以促进焦亡,这可能会影响IVD
later [36].
后期疼痛的致敏[92]。