Self-replicating RNA viruses have become attractive delivery vehicles for therapeutic applications. They are easy to handle, can be rapidly produced in large quantities, and can be delivered as recombinant viral particles, naked or nanoparticle-encapsulated RNA, or plasmid DNA-based vectors. The self-replication of RNA in infected host cells provides the means for generating much higher transgene expression levels and the possibility to apply substantially reduced amounts of RNA to achieve similar expression levels or immune responses compared to conventional synthetic mRNA. Alphaviruses and flaviviruses, possessing a single-stranded RNA genome of positive polarity, as well as measles viruses and rhabdoviruses with a negative-stranded RNA genome. Particularly, oncolytic self-replicating RNA viruses have demonstrated tumor growth inhibition, tumor eradication and cure in animal tumor models. Stable disease and prolonged overall survival have been reported from clinical trials with oncolytic self-replicating RNA viruses.
- recombinant viral particles
- RNA replicons
- DNA replicons
- oncolytic viruses
- cancer vaccines
- cancer immunotherapy
1. Introduction
2. Characterization of Oncolytic Self-Replicating RNA Viruses
Studies on the origin of cancer have indicated that a subpopulation of cells known as cancer stem cells (CSCs) or cancer-initiating cells (CICs) are responsible for tumorigenesis [16][14]. As CICs have been shown to be resistant to conventional anticancer therapies, the potential of oncolytic viruses to destroy CICs have made them attractive for alternative therapeutic applications. Oncolytic viruses of different origin [7,8,9,10,11,12,13,14,15][7][8][9][10][11][12][13][15][16] comprise wild-type viruses, which are unable to infect normal cells but are cytotoxic to cancer cells [17]. Moreover, the deletion of viral genes critical for replication in normal cells but dispensable in cancer cells has generated attenuated oncolytic strains. Serial passaging in cell cultures has also resulted in attenuated viruses. The mechanisms have been postulated to involve RAS pathway activation or take place by genetic modifications [18]. For these reasons, oncolytic viruses present efficient tumor killing, while only minimal toxicity is caused in normal cells. Self-replicating RNA viruses possess a special feature in the ability of self-replicating of their RNA genome in infected host cells, resulting in approximately 200,000-fold RNA amplification [19]. The single-stranded RNA (ssRNA) genome is of positive polarity for alphaviruses [19] and flaviviruses [20]. In contrast, measles viruses [21] and rhabdoviruses [22] possess a negative-stranded genome. This difference is significant, as, in the former case, viral RNA can directly be translated in the cytoplasm of infected cells, whereas, in the latter case, positive-stranded copies need to be generated prior to translation. Among alphaviruses, the naturally oncolytic M1 alphavirus has been used in several cancer therapeutic applications [23,24][23][24]. Moreover, attenuated Sindbis virus (SIN) strains such as SIN AR339 [25] and vectors based on the Semliki Forest virus (SFV) strain SFV-A7(74) [26] have demonstrated oncolytic properties. Additionally, Aura virus (AURAV) has shown oncotropism for certain tumor cell lines [27]. In the context of flaviviruses, the Zika virus (ZIKV) has demonstrated oncolytic activity against glioblastoma stem cells (GSCs) [14,28][15][28]. The negative-stranded measles viruses (MV) have also demonstrated oncolytic activity in several preclinical studies [15][16]. In the case of rhabdoviruses, the vesicular stomatitis virus (VSV) has been utilized for cancer therapy due to its oncolytic activity [11,29][11][29]. Moreover, the oncolytic Maraba virus has been used for the treatment of sarcoma [30]. The delivery of self-replicating RNA viruses is illustrated in Figure 1.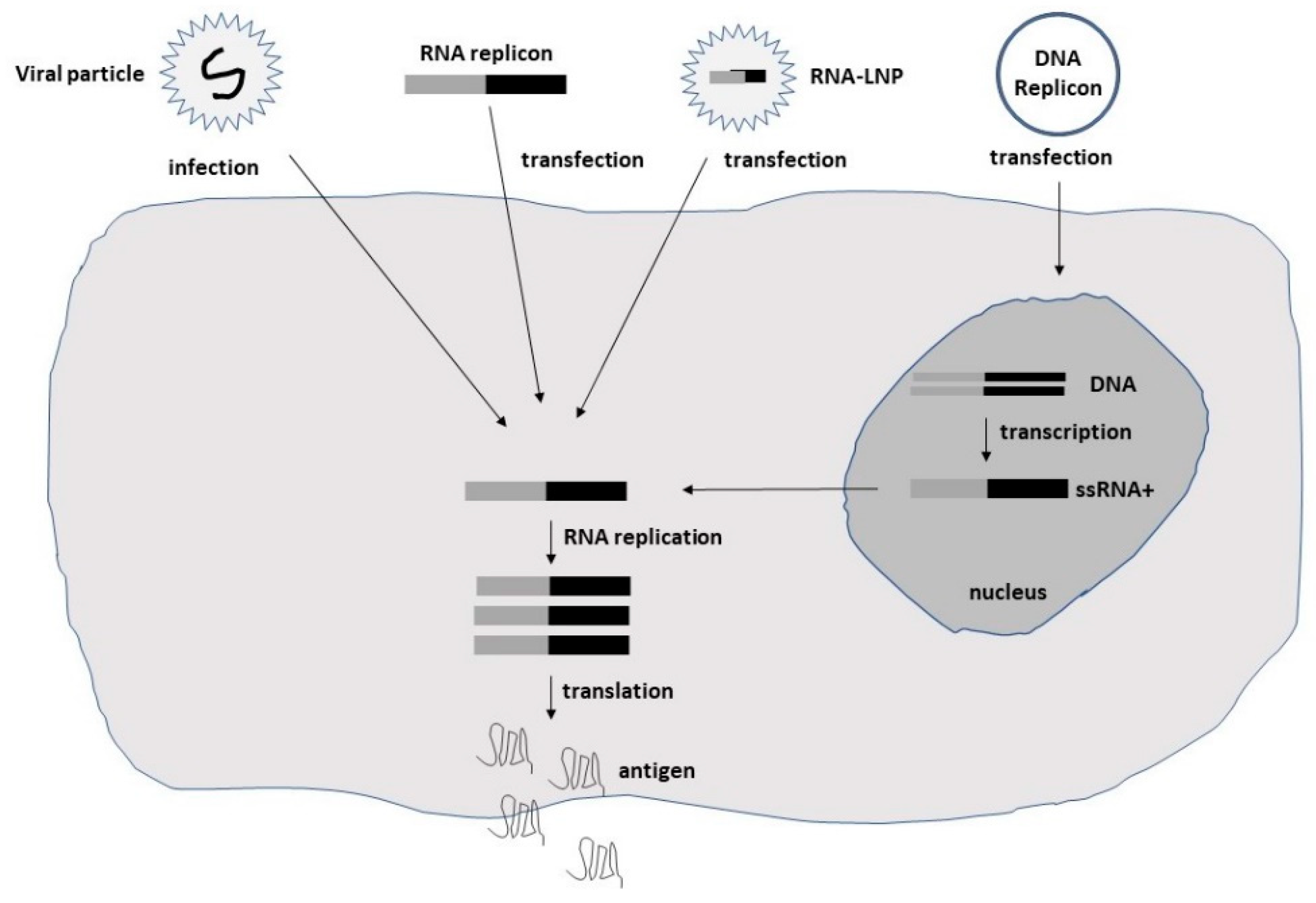
3. Preclinical Studies Using Oncolytic Self-Replicating RNA Viruses
Due to the large number of preclinical studies conducted with oncolytic self-replicating RNA viruses, selected examples for studies using alphaviruses, flaviviruses, measles viruses, and rhabdoviruses are summarized in Table 1.|
Cancer |
Oncolytic Virus |
Gene(s) |
Findings |
Ref. |
|---|---|---|---|---|
|
Alphaviruses |
||||
|
GBM |
SFV VA7 |
EGFP, Rluc |
Tumor eradication, long-term survival in mice |
[31] |
|
Lung A459 |
SFV-VA7 |
EGFP |
Prolonged survival in mice |
[26] |
|
Prostate LNCaP |
SFV-VA7 |
EGFP |
Tumor cell killing, tumor eradication in mice |
[32] |
|
GBM |
SFV-AM6-124T |
miR124 |
Targeting GL261 gliomas, enhanced by anti-PD1 |
[33] |
|
GBM |
SFV4miRT |
miR124,125,134 |
Prolonged survival in mice |
[34] |
|
Cervical |
SIN AR339 |
SIN AR339 |
Tumor cell killing, tumor regression in mice |
[25] |
|
Ovarian |
SIN AR339 |
SIN AR339 |
Tumor cell killing, tumor regression in mice |
[25] |
|
Liver |
M1 |
GFP |
Targeting of liver tumors in mice |
[35] |
|
Glioma |
M1 |
M1 |
Killing of malignant glioma cells in mice, rats |
[36] |
|
Bladder MIBC |
M1 |
GFP |
Tumor growth inhibition in mice |
[37] |
|
Bladder |
M1 |
M1 |
Oncolytic activity in mouse bladder tumor model |
[38] |
|
Breast TNBC |
M1 |
M1 + Dox |
Reduced tumor growth in mice |
[39] |
|
Pancreatic |
M1 |
M1 + IRE |
Superior tumor inhibition, prolonged survival |
[40] |
|
Liver |
M1 |
M-LPO |
Inhibition of Hep3B cancer cell growth in vitro |
[41] |
|
Colorectal |
M1 |
M-LPO |
Inhibition of LoVo cancer cell growth in vitro |
[41] |
|
Flaviviruses |
||||
|
GBM |
ZIKV |
m-ZIKV |
Prolonged survival in mice |
[28] |
|
MB, ependymoma |
ZIKV |
ZIKV |
Infection and killing of GSCs |
[42] |
|
GBM |
ZIKV |
ZIKV + anti-PD1 |
Synergistic effect on survival in mice |
[43] |
|
Embryonal CNS |
ZIKV |
ZIKVBR |
Eradication of brain tumors, no effect on normal cells |
[44] |
|
Spontaneous CNS |
ZIKV |
ZIKVBR |
Tumor eradication, prolonged survival in dogs |
[45] |
|
Prostate |
ZIKV |
ZVp |
Metabolomics to identify PC-3 cancer cell markers |
[46] |
|
Measles viruses |
||||
|
Medulloblastoma |
MV |
GFP |
Complete tumor regression in mice |
[47] |
|
Medulloblastoma |
MV |
GFP |
Significantly prolonged survival in mice |
[48] |
|
Glioma |
MV |
CEA, NIS |
Cytopathic effects in GSC cell lines |
[49] |
|
Breast |
MV |
SLAMblind |
Anti-tumor activity in mice |
[50] |
|
Breast |
MV |
MV |
Infection, killing of MCF-7 and CAL-51 cancer cells |
[51] |
|
Breast |
MV |
MV-Edm |
Re-sensitization of Dox and ironicizing radiation |
[52] |
|
Lung |
MV |
MV Hu-191 |
Suppression of tumor growth in mice |
[53] |
|
Lung. colorectal |
MV |
MV-Schwarz |
Repression of tumor growth in mice |
[54] |
|
Lung |
MV |
CEA |
Tumor growth inhibition in mice |
[55] |
|
Melanoma |
MV |
MV L-16 |
Killing of tumor cells, tumor inhibition in mice |
[56] |
|
Pancreatic |
MV |
SLAMBlind |
Inhibition of tumor growth in mice |
[57] |
|
Pancreatic |
MV |
MV-SCD + Gem |
Reduced tumor mass in pancreatic cell lines |
[58] |
|
Pancreatic |
MV |
MV-miR-148 |
Delayed tumor growth, prolonged survival in mice |
[59] |
|
Prostate |
MV |
CEA |
Delayed tumor growth, prolonged survival in mice |
[60] |
|
Prostate |
MV |
sc-Fv-PSMA |
Killing of prostate cancer cells |
[61] |
|
Prostate |
MV |
MV + MuV |
Superior anti-tumor activity, survival in mice |
[62] |
|
Rhabdoviruses |
||||
|
Glioma, breast |
VSV |
VSVrp30a |
Targeting and eradication of tumors in mice |
[63] |
|
Olfactory bulb |
VSV |
VSVrp30a |
Tumor targeting, no damage to normal cells in mice |
[63] |
|
Glioblastoma |
VSV |
VSV-p1-GFP |
Killing of tumor cells, not normal cells |
[64] |
|
Breast 4T1 |
VSV |
VSV(M51R)-LacZ |
Lesions in breast cancer cells in mice |
[65] |
|
Colon CT-26 |
VSV |
VSV(M51R) |
Prolonged survival in mice |
[66] |
|
Lung LLC-1 |
VSV |
VSV-LCMV GP |
Tumor-to-tumor spread, killing of tumor cells |
[67] |
|
Melanoma |
VSV |
VSV-LCMV GP |
Tumor regression, prolonged survival in mice |
[68] |
|
Ovarian |
VSV |
VSV-LCMV GP |
Superior tumor regression with ruxolitinib |
[69] |
|
Melanoma |
VSV |
VSV-XN2-ΔG |
Tumor regression in mice |
[70] |
|
Ovarian |
VSV |
VSVMP-p DNA |
Tumor weight decrease, prolonged survival in mice |
[71] |
|
Ovarian |
VSV |
VSVMP-p DNA |
87–98% tumor regression, prolonged survival |
[72] |
|
Prostate |
VSV |
VSV(M51R) |
Superior oncolysis after curcumin treatment |
[73] |
|
Melanoma |
Maraba MG1 |
hDCT + Ad-hDCT |
Immune response after prime Ad-hCDT |
[74] |
|
Sarcoma |
Maraba MG1 |
MG1 |
Protection against tumor challenges, cure in mice |
[30] |
|
Breast |
MV, RABV |
rMVEGFP-LDMV |
Blue light induced tumor regression |
[75] |
Ad-hCT, adenovirus hDCT; anti-PD1, anti-programmed death 1; CEA, carcinoembryonic antigen; CNS, central nervous system; Dox, doxorubicin; EGFP, enhanced green fluorescent protein; GBM, glioblastoma multiforme; Gem, gemcitabine; GSCs, glioblastoma stem cells; hDCT, human dopachrome tautomerase; IRE, irreversible electroporation; LCMV GP, lymphocytic choriomeningitis virus glycoprotein; M1, M1 alphavirus; miRNA, microRNA; MB, medulloblastoma; M-LPO, liposome encapsulated M1; MuV, mumps virus; MV, measles virus; MV L-16, MV Leningrad-16 strain: m-ZIKV, mouse adapted ZIKV; NIS, sodium iodide symporter; PSMA, prostate-specific membrane antigen; RABV, rabies virus; rMVEGFP-LDMV, MV with EGFP and controllable Magnet; Rluc, Renilla luciferase; sc-Fv, single-chain antibody; SFV, Semliki Forest virus; SIN, Sindbis virus; SLAMBlind, disenabled signaling lymphocyte activation molecule; TNBC, triple-negative breast cancer; VSV, vesicular stomatitis virus; VSV(M51R), VSV with mutation in matrix protein; VSVMP-p, liposome encapsulated VSV DNA vector with matrix protein ZIKV, Zika virus; ZIKVBR, Brazilian ZIKV strain; ZVp, inactivated ZIKV prototype.
4. Clinical Trials Using Oncolytic Self-Replicating RNA Viruses
A number of clinical trials have also been conducted with oncolytic self-replicating RNA viruses, of which selected examples are listed in Table 2.
Table 2.
Examples of clinical studies using oncolytic self-replicating RNA viruses.
|
Cancer |
|---|
Oncolytic Virus |
Oncolytic Virus |
|---|
Phase |
|---|
Findings |
|---|
Ref |
|---|
|
Ovarian GBM Colorectal Pancreatic CTCL Ovarian Mesothelioma MPNST Head & Neck Myeloma Prostate Breast
|
MV-CEA MV-CEA VEE-CEA VEE-CEA MV-EZ MV-NIS MV-NIS MV-NIS MV-NIS MV.NIS VEE-PSMA VEE-HER2
|
I/II I I I I I I I I I I I
|
No toxicity, SD in patients, 2-fold extended OS Study in progress Antigen-specific responses, extended survival T cell responses, tumor toxicity, extended OS Good safety, complete tumor regression SD in patients, significantly extended OS Study in progress Study in progress Study in progress Complete remission in one patient Safe, but disappointingly weak immune response SD in 1 patient, PR in 2 patients
|
[77] [78.79] [80] [81] [82] [83] [84] [85] [86] [87] [88] [89]
|
||
|
Ovarian |
MV-CEA |
I/II |
No toxicity, SD in patients, 2-fold extended OS |
[76] |
|
GBM |
MV-CEA |
I |
Study in progress |
|
|
Colorectal |
VEE-CEA |
I |
Antigen-specific responses, extended survival |
[79] |
|
Pancreatic |
VEE-CEA |
I |
T cell responses, tumor toxicity, extended OS |
[80] |
|
CTCL |
MV-EZ |
I |
Good safety, complete tumor regression |
[81] |
|
Ovarian |
MV-NIS |
I |
SD in patients, significantly extended OS |
[82] |
|
Mesothelioma |
MV-NIS |
I |
Study in progress |
[83] |
|
MPNST |
MV-NIS |
I |
Study in progress |
[84] |
|
Head & Neck |
MV-NIS |
I |
Study in progress |
[85] |
|
Myeloma |
MV.NIS |
I |
Complete remission in one patient |
[86] |
|
Prostate |
VEE-PSMA |
I |
Safe, but disappointingly weak immune response |
[87] |
|
Breast |
VEE-HER2 |
I |
SD in 1 patient, PR in 2 patients |
[88] |
CEA, carcinoembryonic antigen; CTCL, cutaneous T-cell lymphoma; GBM, glioblastoma multiforme; HER2, human epidermal growth factor receptor 2; MPNSST, malignant peripheral nerve sheath tumor; MV, measles virus; MV-EZ, MV Edmonston-Zagreb strain; NIS, sodium iodide symporter; OS, overall survival; PR, partial response; PSMA, prostate-specific membrane antigen; SD, stable disease; VEE, Venezuelan equine encephalitis virus.
5. Conclusions
Oncolytic self-replicating RNA viruses have been evaluated for the treatment and prevention of various cancers in animal models and clinical trials showing efficient targeting and specific killing of tumor cells. Tumor growth inhibition, tumor regression and cure have been achieved in preclinical studies and stable disease and prolonged survival in clinical trials.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Cancers. CA Cancer J. Clin. 2017, 71, 209–249.
- Magee, M.S.; Snook, A.E.; Marszalowicz, G.P.; Waldman, S.A. Immunotherapeutic Strategies to Target Prognostic and Predictive Markers of Cancer. Biomark. Med. 2013, 7, 23–35.
- Lundstrom, K.; Boulikas, T. Viral and Non-viral Vectors in Gene Therapy: Technology Development and Clinical Trials. Technol. Cancer Res. Treat. 2003, 2, 471–485.
- Fortner, R.T.; Damms-Machado, A.; Kaaks, R. Systematic Review: Tumor-Associated Antigen Autoantibodies and Ovarian Cancer Early Detection. Gynecol. Oncol. 2017, 147, 465–480.
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody Therapy of Cancer. Nat. Rev. Cancer 2012, 12, 278–287.
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662.
- Lin, Y.; Zhang, H.; Liang, J.; Li, K.; Zhu, W.; Fu, L.; Wang, F.; Zheng, X.; Shi, H.; Wu, S.; et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl. Acad. Sci. USA 2014, 111, E4504–E4512.
- Lin, E.; Nemunaitis, J. Oncolytic viral therapies. Cancer Gene Ther. 2004, 11, 643–664.
- Young, B.A.; Spencer, J.F.; Ying, B.; Tollefson, A.E.; Toth, K.; Wold, W.S. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther. 2013, 20, 521–530.
- Li, J.M.; Kao, K.C.; Li., L.F.; Yang, T.M.; Wu, C.P.; Horng, Y.M.; Russelli, A. MicroRNA-145 regulates oncolytic herpes simplex virus-1 for selective killing of human non-small lung cancer cells. Virol. J. 2013, 10, 241.
- Murphy, A.M.; Besmer, D.M.; Moerdyk-Schauwecker, M.; Moestl, N.; Ornelles, D.A.; Mukherjee, P.; Grdzelishvili, V.Z. Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J. Virol. 2012, 86, 3073–3087.
- Zhao, L.; Liu, H. Newcastle disease virus: A promising agent for tumor immunotherapy. Clin. Exp. Pharmacol. Physiol. 2012, 39, 725–730.
- Ehrig, K.; Kilinc, M.O.; Chen, N.G.; Stritzker, J.; Buckel, L.; Zhang, Q.; A Szalay, A. Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68. J. Transl. Med. 2013, 11, 79.
- Cripe, T.P.; Wang, P.-Y.; Marcato, P.; Mahler, Y.Y.; Lee, P.W. Targeting cancer-initiating cells with oncolytic viruses. Mol. Ther. 2009, 17, 1677–1682.
- Francipane, M.G.; Douradinha, P.; Chinnici, C.M.; Russelli, G.; Conaldi, P.G.; Iannolo, G. Zika Virus: A New Therapeutic Candidate for Glioblastoma Treatment. Int. J. Mol. Sci. 2021, 22, 10996.
- Pidelaserra-Marti, G.; Engeland, C.E. Mechanisms of oncolytic measles virus immunotherapy. Cytokine Growth Factor Rev. 2020, 56, 28–38.
- Liu, T.C.; Kirn, D. Gene therapy progress and prospects cancer: Oncolytic viruses. Gene Ther. 2008, 15, 877–884.
- Strong, J.E.; Coffrey, M.C.; Tang, D.; Sabinin, P.; Lee, P.W. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998, 17, 3351–3362.
- Strauss, J.H.; Strauss, E.G. The Alphaviruses: Gene Expression, Replication, and Evolution. Microbiol. Rev. 1994, 58, 491–562.
- Pijlman, G.P.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicons: An RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Exp Opin Biol Ther. 2006, 6, 134–145.
- Donnelly, O.; Errington-Mais, F.; Steele, L.; Hadac, E.; Jennings, V.; Scott, K.; Peach, H.; Phillips, R.M.; Bond, J.; Pandha, H.; et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013, 20, 7–15.
- Osakada, F.; Callaway, E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 2013, 8, 1583–1601.
- Muhuri, M.; Gao, G. Oncolytic Alphavirus M1: A New and Promising Weapon to Fight Cancer. Human Gene Ther. 2021, 32, 136–137.
- Cai, J.; Yan, G. The Identification and Development of a Novel Oncolytic Virus: Alphavirus M1. Human Gene Ther. 2021, 32, 138–149.
- Unno, Y.; Shino, Y.; Kondo, F.; Igarashi, N.; Wang, G.; Shimura, R.; Yamaguchi, T.; Asano, T.; Saisho, H.; Sekiya, S.; et al. Oncolytic Viral Therapy for Cervical and Ovarian Cancer Cells by Sindbis Virus AR339 Strain. Clin. Cancer Res. 2005, 11, 4553–4560.
- Määttä, A.M.; Mäkinen, K.; Ketola, A.; Liimatainen, T.; Yongabi, F.N.; Vähä-Koskela, M. Replication Competent Semliki Forest Virus Prolongs Survival in Experimental Lung Cancer. Int. J. Cancer 2008, 123, 1704–1711.
- Toribio, R.; Diaz-Lopez, I.; Berlanga, J.J.; Molina-Jimenez, F.; Majano, P.; Ventoso, I. Naturally Occurring and Engineered Alphaviruses Sensitive to Double-Stranded-RNA-Activated Protein Kinase Show Restricted Translation in Mammalian Cells, Increased Sensitivity to Interferon, and Marked Oncotropism. J. Virol. 2020, 94, e01630-19.
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017, 214, 2843–2857.
- Urbiola, C.; Santer, F.R.; Petersson, M.; Van Der Pluijm, G.; Horninger, W.; Erlmann, P.; Wollmann, G.; Kimpel, J.; Culig, Z.; Von Laer, D. Oncolytic activity of the rhabdovirus VSV-GP against prostate cancer. Int. J. Cancer 2018, 143, 1786–1796.
- Le Boeuf, F.; Selman, M.; Son, H.H.; Bergeron, A.; Chen, A.; Tsang, J.; Butterwick, D.; Arulanandam, R.; Forbes, N.E.; Tzelepis, F.; et al. Oncolytic Maraba Virus MG1 as a Treatment for Sarcoma. Int. J. Cancer 2017, 141, 1257–1264.
- Heikkilä, J.E.; Vähä-Koskela, M.; Ruotsalainen, J.; Martikainen, M.W.; Stanford, M.M.; McCart, J.A.; Bell, J.C.; Hinkkanen, A.E. Intravenously Administered Alphavirus Vector VA7 Eradicates Orthotopic Human Glioma Xenografts in Nude Mice. PLoS ONE 2010, 5, e8603.
- Martikainen, M.; Ruotsalainen, J.; Tuomela, J.; Härkönen, P.; Essand, M.; Heikkilä, J. Oncolytic alphavirus SFV-A7 efficiently eradicates subcutaneous and orthotopic human prostate tumours in mice. Br. J. Cancer 2017, 117, 51–55.
- Martikainen, M.; Ramachandran, M.; Lugano, R.; Ma, J.; Martikainen, M.-M.; Dimberg, A.; Yu, D.; Merits, A.; Essand, M. IFN-1-tolerant oncolytic Semliki Forest virus in combination with anti-PD1 enhances T cell response against mouse glioma. Mol. Ther. Oncolytics 2021, 21, 37–46.
- Ramachandran, M.; Yu, D.; Dyczynski, M.; Baskaran, S.; Zhang, L.; Lulla, A.; Shimura, E. Safe and Effective Treatment of Experimental Neuroblastoma and Glioblastoma Using Systematically Delivered Triple MicroRNA-Detargeted Oncolytic Semliki Forest Virus. Clin. Cancer Res. 2017, 23, 1519–1530.
- Zhu, W.; Liang, J.; Tan, J.; Guo, L.; Cai, J.; Hu, J.; Yan, G.; Liu, Y.; Zhang, M.J.; Song, D.; et al. Real-Time Visualization and Quantification of Oncolytic M1 Virus In Vitro and In Vivo. Hum. Gene Ther. 2021, 32, 158–165.
- Cai, J.; Zhu, W.; Lin, Y.; Zhang, S.; Chen, M.X.; Gong, S.; He, S.; Hu, J.; Yan, G.; Liang, J. Systematic Characterization of the Biodistribution of the Oncolytic Virus M1. Hum. Gene Ther. 2020, 31, 1203–1213.
- Hu, C.; Liu, Y.; Lin, Y.; Liang, J.-K.; Zhong, W.-W.; Li, K.; Huang, W.-T.; Wang, D.-J.; Yan, G.-M.; Zhu, W.-B.; et al. Intravenous injections of the oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell Death Dis. 2018, 9, 274.
- Liu, Y.; Li, K.; Zhu, W.-B.; Zhang, H.; Huang, W.-T.; Liu, X.-C.; Lin, Y.; Cai, J.; Yan, G.-M.; Qiu, J.-G.; et al. Suppression of CCDC6 sensitizes tumor to oncolytic virus M1. Neoplasia 2021, 23, 158–168.
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.-W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Liang, L.; et al. Necroptotic Virotherapy of Oncolytic Alphavirus M1 Cooperated with Doxorubicin Displays Promising Therapeutic Efficacy in TNBC. Oncogene 2021, 40, 4783–4795.
- Sun, S.; Liu, Y.; He, C.; Hu, W.; Liu, W.; Huang, X.; Wu, J.; Xie, F.; Chen, C.; Wang, J.; et al. Combining Nanoknife with M1 oncolytic virus enhances anticancer activity in pancreatic cancer. Cancer Lett. 2021, 502, 9–24.
- Wang, Y.; Huang, H.; Zou, H.; Tian, X.; Hu, J.; Qiu, P.; Ferreira, L. Liposome Encapsulation of Oncolytic Virus M1 To Reduce Immunogenicity and Immune Clearance in Vivo. Mol. Pharm. 2019, 16, 779–785.
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Kaid, M. Zika Virus Targets Glioblastoma Stem Cells through a SOX-2 Integrin αvβ5 Axis. Cell Stem Cell 2020, 26, 187–204.
- Nair, S.; Mazzoccoli, L.; Jash, A.; Govero, J.; Bais, S.S.; Hu, T.; Fontes-Garfias, C.R.; Shan, C.; Okada, H.; Shresta, S.; et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight 2021, 6, e144619.
- Ferreira, R.O.; Granha, I.; Ferreira, R.S.; Bueno, H.D.S.; Okamoto, O.K.; Kaid, C.; Zatz, M. Effect of Serial Systemic and Intratumoral Injections of Oncolytic ZIKVBR in Mice Bearing Embryonal CNS Tumors. Viruses 2021, 13, 2103.
- Kaid, C.; Madi, R.A.D.S.; Astray, R.; Goulart, E.; Caires-Junior, L.C.; Mitsugi, T.G.; Moreno, A.C.R.; Castro-Amarante, M.F.; Pereira, L.R.; Porchia, B.F.M.M.; et al. Safety, Tumor Redaction, and Clinical Impact of Zika Virus Injection in Dogs with Advanced-Stage Brain Tumors. Mol. Ther. 2020, 28, 1276–1286.
- Delafiori, J.; Lima, E.D.O.; Dabaja, M.Z.; Dias-Audibert, F.L.; de Oliveira, D.N.; Melo, C.F.O.R.; Morishita, K.N.; Sales, G.M.; Ruiz, A.L.T.G.; Da Silva, G.G.; et al. Molecular signatures associated with prostate cancer cell line (PC-3) exposure to inactivated Zika virus. Sci. Rep. 2019, 9, 15351.
- Lal, S.; Carrera, D.; Phillips, J.J.; Weiss, W.A.; Raffel, C. An oncolytic measles virus sensitive Group 3 medulloblastoma model in immune-competent mice. Neuro Oncol. 2018, 20, 1606–1615.
- Studebaker, A.W.; Kreofsky, C.R.; Pierson, C.R.; Russell, S.J.; Glanis, E.; Raffel, C. Treatment of medulloblastoma with a modified measles virus. Neuro Oncol. 2010, 12, 1034–1042.
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo-Musibay, E.; Federspiel, M.J.; Galanis, E. Oncolytic measles virus strains have a significant antitumor activity against glioma stem cells. Gene Ther. 2013, 2, 444–449.
- Sugiyama, T.; Yoneda, M.; Kuraishi, T.; Hattori, S.; Inoue, Y.; Sato, H.; Kai, C. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. 2013, 20, 338–347.
- Abdullah, S.A.; Al-Shammari, A.M.; Lateef, S.A. Attenuated measles vaccine strain have potent oncolytic activity against Iraqi patient derived breast cancer cell line. Saudi J. Biol. Sci. 2020, 27, 865–872.
- Yang, B.; Shi, J.; Sun, Z.; Zhu, D.; Xu, X. Attenuated measles virus overcomes radio and chemoresistance in human breast cancer cells by inhibiting the nonhomologous end joining pathway. Oncol. Rep. 2020, 44, 2253–2264.
- Zhao, D.; Chen, P.; Yang, H.; Wu, Y.; Zeng, X.; Zhao, Y.; Wen, Y.; Zhao, X.; Liu, X.; Wei, Y.; et al. Live attenuated measles virus vaccine induces apoptosis and promotes tumor regression in lung cancer. Oncol. Rep. 2013, 29, 199–204.
- Boisgerault, N.; Guillerme, J.-B.; Pouliquen, D.; Mesel-Lemoine, M.; Achard, C.; Combredet, C.; Fonteneau, J.-F.; Tangy, F.; Grégoire, M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed. Res. Int. 2013, 2013, 387362.
- Patel, M.R.; Jacobson, B.A.; Belgum, H.; Raza, A.; Sadiq, A.; Drees, J.; Wang, H.; Jay-Dixon, J.; Etchison, R.; Federspiel, M.J.; et al. Measles vaccine strains for virotherapy of non-small cell lung carcinoma. J. Thorac. Oncol. 2014, 9, 1101–1110.
- Ammour, Y.; Ryabaya, O.; Shchetinina, Y.; Prokofeva, E.; Gavrilova, M.; Khochenkov, D.; Vorobyev, D.; Faizuloev, E.; Shohin, I.; Zverev, V.V.; et al. The susceptibility of human melanoma cells to infection with the Leningrad-16 vaccine strain of measles virus. Viruses 2020, 12, 173.
- Awano, M.; Fujiyuki, T.; Shoji, K.; Amagai, Y.; Murakami, Y.; Furukawa, Y.; Sato, H.; Yoneda, M.; Kai, C. Measles virus selectively blind to signaling lymphocyte activity molecule has oncolytic efficacy against nectin-4 expressing pancreatic cells. Cancer Sci. 2016, 107, 1647–1652.
- May, V.; Berchtold, S.; Berger, A.; Venturelli, S.; Burkard, M.; Leischner, C.; Malek, N.P.; Lauer, U.M. Chemovirotherapy for pancreatic cancer: Gemcitabine plus oncolytic measles vaccine virus. Oncol. Lett. 2019, 18, 5534–5542.
- Singh, H.M.; Leber, M.F.; Bossow, S.; Engeland, C.E.; Dessila, J.; Grossardt, C. MicroRNA–sensitive oncolytic measles virus for chemovirotherapy of pancreatic cancer. Mol. Ther. Oncolytics 2021, 21, 340–355.
- Msaouel, P.; Iankov, I.D.; Allen, C.; Morris, J.C.; von Messling, V.; Cattaneo, R. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009, 69, 82–91.
- Liu, C.; Hasegawa, K.; Russell, S.J.; Sadelain, M.; Peng, K.-W. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate 2009, 69, 1128–1141.
- Son, H.A.; Zhang, L.; Cuong, B.K.; Van Tong, H.; Cuong, L.D.; Hang, N.T.; Wu, J. Combination of vaccine-strain measles and mumps viruses enhances oncolytic activity against human solid malignancies. Cancer Investig. 2018, 7, 106–117.
- Ozduman, K.; Wollmann, G.; Piepmeier, J.M.; van den Pol, A.N. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J. Neurosci. 2008, 28, 1882–1893.
- Wollmann, G.; Rogulin, V.; Simon, I.; Rose, J.K.; van den Pol, A.N. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J. Virol. 2010, 84, 1563–1573.
- Ebert, O.; Harbaran, S.; Shinozaki, K.; Woo, S.L.C. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immunocompetent mice. Cancer Gene Ther. 2005, 12, 350–358.
- Day, G.L.; Bryan, M.L.; Northrup, S.A.; Lyles, D.S.; Westcott, M.M.; Stewart, J.H., 4th. Immune effects of M51R vesicular stomatitis virus treatment of carcinomatosis from colon cancer. J. Surg. Res. 2020, 245, 127–135.
- Schreiber, L.-M.; Urbiola, C.; Das, K.; Spiesschaert, B.; Kimpel, J.; Heinemann, F.; Stierstorfer, B.; Müller, P.; Petersson, M.; Erlmann, P.; et al. The lytic activity of VSV.GP treatment dominates the therapeutic effects in a syngeneic model of lung cancer. Br. J. Cancer 2019, 121, 647–658.
- Kimpel, J.; Urbiola, C.; Koske, I.; Tober, R.; Banki, Z.; Wollmann, G. The Oncolytic virus VSV-GP is effective against malignant melanoma. Viruses 2018, 10, 108.
- Long, J.; Yang, Y.; Kang, T.; Zhao, W.; Cheng, H.; Wu, Y.; Du, T.; Liu, B.; Li, Y.; Luo, F.; et al. Ovarian cancer therapy by VSVMP gene mediated by a paclitaxel-enhanced nanoparticle. ACS Appl. Mater. Interfaces 2017, 9, 39152–39164.
- Galivo, F.; Diaz, R.M.; Wongthida, P.; Thompson, J.; Kottke, T.; Barber, G.; Melcher, A.; Vile, R. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010, 17, 158–170.
- Lin, X.; Chen, X.; Wei, Y.; Zhao, J.; Fan, L.; Wen, Y.; Wu, H.; Zhao, X. Efficient inhibition of intraperitoneal human ovarian cancer growth and prolonged survival by gene transfer of vesicular stomatitis virus matrix protein in nude mice. Gynecol. Oncol. 2007, 104, 540–546.
- Zhong, Q.; Wen, Y.J.; Yang, H.S.; Luo, H.; Fu, A.F.; Yang, F.; Dowdyl, E. Efficient cisplatin-resistant human ovarian cancer growth and prolonged survival by gene transferred vesicular stomatitis virus matrix protein in nude mice. Ann. Oncol. 2008, 19, 1584–1591.
- Fehl, D.J.; Ahmed, M. Curcumin promotes the oncolytic capacity of vesicular stomatitis virus for the treatment of prostate cancers. Virus Res. 2017, 228, 14–23.
- Pol, J.G.; Zhang, L.; Bridle, B.W.; Stephenson, K.B.; Rességuier, J.; Hanson, S.; Chen, L.; Kazdhan, N.; Bramson, J.; Stojdl, D.F.; et al. Maraba virus as a potent oncolytic vaccine vector. Mol. Ther. 2014, 22, 420–429.
- Tahara, M.; Takishima, Y.; Miyamoto, S.; Nakatsu, Y.; Someya, K.; Sato, M.; Tani, K.; Takeda, M. Photocontrollable mononegaviruses. Proc. Natl. Acad. Sci. USA 2019, 116, 11587–11589.
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882.
- Msaouel, P.; Dispenzieri, A.; Galanis, E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: An overview. Curr. Opin. Mol. Ther. 2009, 11, 43–53.
- Viral Therapy in Treating Patients with Recurrent Glioblastoma Multiforme. Available online: www.clinicaltrials.govNCT00390299 (accessed on 7 November 2022).
- Crosby, E.J.; Hobeika, A.C.; Niedzwiecki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Iii, W.R.G.; Hartman, Z.C.; et al. Long-term Survival of Patients with Stage III colon Cancer Treated with VRP-CEA(6D), an Alphavirus Vector that Increases the CD8+ Effector Memory T Cell to Treg Ratio. J. Immunother. Cancer 2020, 8, e001662.
- Morse, M.A.; Hobeika, A.C.; Osada, T.; Berglund, P.; Hubby, B.; Negri, S.; Niedzwiecki, D.; Devi, G.R.; Burnett, B.K.; Clay, T.M.; et al. An Alphavirus Vector Overcomes the Presence of Neutralizing Antibodies and Elevated Numbers of Tregs to Induce Immune Responses in Humans with Advanced Cancer. J. Clin. Investig. 2010, 120, 3234–3241.
- Heinzerling, L.; Künzi, V.; Oberholzer, P.A.; Kündig, T.; Naim, H.; Dummer, R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumors. Blood 2005, 106, 2287–2294.
- Galanis, E.; Atherton, P.J.; Maurer, M.J.; Knutson, K.L.; Dowdy, S.C.; Cliby, W.A.; Haluska, P.; Long, H.J.; Oberg, A.; Aderca, I.; et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015, 75, 22–30.
- Intrapleural Measles Virus Therapy in Patients with Malignant Pleural Mesothelioma. Available online: www.clinicaltrials.govNCT01503177 (accessed on 7 November 2022).
- Vaccine Therapy in Treating Patients with Malignant Peripheral Nerve Sheath Tumor That Is Recurrent of Cannot Be Removed by Surgery. Available online: www.clinicaltrials.govNCT02700230 (accessed on 7 November 2022).
- Viral Therapy in Treating Patients with Recurrent of Metastatic Squamous Cell Carcinoma of the Head and Neck Cancer or Metastatic Breast Cancer. Available online: www.clinicaltrials.govNCT01846091 (accessed on 7 November 2022).
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Nijman, H. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 2020, 34, 3310–3322.
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Rushing, L. A Phase I Dose Escalation Trial of Vaccine Replicon Particles (VRP) Expressing Prostate-specific Membrane Antigen (PSMA) in Subjects with Prostate Cancer. Vaccine 2013, 31, 943–949.
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Rooney, C.M.; Dispenzieri, A.; Peng, K.-W.; Russell, S.J. Vaccine-induced memory CD8(+) T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clin. Cancer Res. 2019, 25, 2725–2736.
