Self-replicating RNA viruses have become attractive delivery vehicles for therapeutic applications. They are easy to handle, can be rapidly produced in large quantities, and can be delivered as recombinant viral particles, naked or nanoparticle-encapsulated RNA, or plasmid DNA-based vectors. The self-replication of RNA in infected host cells provides the means for generating much higher transgene expression levels and the possibility to apply substantially reduced amounts of RNA to achieve similar expression levels or immune responses compared to conventional synthetic mRNA. Alphaviruses and flaviviruses, possessing a single-stranded RNA genome of positive polarity, as well as measles viruses and rhabdoviruses with a negative-stranded RNA genome. Particularly, oncolytic self-replicating RNA viruses have demonstrated tumor growth inhibition, tumor eradication and cure in animal tumor models. Stable disease and prolonged overall survival have been reported from clinical trials with oncolytic self-replicating RNA viruses.
- recombinant viral particles
- RNA replicons
- DNA replicons
- oncolytic viruses
- cancer vaccines
- cancer immunotherapy
1. Introduction
2. Characterization of Oncolytic Self-Replicating RNA Viruses
Studies on the origin of cancer have indicated that a subpopulation of cells known as cancer stem cells (CSCs) or cancer-initiating cells (CICs) are responsible for tumorigenesis [14][16]. As CICs have been shown to be resistant to conventional anticancer therapies, the potential of oncolytic viruses to destroy CICs have made them attractive for alternative therapeutic applications. Oncolytic viruses of different origin [7][8][9][10][11][12][13][15][16][7,8,9,10,11,12,13,14,15] comprise wild-type viruses, which are unable to infect normal cells but are cytotoxic to cancer cells [17]. Moreover, the deletion of viral genes critical for replication in normal cells but dispensable in cancer cells has generated attenuated oncolytic strains. Serial passaging in cell cultures has also resulted in attenuated viruses. The mechanisms have been postulated to involve RAS pathway activation or take place by genetic modifications [18]. For these reasons, oncolytic viruses present efficient tumor killing, while only minimal toxicity is caused in normal cells. Self-replicating RNA viruses possess a special feature in the ability of self-replicating of their RNA genome in infected host cells, resulting in approximately 200,000-fold RNA amplification [19]. The single-stranded RNA (ssRNA) genome is of positive polarity for alphaviruses [19] and flaviviruses [20]. In contrast, measles viruses [21] and rhabdoviruses [22] possess a negative-stranded genome. This difference is significant, as, in the former case, viral RNA can directly be translated in the cytoplasm of infected cells, whereas, in the latter case, positive-stranded copies need to be generated prior to translation. Among alphaviruses, the naturally oncolytic M1 alphavirus has been used in several cancer therapeutic applications [23][24][23,24]. Moreover, attenuated Sindbis virus (SIN) strains such as SIN AR339 [25] and vectors based on the Semliki Forest virus (SFV) strain SFV-A7(74) [26] have demonstrated oncolytic properties. Additionally, Aura virus (AURAV) has shown oncotropism for certain tumor cell lines [27]. In the context of flaviviruses, the Zika virus (ZIKV) has demonstrated oncolytic activity against glioblastoma stem cells (GSCs) [15][28][14,28]. The negative-stranded measles viruses (MV) have also demonstrated oncolytic activity in several preclinical studies [16][15]. In the case of rhabdoviruses, the vesicular stomatitis virus (VSV) has been utilized for cancer therapy due to its oncolytic activity [11][29][11,29]. Moreover, the oncolytic Maraba virus has been used for the treatment of sarcoma [30]. The delivery of self-replicating RNA viruses is illustrated in Figure 1.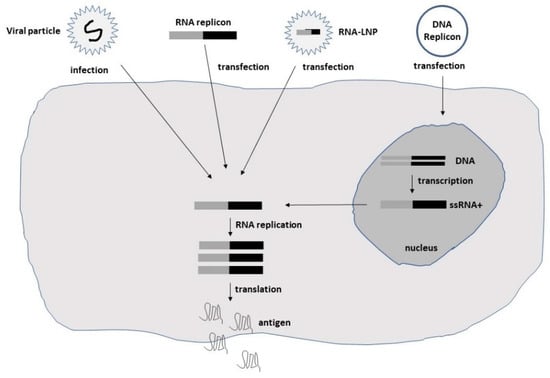
3. Preclinical Studies Using Oncolytic Self-Replicating RNA Viruses
Due to the large number of preclinical studies conducted with oncolytic self-replicating RNA viruses, selected examples for studies using alphaviruses, flaviviruses, measles viruses, and rhabdoviruses are summarized in Table 1.|
Cancer |
Oncolytic Virus |
Gene(s) |
Findings |
Ref. |
|---|---|---|---|---|
|
Alphaviruses |
||||
|
GBM |
SFV VA7 |
EGFP, Rluc |
Tumor eradication, long-term survival in mice |
[31] |
|
Lung A459 |
SFV-VA7 |
EGFP |
Prolonged survival in mice |
[26] |
|
Prostate LNCaP |
SFV-VA7 |
EGFP |
Tumor cell killing, tumor eradication in mice |
[32] |
|
GBM |
SFV-AM6-124T |
miR124 |
Targeting GL261 gliomas, enhanced by anti-PD1 |
[33] |
|
GBM |
SFV4miRT |
miR124,125,134 |
Prolonged survival in mice |
[34] |
|
Cervical |
SIN AR339 |
SIN AR339 |
Tumor cell killing, tumor regression in mice |
[25] |
|
Ovarian |
SIN AR339 |
SIN AR339 |
Tumor cell killing, tumor regression in mice |
[25] |
|
Liver |
M1 |
GFP |
Targeting of liver tumors in mice |
[35] |
|
Glioma |
M1 |
M1 |
Killing of malignant glioma cells in mice, rats |
[36] |
|
Bladder MIBC |
M1 |
GFP |
Tumor growth inhibition in mice |
[37] |
|
Bladder |
M1 |
M1 |
Oncolytic activity in mouse bladder tumor model |
[38] |
|
Breast TNBC |
M1 |
M1 + Dox |
Reduced tumor growth in mice |
[39] |
|
Pancreatic |
M1 |
M1 + IRE |
Superior tumor inhibition, prolonged survival |
[40] |
|
Liver |
M1 |
M-LPO |
Inhibition of Hep3B cancer cell growth in vitro |
[41] |
|
Colorectal |
M1 |
M-LPO |
Inhibition of LoVo cancer cell growth in vitro |
[41] |
|
Flaviviruses |
||||
|
GBM |
ZIKV |
m-ZIKV |
Prolonged survival in mice |
[28] |
|
MB, ependymoma |
ZIKV |
ZIKV |
Infection and killing of GSCs |
[42] |
|
GBM |
ZIKV |
ZIKV + anti-PD1 |
Synergistic effect on survival in mice |
[43] |
|
Embryonal CNS |
ZIKV |
ZIKVBR |
Eradication of brain tumors, no effect on normal cells |
[44] |
|
Spontaneous CNS |
ZIKV |
ZIKVBR |
Tumor eradication, prolonged survival in dogs |
[45] |
|
Prostate |
ZIKV |
ZVp |
Metabolomics to identify PC-3 cancer cell markers |
[46] |
|
Measles viruses |
||||
|
Medulloblastoma |
MV |
GFP |
Complete tumor regression in mice |
[47] |
|
Medulloblastoma |
MV |
GFP |
Significantly prolonged survival in mice |
[48] |
|
Glioma |
MV |
CEA, NIS |
Cytopathic effects in GSC cell lines |
[49] |
|
Breast |
MV |
SLAMblind |
Anti-tumor activity in mice |
[50] |
|
Breast |
MV |
MV |
Infection, killing of MCF-7 and CAL-51 cancer cells |
[51] |
|
Breast |
MV |
MV-Edm |
Re-sensitization of Dox and ironicizing radiation |
[52] |
|
Lung |
MV |
MV Hu-191 |
Suppression of tumor growth in mice |
[53] |
|
Lung. colorectal |
MV |
MV-Schwarz |
Repression of tumor growth in mice |
[54] |
|
Lung |
MV |
CEA |
Tumor growth inhibition in mice |
[55] |
|
Melanoma |
MV |
MV L-16 |
Killing of tumor cells, tumor inhibition in mice |
[56] |
|
Pancreatic |
MV |
SLAMBlind |
Inhibition of tumor growth in mice |
[57] |
|
Pancreatic |
MV |
MV-SCD + Gem |
Reduced tumor mass in pancreatic cell lines |
[58] |
|
Pancreatic |
MV |
MV-miR-148 |
Delayed tumor growth, prolonged survival in mice |
[59] |
|
Prostate |
MV |
CEA |
Delayed tumor growth, prolonged survival in mice |
[60] |
|
Prostate |
MV |
sc-Fv-PSMA |
Killing of prostate cancer cells |
[61] |
|
Prostate |
MV |
MV + MuV |
Superior anti-tumor activity, survival in mice |
[62] |
|
Rhabdoviruses |
||||
|
Glioma, breast |
VSV |
VSVrp30a |
Targeting and eradication of tumors in mice |
[63] |
|
Olfactory bulb |
VSV |
VSVrp30a |
Tumor targeting, no damage to normal cells in mice |
[63] |
|
Glioblastoma |
VSV |
VSV-p1-GFP |
Killing of tumor cells, not normal cells |
[64] |
|
Breast 4T1 |
VSV |
VSV(M51R)-LacZ |
Lesions in breast cancer cells in mice |
[65] |
|
Colon CT-26 |
VSV |
VSV(M51R) |
Prolonged survival in mice |
[66] |
|
Lung LLC-1 |
VSV |
VSV-LCMV GP |
Tumor-to-tumor spread, killing of tumor cells |
[67] |
|
Melanoma |
VSV |
VSV-LCMV GP |
Tumor regression, prolonged survival in mice |
[68] |
|
Ovarian |
VSV |
VSV-LCMV GP |
Superior tumor regression with ruxolitinib |
[69] |
|
Melanoma |
VSV |
VSV-XN2-ΔG |
Tumor regression in mice |
[70] |
|
Ovarian |
VSV |
VSVMP-p DNA |
Tumor weight decrease, prolonged survival in mice |
[71] |
|
Ovarian |
VSV |
VSVMP-p DNA |
87–98% tumor regression, prolonged survival |
[72] |
|
Prostate |
VSV |
VSV(M51R) |
Superior oncolysis after curcumin treatment |
[73] |
|
Melanoma |
Maraba MG1 |
hDCT + Ad-hDCT |
Immune response after prime Ad-hCDT |
[74] |
|
Sarcoma |
Maraba MG1 |
MG1 |
Protection against tumor challenges, cure in mice |
[30] |
|
Breast |
MV, RABV |
rMVEGFP-LDMV |
Blue light induced tumor regression |
[75] |
Ad-hCT, adenovirus hDCT; anti-PD1, anti-programmed death 1; CEA, carcinoembryonic antigen; CNS, central nervous system; Dox, doxorubicin; EGFP, enhanced green fluorescent protein; GBM, glioblastoma multiforme; Gem, gemcitabine; GSCs, glioblastoma stem cells; hDCT, human dopachrome tautomerase; IRE, irreversible electroporation; LCMV GP, lymphocytic choriomeningitis virus glycoprotein; M1, M1 alphavirus; miRNA, microRNA; MB, medulloblastoma; M-LPO, liposome encapsulated M1; MuV, mumps virus; MV, measles virus; MV L-16, MV Leningrad-16 strain: m-ZIKV, mouse adapted ZIKV; NIS, sodium iodide symporter; PSMA, prostate-specific membrane antigen; RABV, rabies virus; rMVEGFP-LDMV, MV with EGFP and controllable Magnet; Rluc, Renilla luciferase; sc-Fv, single-chain antibody; SFV, Semliki Forest virus; SIN, Sindbis virus; SLAMBlind, disenabled signaling lymphocyte activation molecule; TNBC, triple-negative breast cancer; VSV, vesicular stomatitis virus; VSV(M51R), VSV with mutation in matrix protein; VSVMP-p, liposome encapsulated VSV DNA vector with matrix protein ZIKV, Zika virus; ZIKVBR, Brazilian ZIKV strain; ZVp, inactivated ZIKV prototype.
4. Clinical Trials Using Oncolytic Self-Replicating RNA Viruses
A number of clinical trials have also been conducted with oncolytic self-replicating RNA viruses, of which selected examples are listed in Table 2.
Table 2.
Examples of clinical studies using oncolytic self-replicating RNA viruses.
|
Cancer |
Oncolytic Virus |
|---|
Oncolytic Virus |
Phase |
Findings |
Ref |
Ovarian |
|
Mesothelioma MPNST Head & Neck |
|
Ovarian |
[ |
] |
|
Ovarian GBM Colorectal Pancreatic CTCL Myeloma Prostate Breast
|
MV-CEA MV-CEA VEE-CEA VEE-CEA MV-EZ MV-NIS MV-NIS MV-NIS MV-NIS MV.NIS VEE-PSMA VEE-HER2
|
I/II I I I I I I I I I I I
|
No toxicity, SD in patients, 2-fold extended OS Study in progress Antigen-specific responses, extended survival T cell responses, tumor toxicity, extended OS Good safety, complete tumor regression SD in patients, significantly extended OS Study in progress Study in progress Study in progress Complete remission in one patient Safe, but disappointingly weak immune response SD in 1 patient, PR in 2 patients
|
[77] [78.79] [80] [81] [82] [83] [84] [85] [86] [87] [88] [89]
|
||
MV-CEA |
I/II |
No toxicity, SD in patients, 2-fold extended OS |
[76] |
|
|
GBM |
MV-CEA |
I |
Study in progress |
|
|
Colorectal |
VEE-CEA |
I |
Antigen-specific responses, extended survival |
[79] |
|
Pancreatic |
VEE-CEA |
I |
T cell responses, tumor toxicity, extended OS |
[80] |
|
CTCL |
MV-EZ |
I |
Good safety, complete tumor regression |
[81] |
|
Ovarian |
MV-NIS |
I |
SD in patients, significantly extended OS |
[82] |
|
Mesothelioma |
MV-NIS |
I |
Study in progress |
[83] |
|
MPNST |
MV-NIS |
I |
Study in progress |
[84] |
|
Head & Neck |
MV-NIS |
I |
Study in progress |
[85] |
|
Myeloma |
MV.NIS |
I |
Complete remission in one patient |
[86] |
|
Prostate |
VEE-PSMA |
I |
Safe, but disappointingly weak immune response |
[87] |
|
Breast |
VEE-HER2 |
I |
SD in 1 patient, PR in 2 patients |
CEA, carcinoembryonic antigen; CTCL, cutaneous T-cell lymphoma; GBM, glioblastoma multiforme; HER2, human epidermal growth factor receptor 2; MPNSST, malignant peripheral nerve sheath tumor; MV, measles virus; MV-EZ, MV Edmonston-Zagreb strain; NIS, sodium iodide symporter; OS, overall survival; PR, partial response; PSMA, prostate-specific membrane antigen; SD, stable disease; VEE, Venezuelan equine encephalitis virus.
5. Conclusions
Oncolytic self-replicating RNA viruses have been evaluated for the treatment and prevention of various cancers in animal models and clinical trials showing efficient targeting and specific killing of tumor cells. Tumor growth inhibition, tumor regression and cure have been achieved in preclinical studies and stable disease and prolonged survival in clinical trials.
